User:Simoncaulton/sandbox: Difference between revisions
Simoncaulton (talk | contribs) |
Simoncaulton (talk | contribs) No edit summary |
||
| (16 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
[[Image:Contact system.svg|400px|thumb|right|The two arms of the contact system. PKa's cleavage of HK liberates BK and promotes inflammation. FXIIa's cleavage of FXI initiates coagulation.]] |
|||
{{about|cancer cells|the scientific journal|Cancer Cell (journal)}} |
|||
The '''contact activation system''' or '''CAS''' involves three proteins: [[factor XII]] (FXII), [[prekallikrein]] (PK) and [[kininogen|high molecular weight kiningogen]] (HK). FXII and PK are [[protease]]s and HK is a non-enzymatic co-factor. The CAS can activate the [[kinin–kallikrein system]] and [[blood coagulation]] through its ability to activate multiple downstream proteins. The CAS is initiated when FXII binds to a surface and reciprocal activation of FXII and PK occurs, forming FXIIa and PKa. FXIIa can initiate the [[coagulation cascade]] by cleaving and activating [[factor XI]] (FXI), which leads to formation of a blood clot. Additionally, the CAS can activate the kinin–kallikrein system. Here, PKa cleaves HK to form cHK, releasing a peptide known as [[bradykinin]] (BK). BK and its derivatives bind to bradykinin receptors [[bradykinin receptor B1|B1]] and [[bradykinin receptor B2|B2]] to mediate [[inflammation]].<ref>{{cite journal |last1=Schmaier |first1=AH |title=Physiologic activities of the contact activation system. |journal=Thrombosis research |date=May 2014 |volume=133 Suppl 1 |pages=S41-4 |doi=10.1016/j.thromres.2014.03.018 |pmid=24759141}}</ref><ref>{{cite journal |last1=de Maat |first1=S |last2=Tersteeg |first2=C |last3=Herczenik |first3=E |last4=Maas |first4=C |title=Tracking down contact activation - from coagulation in vitro to inflammation in vivo. |journal=International journal of laboratory hematology |date=June 2014 |volume=36 |issue=3 |pages=374-81 |doi=10.1111/ijlh.12222 |pmid=24750684}}</ref> |
|||
{{Expert-subject|date=March 2011}} |
|||
==Surfaces and activation== |
|||
[[File:Breast_cancer_cell_(2).jpg|thumb|right|[[Scanning electron microscopy]] image of a [[breast cancer]] cell]] |
|||
Artificial negatively charged substances that activate FXII include L-homocysteine, heparan sulfates, chondroitin sulfates, dermatan sulfate, uric acid crystals, lipoproteins, ferritin and porphyrins. However, the physiological substances or surfaces that activate FXII are still under debate. These may include proteins, such as gC1q-R, aggregated proteins, amyloid, collagen, nucleic acids, and polyphosphates.<ref>{{cite journal |last1=Ghebrehiwet |first1=B |last2=Kaplan |first2=AP |last3=Joseph |first3=K |last4=Peerschke |first4=EI |title=The complement and contact activation systems: partnership in pathogenesis beyond angioedema. |journal=Immunological reviews |date=November 2016 |volume=274 |issue=1 |pages=281-289 |doi=10.1111/imr.12469 |pmid=27782339}}</ref><ref>{{cite journal |last1=Naudin |first1=C |last2=Burillo |first2=E |last3=Blankenberg |first3=S |last4=Butler |first4=L |last5=Renné |first5=T |title=Factor XII Contact Activation. |journal=Seminars in thrombosis and hemostasis |date=November 2017 |volume=43 |issue=8 |pages=814-826 |doi=10.1055/s-0036-1598003 |pmid=28346966}}</ref><ref>{{cite journal |last1=Schmaier |first1=AH |title=The contact activation and kallikrein/kinin systems: pathophysiologic and physiologic activities. |journal=Journal of thrombosis and haemostasis : JTH |date=January 2016 |volume=14 |issue=1 |pages=28-39 |doi=10.1111/jth.13194 |pmid=26565070}}</ref> The ability of FXII to bind to negatively charged surfaces and activate coagulation forms the basis of the [[aPTT]] test, in which artificial materials act as a surface for contact activation. This test is used to measure the contact activation pathway (intrinsic pathway) and the common pathway of clotting.<ref>{{cite journal |last1=Naudin |first1=C |last2=Burillo |first2=E |last3=Blankenberg |first3=S |last4=Butler |first4=L |last5=Renné |first5=T |title=Factor XII Contact Activation. |journal=Seminars in thrombosis and hemostasis |date=November 2017 |volume=43 |issue=8 |pages=814-826 |doi=10.1055/s-0036-1598003 |pmid=28346966}}</ref> FXII is a [[zymogen]], which means that it requires processing to attain its catalytic protease activity. Upon binding to surfaces, FXII alters in its conformation, giving it low-level protease activity. This change in conformation also promotes its cleavage by PKa and cleavage by FXIIa itself. FXIIa can cleave PK producing PKa, producing a positive feed-back to activate both enzymes. HK binds to PK and is required to locate PK at the surface for activation by FXII.<ref>{{cite journal |last1=de Maat |first1=S |last2=Tersteeg |first2=C |last3=Herczenik |first3=E |last4=Maas |first4=C |title=Tracking down contact activation - from coagulation in vitro to inflammation in vivo. |journal=International journal of laboratory hematology |date=June 2014 |volume=36 |issue=3 |pages=374-81 |doi=10.1111/ijlh.12222 |pmid=24750684}}</ref> |
|||
[[File:Cáncer1EN.png|right|thumb]] |
|||
[[File:Cancer stem cells.svg|right|thumb|A diagram illustrating the distinction between [[cancer stem cell]] targeted and conventional cancer therapies]] |
|||
'''Cancer cells''' are cells that grow and divide at an unregulated, quickened pace. Although [[cancer]] cells can be quite common in a person they are only malignant when the other cells (particularly [[natural killer cell]]s) fail to recognize and/or destroy them.<ref name="ccbcmd1">[http://student.ccbcmd.edu/courses/bio141/lecguide/unit4/innate/nkcell.html The Innate Immune System: NK Cells]. Student.ccbcmd.edu. Retrieved on 2010-12-01.</ref> In the past a common belief was that cancer cells failed to be recognized and destroyed because of a weakness in the [[immune system]]. However more recent research has shown that the failure to recognize cancer cells is caused by the lack of particular co-stimulated molecules that aid in the way [[antigen]]s react with [[lymphocyte]]s.<ref name="autogenerated1">[http://www.research.uky.edu/odyssey/fall99/cancerkllers.html Creating Cancer Killers]. Research.uky.edu. Retrieved on 2010-12-01.</ref> |
|||
==Phyiological roles== |
|||
==The cell and cell division== |
|||
Although the contact system can activate FXI and the subsequent clotting cascade, and it is routinely observed to activate coagulation in the presence of medical devices,<ref>{{cite journal |last1=Jaffer |first1=IH |last2=Fredenburgh |first2=JC |last3=Hirsh |first3=J |last4=Weitz |first4=JI |title=Medical device-induced thrombosis: what causes it and how can we prevent it? |journal=Journal of thrombosis and haemostasis : JTH |date=June 2015 |volume=13 Suppl 1 |pages=S72-81 |doi=10.1111/jth.12961 |pmid=26149053}}</ref> the actual role of the contact system in normal physiological coagulation remains contentious. This is primarily due to the fact that deficiencies in the contact system proteins FXII, PK and HK do not produce bleeding disorders.<ref>{{cite journal |last1=de Maat |first1=S |last2=Tersteeg |first2=C |last3=Herczenik |first3=E |last4=Maas |first4=C |title=Tracking down contact activation - from coagulation in vitro to inflammation in vivo. |journal=International journal of laboratory hematology |date=June 2014 |volume=36 |issue=3 |pages=374-81 |doi=10.1111/ijlh.12222 |pmid=24750684}}</ref> |
|||
Cells are the individual units that make up an organism. In the human body there are trillions of cells that work together, each with their different roles. Physically, cells can look very different, but most will have the same features: an outer [[cell membrane|membrane]] containing fluid ([[cytoplasm]]), a nucleus that contains [[DNA]] and other structures including [[mitochondreon|mitochondrea]], the [[cytoskeleton]] and [[Golgi apparatus]]. The individual structures that make up a cell are known as [[organelle]]s. Although cancer cells will have almost identical features to normal cells, they are subtly different in due to mutations in their DNA, which cause alterations in the way they function. |
|||
The contact activation system's physiological role in the kinin-kallikrein system is more clear. Here, after activation of PK to PKa by FXIIa, PKa cleaves HK. This produces cleaved HK (cHK), releasing a small peptide known as bradykinin. This peptide binds to bradykinin receptor B2 and its derivative, Des-Arg9-bradykinin binds to bradykinin receptor B1. Upon ligand binding, these receptors mediate inflammatory responses.<ref>{{cite journal |last1=Schmaier |first1=AH |title=The contact activation and kallikrein/kinin systems: pathophysiologic and physiologic activities. |journal=Journal of thrombosis and haemostasis : JTH |date=January 2016 |volume=14 |issue=1 |pages=28-39 |doi=10.1111/jth.13194 |pmid=26565070}}</ref> |
|||
During the lifetime of an organism cells will either become damaged or die and will be replaced by other cells that divide by a process known as [[mitosis]]. This process is well regulated and normal cells will not divide when it is not necessary to do so. Cancer cells are abnormal - they grow and divide uncontrollably. The reason for this is that DNA controls the processes within the cell and when the DNA is damaged, it can no longer control these processes properly. Some changes to the cell can be seen visually, such as alterations to the size and shape of the whole cell or certain organelles. Other changes occur, such as those on the molecular level, that cannot be seen without the aid of laboratory techniques. |
|||
==Roles in disease== |
|||
==Causes== |
|||
Activation of the CAS is associated with [[hereditary angioedema]], a disorder characterised by episodes of swelling.<ref>{{cite journal |last1=De Maat |first1=S |last2=Hofman |first2=ZLM |last3=Maas |first3=C |title=Hereditary angioedema: the plasma contact system out of control. |journal=Journal of thrombosis and haemostasis : JTH |date=19 June 2018 |doi=10.1111/jth.14209 |pmid=29920929}}</ref> |
|||
By researching stem cells scientists have suggested that too much [[SP2 protein]] may turn [[stem cells]] into cancer |
|||
cells.<ref>[http://www.sciencedaily.com/releases/2010/10/101027151209.htm Too much SP2 protein turns stem cells into 'evil twin' cancer cells]. Sciencedaily.com (2010-10-27). Retrieved on 2010-12-01.</ref> Other issues thought to play a role in the spread of cancer include [[virus]]es, immune system issues, genetics, environment and age.<ref>[http://www.cancerhelp.org.uk/about-cancer/causes-symptoms/causes/what-causes-cancer What causes cancer? : Cancer Research UK : CancerHelp UK]. Cancerhelp.org.uk (2010-07-15). Retrieved on 2010-12-01.</ref> However, a lack of particular co-stimulated molecules that aid in the way antigens react with lymphocytes can impair the natural killer cells ability and ultimately cause cancer.<ref name="autogenerated1"/> |
|||
==References== |
|||
All cancers begin in cells, the body's basic unit of life. To understand cancer, it's helpful to know what happens when normal cells become cancer cells. |
|||
The body is made up of many types of cells. These cells grow and are controlled to produce more cells as they are needed to keep the body healthy. When cells become old or damaged, they die and are replaced with new cells. |
|||
Sometimes this process of controlled production of cells goes wrong. The genetic material (DNA) of a cell start producing mutations that affect normal cell growth and division by being damaged. When this happens, sometimes these cells do not die but form a mass of tissue called a tumor. Said mutations accumulate, being another reason that cancer is found more often in older people. |
|||
==Pathology== |
|||
White Blood cells are thought to use a dual receptor system when they determine whether or not to kill human cells. If a cell is under stress, turning into tumors, or infected, molecules including MIC-A and MIC-B are produced to put on the surface of the cell.<ref name="ccbcmd1"/> These work to detect and kill cancer cells.<ref>[http://student.ccbcmd.edu/courses/bio141/lecguide/unit5/humoral/abydefense/adcc/adcc.html The Adaptive Immune System: Ways That Antibodies Help to Defend the Body - Antibody-Dependent Cellular Cytotoxicity (ADCC)]. Student.ccbcmd.edu. Retrieved on 2010-12-01.</ref> |
|||
==Discovery== |
|||
Some descriptions of cancer, not all descriptions, go back to [[ancient Egypt]] as far back as 1600 BC and the understanding of cancer was significantly advanced during the [[Renaissance period]]. However, Sir [[Rudolf Virchow]], a German [[biologist]] and [[politician]], is generally credited with discovering the first cancer cells. As [[Giovanni Morgagni]] had linked [[autopsy]] findings seen with the unaided eye with the clinical course of illness, so Virchow correlated microscopic pathology.<ref>[http://www.uib.no/med/avd/miapr/arvid/MOD2/Arvid/Patofysiologi/history_of_cancer.pdf History of cancer]</ref> |
|||
==Telomerase== |
|||
Cancer cells have unique features that make them "immortal" according to some researchers. The enzyme telomerase is used to extend the cancer cell's life span. While the telomeres of most cells shortens after each division eventually causing the cell to die, telomerase extends the cell's telomeres. This is a major reason that cancer cells can accumulate over time creating tumors. |
|||
== Cancer stem cells and drug resistance == |
|||
Scientists have discovered a molecule on the surface of tumors that appears to promote drug resistance—by converting the tumor cells back into a [[stem cell]]-like state. |
|||
When the tumor cells began to exhibit drug resistance, the cells were '''simultaneously''' transforming into a stem cell-like state, which made them impervious to the drugs. It appeared that the treatment itself was driving this transformation by activating a specific molecular pathway. Luckily, several existing drugs can attack this pathway and reverse the cellular transformation, thus ‘re-sensitizing’ the tumor to treatment.<ref>[http://www.eurekalert.org/pub_releases/2014-04/uoc--csc041614.php Cancer stem cells linked to drug resistance]</ref><ref>[http://cirmresearch.blogspot.com/2014/04/tumor-cells-become-drug-resistant-by.html Tumor Cells Become Drug Resistant by Reverting to a Stem Cell-Like State]</ref><ref>Laetitia Seguin, Shumei Kato, Aleksandra Franovic, et al., & David A. Cheresh (2014). [http://www.nature.com/ncb/journal/vaop/ncurrent/full/ncb2953.html An integrin β3–KRAS–RalB complex drives tumour stemness and resistance to EGFR inhibition]. Nature Cell Biology {{doi|10.1038/ncb2953}}</ref> |
|||
==reflist== |
|||
Latest revision as of 10:59, 31 July 2018
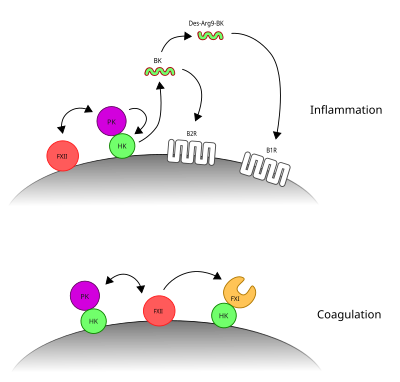
The contact activation system or CAS involves three proteins: factor XII (FXII), prekallikrein (PK) and high molecular weight kiningogen (HK). FXII and PK are proteases and HK is a non-enzymatic co-factor. The CAS can activate the kinin–kallikrein system and blood coagulation through its ability to activate multiple downstream proteins. The CAS is initiated when FXII binds to a surface and reciprocal activation of FXII and PK occurs, forming FXIIa and PKa. FXIIa can initiate the coagulation cascade by cleaving and activating factor XI (FXI), which leads to formation of a blood clot. Additionally, the CAS can activate the kinin–kallikrein system. Here, PKa cleaves HK to form cHK, releasing a peptide known as bradykinin (BK). BK and its derivatives bind to bradykinin receptors B1 and B2 to mediate inflammation.[1][2]
Surfaces and activation
[edit]Artificial negatively charged substances that activate FXII include L-homocysteine, heparan sulfates, chondroitin sulfates, dermatan sulfate, uric acid crystals, lipoproteins, ferritin and porphyrins. However, the physiological substances or surfaces that activate FXII are still under debate. These may include proteins, such as gC1q-R, aggregated proteins, amyloid, collagen, nucleic acids, and polyphosphates.[3][4][5] The ability of FXII to bind to negatively charged surfaces and activate coagulation forms the basis of the aPTT test, in which artificial materials act as a surface for contact activation. This test is used to measure the contact activation pathway (intrinsic pathway) and the common pathway of clotting.[6] FXII is a zymogen, which means that it requires processing to attain its catalytic protease activity. Upon binding to surfaces, FXII alters in its conformation, giving it low-level protease activity. This change in conformation also promotes its cleavage by PKa and cleavage by FXIIa itself. FXIIa can cleave PK producing PKa, producing a positive feed-back to activate both enzymes. HK binds to PK and is required to locate PK at the surface for activation by FXII.[7]
Phyiological roles
[edit]Although the contact system can activate FXI and the subsequent clotting cascade, and it is routinely observed to activate coagulation in the presence of medical devices,[8] the actual role of the contact system in normal physiological coagulation remains contentious. This is primarily due to the fact that deficiencies in the contact system proteins FXII, PK and HK do not produce bleeding disorders.[9]
The contact activation system's physiological role in the kinin-kallikrein system is more clear. Here, after activation of PK to PKa by FXIIa, PKa cleaves HK. This produces cleaved HK (cHK), releasing a small peptide known as bradykinin. This peptide binds to bradykinin receptor B2 and its derivative, Des-Arg9-bradykinin binds to bradykinin receptor B1. Upon ligand binding, these receptors mediate inflammatory responses.[10]
Roles in disease
[edit]Activation of the CAS is associated with hereditary angioedema, a disorder characterised by episodes of swelling.[11]
References
[edit]- ^ Schmaier, AH (May 2014). "Physiologic activities of the contact activation system". Thrombosis research. 133 Suppl 1: S41-4. doi:10.1016/j.thromres.2014.03.018. PMID 24759141.
- ^ de Maat, S; Tersteeg, C; Herczenik, E; Maas, C (June 2014). "Tracking down contact activation - from coagulation in vitro to inflammation in vivo". International journal of laboratory hematology. 36 (3): 374–81. doi:10.1111/ijlh.12222. PMID 24750684.
- ^ Ghebrehiwet, B; Kaplan, AP; Joseph, K; Peerschke, EI (November 2016). "The complement and contact activation systems: partnership in pathogenesis beyond angioedema". Immunological reviews. 274 (1): 281–289. doi:10.1111/imr.12469. PMID 27782339.
- ^ Naudin, C; Burillo, E; Blankenberg, S; Butler, L; Renné, T (November 2017). "Factor XII Contact Activation". Seminars in thrombosis and hemostasis. 43 (8): 814–826. doi:10.1055/s-0036-1598003. PMID 28346966.
- ^ Schmaier, AH (January 2016). "The contact activation and kallikrein/kinin systems: pathophysiologic and physiologic activities". Journal of thrombosis and haemostasis : JTH. 14 (1): 28–39. doi:10.1111/jth.13194. PMID 26565070.
- ^ Naudin, C; Burillo, E; Blankenberg, S; Butler, L; Renné, T (November 2017). "Factor XII Contact Activation". Seminars in thrombosis and hemostasis. 43 (8): 814–826. doi:10.1055/s-0036-1598003. PMID 28346966.
- ^ de Maat, S; Tersteeg, C; Herczenik, E; Maas, C (June 2014). "Tracking down contact activation - from coagulation in vitro to inflammation in vivo". International journal of laboratory hematology. 36 (3): 374–81. doi:10.1111/ijlh.12222. PMID 24750684.
- ^ Jaffer, IH; Fredenburgh, JC; Hirsh, J; Weitz, JI (June 2015). "Medical device-induced thrombosis: what causes it and how can we prevent it?". Journal of thrombosis and haemostasis : JTH. 13 Suppl 1: S72-81. doi:10.1111/jth.12961. PMID 26149053.
- ^ de Maat, S; Tersteeg, C; Herczenik, E; Maas, C (June 2014). "Tracking down contact activation - from coagulation in vitro to inflammation in vivo". International journal of laboratory hematology. 36 (3): 374–81. doi:10.1111/ijlh.12222. PMID 24750684.
- ^ Schmaier, AH (January 2016). "The contact activation and kallikrein/kinin systems: pathophysiologic and physiologic activities". Journal of thrombosis and haemostasis : JTH. 14 (1): 28–39. doi:10.1111/jth.13194. PMID 26565070.
- ^ De Maat, S; Hofman, ZLM; Maas, C (19 June 2018). "Hereditary angioedema: the plasma contact system out of control". Journal of thrombosis and haemostasis : JTH. doi:10.1111/jth.14209. PMID 29920929.
