Aldol reaction: Difference between revisions
m →Mechanisms: changed the word "compounds" to the word "species" when describing the tautomeric form |
m Disambiguating links to Base (link changed to Base (chemistry)) using DisamAssist. |
||
| (235 intermediate revisions by 81 users not shown) | |||
| Line 1: | Line 1: | ||
{{Short description|Chemical reaction}} |
|||
{{Featured article}} |
|||
{{distinguish|Aldol reactions}} |
|||
{{pp-move-indef}} |
|||
{{Reactionbox |
|||
The '''aldol reaction''' is a means of forming [[carbon–carbon bond]]s in [[organic chemistry]].<ref name=Wade> |
|||
| Name = Aldol Addition |
|||
{{cite book |
|||
| Type = Coupling reaction |
|||
| last = Wade | first = L. G. |
|||
| Section3 = {{Reactionbox Identifiers |
|||
| title = Organic Chemistry |
|||
| OrganicChemistryNamed = aldol-addition |
|||
| publisher = Prentice Hall |
|||
| RSC_ontology_id = 0000016 |
|||
| year = 2005 |
|||
}} |
|||
| edition = 6th |
|||
|Reaction={{Reactionbox Reaction |
|||
| location = Upper Saddle River, New Jersey |
|||
| Reactant1 = [[Ketone]] or [[Aldehyde]] |
|||
| pages = 1056–66 |
|||
| Reactant2 = [[Ketone]] or [[Aldehyde]] |
|||
| isbn = 0-13-236731-9 }}</ref><ref name=March> |
|||
| Reagent1= |
|||
{{cite book |
|||
| Product1 =[[Aldol|β-hydroxy Aldehyde]] {{br}}or{{br}} [[Aldol|β-hydroxy Ketone]] |
|||
| last1 = Smith | first1 = M. B. |
|||
| Sideproduct1 = |
|||
| last2=March | first2= J. |
|||
}}|Section1={{Reactionbox Conditions |
|||
| title = Advanced Organic Chemistry |
|||
| Reference = |
|||
| publisher = Wiley Interscience |
|||
| Solvent = |
|||
| Catalyst = {{center|<sup>-</sup>OH or H<sup>+</sup>}} |
|||
| edition = 5th |
|||
| Temperature = {{center|-Δ, ~-70°C{{Efn| It is typically best to minimize heat for this reaction. As removal of water from excess heat risks shifting the equilibrium in favor of a dehydration reaction, leading to the aldol condensation product.<br>By avoiding heat, it can help avoid dehydration so that the majority of product produced is the aldol addition product.<ref>{{Cite book |last=Klein |first=David R. |url=https://www.worldcat.org/oclc/1201694230 |title=Organic chemistry |date=December 22, 2020 |publisher=Wiley |isbn=978-1-119-65959-4 |edition=4th |location=Hoboken, NJ |pages=1014 |oclc=1201694230}}</ref>}}}} |
|||
| location = New York |
|||
}}}} |
|||
| pages = 1218–23 |
|||
| isbn = 0-471-58589-0}} |
|||
</ref><ref name=Mahrwald2004> |
|||
{{cite book |
|||
| last = Mahrwald | first = R. |
|||
| title = Modern Aldol Reactions, Volumes 1 and 2 |
|||
| publisher = Wiley-VCH Verlag GmbH & Co. KGaA |
|||
| year = 2004 |
|||
| location = Weinheim, Germany |
|||
| pages = 1218–23 |
|||
| isbn = 3-527-30714-1}} |
|||
</ref> |
|||
Discovered independently by [[Charles-Adolphe Wurtz]]<ref name=Wurtz1872> |
|||
{{cite journal |
|||
| last = Wurtz | first = C. A. |
|||
| authorlink = Charles-Adolphe Wurtz |
|||
| journal = Bull. Soc. Chim. Fr. |
|||
| year =1872 |
|||
| title = |
|||
| volume = 17 |
|||
| pages =436–442}}</ref><ref name=Wurtz1872b> |
|||
{{cite journal |
|||
| last = Wurtz | first = C. A. |
|||
| authorlink = Charles-Adolphe Wurtz |
|||
| journal = Journal für Praktische Chemie |
|||
| year = 1872 |
|||
| title = Ueber einen Aldehyd-Alkohol |
|||
| volume = 5 |
|||
| issue = 1 |
|||
| pages = 457–464 |
|||
| doi = 10.1002/prac.18720050148 |
|||
}}</ref><ref name=Wurtz1872c> |
|||
{{cite journal |
|||
| last = Wurtz | first = C. A. |
|||
| authorlink = Charles-Adolphe Wurtz |
|||
| journal = [[Comptes rendus de l'Académie des sciences]] |
|||
| year = 1872 |
|||
| title = Sur un aldéhyde-alcool |
|||
| volume = 74 |
|||
| pages =1361 |
|||
| language = French |
|||
| url = http://gallica.bnf.fr/ark:/12148/bpt6k3031q/f1361.table }}</ref> and [[Alexander Porfyrevich Borodin|Alexander Borodin]] in 1872,<ref>Borodin observed the dimerization of acetaldehyde to 3-hydroxybutanal under acidic conditions</ref> the reaction combines two [[carbonyl]] compounds (the original experiments used [[aldehyde]]s) to form a new β-hydroxy carbonyl compound. These products are known as ''[[aldol]]s'', from the ''ald''ehyde + alcoh''ol'', a structural motif seen in many of the products. Aldol structural units are found in many important molecules, whether naturally occurring or synthetic.<ref name=Heathcock1991>{{cite book |
|||
| authorlink = Clayton Heathcock |
|||
| last = Heathcock | first = C. H. |
|||
| editor = Trost, B. M.; Fleming, I |
|||
| chapter = The Aldol Reaction: Acid and General Base Catalysis |
|||
| title = Comprehensive Organic Synthesis |
|||
| volume = 2 |
|||
| publisher = Elsevier Science |
|||
| year = 1991 |
|||
| url = http://www.sciencedirect.com/science/article/pii/B9780080523491000275 |
|||
| pages = 133–179 |
|||
| isbn = 978-0-08-052349-1 |
|||
| doi = 10.1016/B978-0-08-052349-1.00027-5}} |
|||
</ref><ref name=Mukaiyama1982> |
|||
{{cite journal |
|||
| title = The Directed Aldol Reaction |
|||
| author = Mukaiyama T. |
|||
| journal = Org. React. |
|||
| year = 1982 |
|||
| volume = 28 |
|||
| pages = 203–331 |
|||
| doi = 10.1002/0471264180.or028.03 }} |
|||
</ref><ref name=Paterson1988> |
|||
{{cite journal |
|||
| title = New Asymmetric Aldol Methodology Using Boron Enolates |
|||
| author = Paterson, I. |
|||
| journal = Chem. Ind. |
|||
| year = 1988 |
|||
| volume = 12 |
|||
| pages = 390–394}}</ref> |
|||
For example, the aldol reaction has been used in the large-scale production of the commodity chemical [[pentaerythritol]]<ref name=mestres> |
|||
{{cite journal |
|||
| title = A green look at the aldol reaction |
|||
| author = Mestres R. |
|||
| journal = Green Chemistry |
|||
| year = 2004 |
|||
| volume = 6 |
|||
| pages = 583–603 |
|||
| doi = 10.1039/b409143b |
|||
| issue = 12 }}</ref> |
|||
and the synthesis of the heart disease drug Lipitor ([[atorvastatin]], calcium salt).<ref name=Braun5031>{{cite journal |
|||
| title = (R) and (S)-2-acetoxy-1,1,2-triphenylethanol – effective synthetic equivalents of a chiral acetate enolate |
|||
| author = M. Braun |
|||
|author2=R. Devant |
|||
| journal = Tetrahedron Letters |
|||
| year = 1984 |
|||
| volume = 25 |
|||
| pages = 5031–4 |
|||
| doi = 10.1016/S0040-4039(01)91110-4 |
|||
| issue = 44 |
|||
}} |
|||
</ref><ref name=jackli2004>{{cite book |
|||
| author = Jie Jack Li et al. |
|||
| title = Contemporary Drug Synthesis |
|||
| publisher = Wiley-Interscience |
|||
| year = 2004 |
|||
| location = |
|||
| pages = 118– |
|||
| isbn = 0-471-21480-9}} |
|||
</ref> |
|||
The '''aldol reaction''' ('''aldol addition''') is a [[Chemical reaction|reaction]] in [[organic chemistry]] that combines two [[Carbonyl group|carbonyl]] compounds (e.g. [[aldehyde]]s or [[ketone]]s) to form a new β-hydroxy carbonyl compound. Its simplest form might involve the [[nucleophilic addition]] of an [[Enolate|enolized]] [[ketone]] to another: |
|||
The aldol reaction unites two relatively simple molecules into a more complex one. Increased complexity arises because up to two new [[stereogenic center]]s (on the [[Alpha carbon|α- and β-carbon]] of the aldol adduct, marked with asterisks in the scheme below) are formed. Modern methodology is capable of not only allowing aldol reactions to proceed in high yield but also controlling both the relative and absolute stereochemical configuration of these stereocenters. This ability to selectively synthesize a particular [[stereoisomer]] is significant because different stereoisomers can have very different chemical and biological properties. |
|||
[[File:Aldolsimple.svg|thumb|center|423x423px|Prototype aldol reaction]] |
|||
For example, stereogenic aldol units are especially common in [[polyketide]]s, a class of [[natural products|molecules found in biological organisms]]. In nature, polyketides are synthesized by enzymes that effect iterative [[Claisen condensation]]s. The 1,3-dicarbonyl products of these reactions can then be variously derivatized to produce a wide variety of interesting structures. Often, such derivitization involves the reduction of one of the carbonyl groups, producing the aldol subunit. Some of these structures have potent biological properties: the immunosuppressant [[FK506]], the anti-tumor agent [[discodermolide]], or the antifungal agent [[amphotericin B]], for example. Although the synthesis of many such compounds was once considered nearly impossible, aldol methodology has allowed their efficient [[total synthesis|synthesis]] in many cases.<ref name=MahrwaldSchetter2006> |
|||
{{cite journal |
|||
| title = Modern Aldol Methods for the Total Synthesis of Polyketides |
|||
| author = Schetter, B. |
|||
|author2=Mahrwald, R. |
|||
| journal =Angew. Chem. Int. Ed. |
|||
| year =2006 |
|||
| volume =45 |
|||
| pages =7506–7525 |
|||
| doi = 10.1002/anie.200602780 |
|||
| pmid = 17103481 |
|||
| issue = 45 }}</ref> |
|||
These products are known as ''[[aldol]]s'', from the ''ald''ehyde + alcoh''ol'', a structural motif seen in many of the products. The use of aldehyde in the name comes from its history: aldehydes are more reactive than ketones, so that the reaction was discovered first with them.<ref name="Wurtz1872" /><ref name="Wurtz1872b" /><ref name="Wurtz1872c" /> |
|||
[[File:Typical aldol-en.svg|600px|center]] |
|||
The aldol reaction is [[paradigmatic]] in organic chemistry and one of the most common means of forming [[carbon–carbon bond]]s in [[organic chemistry]].<ref name="Wade">{{cite book |last=Wade |first=L. G. |title=Organic Chemistry |publisher=Prentice Hall |year=2005 |isbn=978-0-13-236731-8 |edition=6th |location=Upper Saddle River, New Jersey |pages=1056–66}}</ref><ref name="March">{{cite book |last1=Smith |first1=Michael B. |title=March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure |last2=March |first2=Jerry |year=2006 |isbn=9780470084960 |doi=10.1002/0470084960}}</ref><ref name="Mahrwald2004">{{cite book |last=Mahrwald |first=R. |url=https://archive.org/details/modernaldolreact00rain/page/1218 |title=Modern Aldol Reactions, Volumes 1 and 2 |publisher=Wiley-VCH Verlag GmbH & Co. KGaA |year=2004 |isbn=978-3-527-30714-2 |location=Weinheim, Germany |pages=[https://archive.org/details/modernaldolreact00rain/page/1218 1218–23] |url-access=registration}}</ref> It lends its name to the family of [[aldol reactions]] and similar techniques analyze a whole family of [[carbonyl α-substitution reactions]], as well as the [[Claisen condensation|diketone condensations]]. |
|||
A typical modern aldol [[addition reaction]], shown above, might involve the [[nucleophilic addition]] of a [[ketone enolate]] to an [[aldehyde]]. Once formed, the aldol product can sometimes [[Dehydration reaction|lose a molecule of water]] to form an [[α,β-unsaturated carbonyl compound]]. This is called ''[[aldol condensation]]''. A variety of nucleophiles may be employed in the aldol reaction, including the [[enol]]s, [[enolate]]s, and enol [[ether]]s of ketones, aldehydes, and many other [[carbonyl]] compounds. The [[electrophile|electrophilic]] partner is usually an aldehyde or ketone (many variations, such as the [[Mannich reaction]], exist). When the nucleophile and electrophile are different, the reaction is called a ''crossed aldol reaction''; on the converse, when the nucleophile and electrophile are the same, the reaction is called an ''aldol [[Dimer (chemistry)|dimerization]]''. |
|||
[[File:aldolrxnpic.jpg|thumb|right|300px|A typical experimental setup for an aldol reaction.<br /> The flask on the right is a solution of [[lithium diisopropylamide]] (LDA) in [[tetrahydrofuran]] (THF). The flask on the left is a solution of the lithium enolate of ''tert''-butyl propionate (formed by addition of LDA to ''tert''-butyl propionate). An aldehyde can then be added to the enolate flask to initiate an aldol addition reaction.<br /> Both flasks are submerged in a dry ice/acetone [[cooling bath]] (−78 °C) the temperature of which is being monitored by a thermocouple (the wire on the left).]] |
|||
== |
==Scope== |
||
Aldol structural units are found in many important molecules, whether naturally occurring or synthetic.<ref name="Heathcock1991">{{cite book |last=Heathcock |first=C. H. |title=Comprehensive Organic Synthesis |publisher=Elsevier Science |year=1991 |isbn=978-0-08-052349-1 |editor1=Trost, B. M. |editor-link1=Barry Trost |volume=2 |pages=133–179 |chapter=The Aldol Reaction: Acid and General Base Catalysis |doi=10.1016/B978-0-08-052349-1.00027-5 |author-link=Clayton Heathcock |editor2=Fleming, I. |editor-link2=Ian Fleming (chemist)}}</ref><ref name="Paterson1988">{{cite journal |author=Paterson, I. |year=1988 |title=New Asymmetric Aldol Methodology Using Boron Enolates |journal=Chem. Ind. |volume=12 |pages=390–394}}</ref> The reaction is well used on an industrial scale, notably of [[pentaerythritol]],<ref name="mestres">{{cite journal| title = A green look at the aldol reaction| author = Mestres R.| journal = Green Chemistry| year = 2004| volume = 6| pages = 583–603| doi = 10.1039/b409143b| issue = 12}}</ref> [[trimethylolpropane]], the plasticizer precursor [[2-Ethylhexanol|2-ethylhexanol]], and the drug Lipitor ([[atorvastatin]], calcium salt).<!--what is this here for?<ref name=Braun5031>{{cite journal| title = (R) and (S)-2-acetoxy-1,1,2-triphenylethanol – effective synthetic equivalents of a chiral acetate enolate| author = M. Braun|author2=R. Devant| journal = Tetrahedron Letters| year = 1984| volume = 25| pages = 5031–4| doi = 10.1016/S0040-4039(01)91110-4| issue = 44}} |
|||
The aldol reaction may proceed via two fundamentally different mechanisms. Carbonyl compounds, such as aldehydes and ketones, can be converted to enols or enol ethers. These species, being nucleophilic at the [[Alpha-carbon|α-carbon]], can attack especially reactive protonated carbonyls such as protonated aldehydes. This is the 'enol mechanism'. Carbonyl compounds, being [[carbon acid]]s, can also be deprotonated to form enolates, which are much more nucleophilic than enols or enol ethers and can attack electrophiles directly. The usual electrophile is an aldehyde, since ketones are much less reactive. This is the 'enolate mechanism'. |
|||
</ref>--><ref name="jackli2004">{{cite book| author = Jie Jack Li| title = Contemporary Drug Synthesis| publisher = Wiley-Interscience| year = 2004| pages = 118–| isbn = 978-0-471-21480-9| display-authors= etal}}</ref> For many of the commodity applications, the stereochemistry of the aldol reaction is unimportant, but the topic is of intense interest for the synthesis of many specialty chemicals. |
|||
===Aldol dimerization=== |
|||
If the conditions are particularly harsh (e.g.: NaOMe/MeOH/[[reflux]]), condensation may occur, but this can usually be avoided with mild reagents and low temperatures (e.g., LDA (a strong base), THF, −78 °C). Although the aldol addition usually proceeds to near completion under irreversible conditions, the isolated aldol adducts are sensitive to base-induced retro-aldol cleavage to return starting materials. In contrast, retro-aldol condensations are rare, but possible.<ref name=Guthrie1984>{{cite journal |
|||
In its simplest implementation, base induces conversion of an aldehyde or a ketone to the aldol product. One example involves the aldol condensation of [[propionaldehyde]]: |
|||
| title = The retroaldol reaction of cinnamaldehyde |
|||
:{{chem2|2 CH3CH2CHO -> CH3CH2CH(OH)CH(CH3)CHO}} |
|||
| author = Guthrie, J.P. |
|||
Featuring the RCH(OH)CHR'C(O)R" grouping, the product is an [[aldol]]. In this case {{chem2|R = CH3CH2, R' = CH3, and R'' = H}}. Such reactions are called '''aldol [[Dimer (chemistry)|aldol dimerization]]'''. |
|||
|author2= Cooper, K.J.|author3= Cossar, J.|author4= Dawson, B.A.|author5= Taylor, K.F. |
|||
| doi = 10.1139/v84-243 |
|||
| journal =[[Can. J. Chem.]] |
|||
| year =1984 |
|||
| volume =62 |
|||
| pages =1441–1445 |
|||
| issue = 8}}</ref> |
|||
===Cross-aldol=== |
|||
[[File:Simple aldol reaction.svg|750px|A generalized view of the Aldol reaction|center]] |
|||
With a mixture of carbonyl precursors, complicated mixtures can occur. Addition of base to a mixture of propionaldehyde and acetaldehyde, one obtains four products: |
|||
:{{chem2|CH3CH2CH(OH)CH(CH3)CHO (from two propionaldehydes), CH3CH(OH)CH2CHO (from two acetaldehydes, and both CH3CH2CH(OH)CH2CHO and CH3CH(OH)CH(CH3)CHO}} |
|||
The first two products are the result of aldol dimerization but the latter two result from a '''crossed aldol reaction'''. Complicated mixtures from cross aldol reactions can be avoided by using one component that cannot form an enolate, examples being [[formaldehyde]] and [[benzaldehyde]]. This approach is used in one stage in the production of [[trimethylolethane]], which entails crossed aldol condensation of butyraldehyde and formaldehyde: |
|||
:{{chem2|CH3CH2CH2CHO + 2 CH2O → CH3CH2C(CH2OH)2CHO}} |
|||
=== |
===Reactions of aldols=== |
||
Aldols dehydrate: |
|||
When an acid catalyst is used, the initial step in the [[reaction mechanism]] involves acid-catalyzed [[tautomer]]ization of the carbonyl compound to the enol. The acid also serves to activate the carbonyl group of ''another molecule'' by protonation, rendering it highly [[electrophile|electrophilic]]. The enol is [[nucleophile|nucleophilic]] at the α-carbon, allowing it to attack the protonated carbonyl compound, leading to the aldol after [[deprotonation]]. This usually [[Dehydration reaction|dehydrates]] to give the unsaturated carbonyl compound. The scheme shows a typical acid-catalyzed self-condensation of an aldehyde. |
|||
:{{chem2|CH3CH2CH(OH)CH(CH3)CHO -> CH3CH2CH\dC(CH3)CHO + H2O}} |
|||
Because this conversion is facile, it is sometimes assumed. It is for this reason that the aldol reaction is sometimes called the aldol condensation. |
|||
==Mechanisms== |
|||
'''Acid-catalyzed aldol mechanism''' |
|||
[[File:aldolrxnpic.jpg|thumb|right|300px|A typical experimental setup for an aldol reaction in a research laboratory.<br /> The flask on the right is a solution of [[lithium diisopropylamide]] (LDA) in [[tetrahydrofuran]] (THF). The flask on the left is a solution of the lithium enolate of ''tert''-butyl propionate (formed by addition of LDA to ''tert''-butyl propionate). An aldehyde can then be added to the enolate flask to initiate an aldol addition reaction.<br /> Both flasks are submerged in a dry ice/acetone [[cooling bath]] (−78 °C) the temperature of which is being monitored by a thermocouple (the wire on the left).]] |
|||
:[[File:Enol aldol formation mechanism.png|500px|Mechanism for acid-catalyzed aldol reaction of an aldehyde with itself|center]] |
|||
The aldol reaction has one underlying mechanism: a carbanion-like nucleophile attacks a carbonyl center.<ref>{{Cite book |last=Grossmann |first=Robert B. |title=The Art of Writing Reasonable Organic Reaction Mechanisms |date=Jan 2002 |publisher=Springer |isbn=0-387-95468-6 |edition=2nd |location=New York |pages=133}}</ref> |
|||
'''Acid-catalyzed dehydration''' |
|||
[[File:Enol aldol dehydration mechanism.png|500px|Mechanism for acid-catalyzed dehydration of an aldol|center]] |
|||
If the [[Base (chemistry)|base]] is of only moderate strength such as [[hydroxide]] ion or an [[alkoxide]], the aldol reaction occurs via nucleophilic attack by the [[Resonance (chemistry)|resonance-stabilized]] enolate on the carbonyl group of another molecule. The product is the [[alkoxide]] salt of the aldol product. The aldol itself is then formed, and it may then undergo dehydration to give the unsaturated carbonyl compound. The scheme shows a simple mechanism for the base-catalyzed aldol reaction of an aldehyde with itself.[[File:Aldol addition base-catalyzed.svg|795x795px|Simple mechanism for base-catalyzed aldol reaction of an aldehyde with itself|center]] |
|||
===Enolate mechanism=== |
|||
If the [[catalyst]] is a moderate base such as [[hydroxide]] ion or an [[alkoxide]], the aldol reaction occurs via nucleophilic attack by the [[Resonance (chemistry)|resonance-stabilized]] enolate on the carbonyl group of another molecule. The product is the [[alkoxide]] salt of the aldol product. The aldol itself is then formed, and it may then undergo dehydration to give the unsaturated carbonyl compound. The scheme shows a simple mechanism for the base-catalyzed aldol reaction of an aldehyde with itself. |
|||
'''Base-catalyzed aldol reaction''' (shown using [[methoxide|<sup>−</sup>OCH<sub>3</sub>]] as base) |
|||
[[File:Enolate aldol formation mechanism.png|500px|Simple mechanism for base-catalyzed aldol reaction of an aldehyde with itself|center]] |
|||
'''Base-catalyzed dehydration''' (frequently written incorrectly as a single step, see [[E1cB elimination reaction]]) |
|||
[[File:Enolate aldol dehydration mechanism.png|500px|Simple mechanism for the dehydration of an aldol product|center]] |
|||
Although only a catalytic amount of base is required in some cases, the more usual procedure is to use a [[stoichiometric]] amount of a strong base such as [[Lithium diisopropylamide|LDA]] or [[Sodium hexamethyldisilazide|NaHMDS]]. In this case, enolate formation is irreversible, and the aldol product is not formed until the metal alkoxide of the aldol product is protonated in a separate workup step. |
Although only a catalytic amount of base is required in some cases, the more usual procedure is to use a [[stoichiometric]] amount of a strong base such as [[Lithium diisopropylamide|LDA]] or [[Sodium hexamethyldisilazide|NaHMDS]]. In this case, enolate formation is irreversible, and the aldol product is not formed until the metal alkoxide of the aldol product is protonated in a separate workup step. |
||
When an acid catalyst is used, the initial step in the [[reaction mechanism]] involves acid-catalyzed [[tautomer]]ization of the carbonyl compound to the enol. The acid also serves to activate the carbonyl group of ''another molecule'' by protonation, rendering it highly electrophilic. The enol is nucleophilic at the α-carbon, allowing it to attack the protonated carbonyl compound, leading to the aldol after [[deprotonation]]. Some may also dehydrate past the intended product to give the unsaturated carbonyl compound through [[aldol condensation]].[[File:Aldol addition acid-catalyzed.svg|880x880px|Mechanism for acid-catalyzed aldol reaction of an aldehyde with itself|center]] |
|||
===Zimmerman–Traxler model=== |
|||
More refined forms of the mechanism are known. In 1957, [[Howard Zimmerman|Zimmerman]] and Traxler proposed that some aldol reactions have "six-membered transition states having a [[chair conformation]]."<ref name=Zimmerman1920>{{cite journal |
|||
| title = The Stereochemistry of the Ivanov and Reformatsky Reactions. I |
|||
| author = Zimmerman, H. E. |
|||
|author2=Traxler, M. D. |
|||
| doi = 10.1021/ja01565a041 |
|||
| journal = [[Journal of the American Chemical Society]] |
|||
| year =1957 |
|||
| volume =79 |
|||
| pages =1920–1923 |
|||
| issue = 8}}</ref> This is now known as the '''Zimmerman–Traxler model'''. ''E''-enolates give rise to [[anti isomer|anti products]], whereas ''Z''-enolates give rise to [[syn addition|syn products]]. The factors that control selectivity are the preference for placing substituents equatorially in six-membered transition states and the avoidance of [[pentane interference|syn-pentane interactions]], respectively.<ref name=Heathcock1980>{{cite journal |
|||
|title = Acyclic stereoselection. 7. Stereoselective synthesis of 2-alkyl-3-hydroxy carbonyl compounds by aldol condensation |
|||
| authorlink = Clayton Heathcock |
|||
| last1 = Heathcock | first1 = C. H. |
|||
| last2 = Buse |first2 =C. T. |
|||
| last3=Kleschnick|first3=W. A. |
|||
| last4=Pirrung| first4= M. C. |
|||
| last5=Sohn|first5=J. E. |
|||
| last6= Lampe first6=J. |
|||
| doi = 10.1021/jo01294a030 |
|||
| journal = [[Journal of Organic Chemistry]] |
|||
| year = 1980 |
|||
| volume =45 |
|||
| pages = 1066–1081 |
|||
|issue = 6}}</ref> [[E-Z notation|E and Z]] refer to the [[cis-trans isomerism|cis-trans stereochemical relationship]] between the enolate oxygen bearing the positive counterion and the highest priority group on the alpha carbon. In reality, only some metals such as lithium and boron reliably follow the Zimmerman–Traxler model. Thus, in some cases, the [[stereochemistry|stereochemical]] outcome of the reaction may be unpredictable. |
|||
[[File:Zimmerman-Traxler model scheme.svg|The Zimmerman–Traxler model|center]] |
|||
==Crossed-aldol reactant control== |
==Crossed-aldol reactant control== |
||
The problem of "control" in the aldol addition is best demonstrated by an example. Consider the outcome of this hypothetical reaction: |
|||
Despite the attractiveness of the aldol manifold, there are several problems that need to be addressed to render the process effective. The first problem is a thermodynamic one: most aldol reactions are reversible. Furthermore, the equilibrium is also just barely on the side of the products in the case of simple aldehyde–ketone aldol reactions.<ref>{{Cite book |url=http://www.thieme-connect.de/DOI/DOI?10.1055/b-003-125712 |title=Stereoselective Synthesis 2: Stereoselective Reactions of Carbonyl and Imino Groups |date=2011 |publisher=Georg Thieme Verlag |isbn=978-3-13-154121-5 |editor-last=Molander |editor-first=G. A. |edition=1 |location=Stuttgart |language=en |doi=10.1055/sos-sd-202-00331}}</ref> If the conditions are particularly harsh (e.g.: NaOMe/MeOH/[[reflux]]), condensation may occur, but this can usually be avoided with mild reagents and low temperatures (e.g., LDA (a strong base), THF, −78 °C). Although the aldol addition usually proceeds to near completion under irreversible conditions, the isolated aldol adducts are sensitive to base-induced retro-aldol cleavage to return starting materials. In contrast, retro-aldol condensations are rare, but possible.<ref name="Guthrie1984">{{cite journal |author=Guthrie, J.P. |author2=Cooper, K.J. |author3=Cossar, J. |author4=Dawson, B.A. |author5=Taylor, K.F. |year=1984 |title=The retroaldol reaction of cinnamaldehyde |journal=[[Can. J. Chem.]] |volume=62 |issue=8 |pages=1441–1445 |doi=10.1139/v84-243 |doi-access=free}}</ref> This is the basis of the catalytic strategy of class I aldolases in nature, as well as numerous small-molecule amine catalysts.<ref>{{Cite book |url=http://www.thieme-connect.de/products/ebooks/book/10.1055/b-003-125712 |title=Stereoselective Synthesis 2: Stereoselective Reactions of Carbonyl and Imino Groups |date=2011 |publisher=Georg Thieme Verlag |isbn=978-3-13-154121-5 |editor-last=Molander |edition=1 |location=Stuttgart |language=en |doi=10.1055/sos-sd-202-00331}}</ref> |
|||
[[File:Aldol control 1.svg|center|Hypothetical aldol reaction]] |
|||
When a mixture of unsymmetrical ketones are reacted, four crossed-aldol ([[Addition reaction|addition]]) products can be anticipated: [[File:Aldol_control_2_update.svg|center|625x625px|Crossed aldol (addition) reaction|frameless]] Thus, if one wishes to obtain only one of the cross-products, one must control which carbonyl becomes the nucleophilic enol/enolate and which remains in its electrophilic carbonyl form. |
|||
In this reaction, two unsymmetrical ketones are being condensed using [[sodium ethoxide]]. The basicity of sodium ethoxide is such that it cannot fully deprotonate either of the ketones, but can produce small amounts of the sodium enolate of both ketones. This means that, in addition to being potential aldol electrophiles, both ketones may also act as nucleophiles via their sodium enolate. Two electrophiles and two nucleophiles, then, have potential to result in four possible products: |
|||
The simplest control is if only one of the reactants has acidic protons, and only this molecule forms the enolate. For example, the addition of [[diethyl malonate]] into [[benzaldehyde]] produces only one product: [[File:Aldol control 3.svg|center|Acidic control of the aldol (addition) reaction]] |
|||
[[File:Aldol control 2.svg|center|Four possible aldol reaction products]] |
|||
If one group is considerably more acidic than the other, the most acidic proton is abstracted by the base and an enolate is formed at that carbonyl while the less-acidic carbonyl remains electrophilic. This type of control works only if the difference in acidity is large enough and base is the [[limiting reactant]]. A typical substrate for this situation is when the deprotonatable position is activated by more than one carbonyl-like group. Common examples include a CH<sub>2</sub> group flanked by two carbonyls or nitriles (see for example the [[Knoevenagel condensation]] and the first steps of the [[malonic ester synthesis]] and [[acetoacetic ester synthesis]]). |
|||
Thus, if one wishes to obtain only one of the cross-products, one must control which carbonyl becomes the nucleophilic enol/enolate and which remains in its electrophilic carbonyl form. |
|||
Otherwise, the most acidic carbonyls are typically also the most active electrophiles: first [[aldehydes]], then [[ketones]], then [[esters]], and finally [[amides]]. Thus cross-aldehyde reactions are typically most challenging because they can [[polymerization|polymerize]] easily or react unselectively to give a statistical mixture of products.<ref>{{cite book|title=Organic synthesis: the disconnection approach|edition=2nd|first1=Stuart|last1=Warren|first2=Paul|last2=Wyatt|publisher=Wiley|year=2008|isbn=978-0-470-71236-8}}</ref> |
|||
===Acidity=== |
|||
The simplest control is if only one of the reactants has acidic protons, and only this molecule forms the enolate. For example, the addition of [[diethyl malonate]] into [[benzaldehyde]] would produce only one product. Only the malonate has α hydrogens, so it is the nucleophilic partner, whereas the non-enolizeable benzaldehyde can only be the electrophile: |
|||
One common solution is to form the enolate of one partner first, and then add the other partner under [[kinetic reaction control|kinetic control]].<ref name="OS1985">Bal, B.; Buse, C. T.; Smith, K.; Heathcock, C. H., [http://www.orgsyn.org/orgsyn/prep.asp?prep=cv7p0185 (2SR,3RS)-2,4-Dimethyl-3-Hydroxypentanoic Acid] {{Webarchive|url=https://web.archive.org/web/20110606033118/http://www.orgsyn.org/orgsyn/prep.asp?prep=cv7p0185 |date=2011-06-06 }}, ''[[Org. Synth.]]'', Coll. Vol. 7, p.185 (1990); Vol. 63, p.89 (1985).</ref> Kinetic control means that the forward aldol addition reaction must be significantly faster than the reverse retro-aldol reaction. For this approach to succeed, two other conditions must also be satisfied; it must be possible to quantitatively form the enolate of one partner, and the forward aldol reaction must be significantly faster than the transfer of the enolate from one partner to another. Common kinetic control conditions involve the formation of the enolate of a ketone with [[Lithium diisopropylamide|LDA]] at −78 °C, followed by the slow addition of an aldehyde. |
|||
[[File:Aldol control 3.svg|center|Acidic control of the aldol reaction]] |
|||
== Stereoselectivity == |
|||
The malonate is particularly easy to deprotonate because the α position is flanked by more than one carbonyl. Double-activation makes the enolate more stable, so not as strong a base is required to form it. An extension of this effect can allow control over which of the two carbonyl reactants becomes the enolate even if both do have α hydrogens. If one partner is considerably more acidic than the other, the most acidic proton is abstracted by the base and an enolate is formed at that carbonyl while the carbonyl that is less acidic is not affected by the base. This type of control works only if the difference in acidity is large enough and no excess of base is used for the reaction. A typical substrate for this situation is when the deprotonatable position is activated by more than one carbonyl-like group. Common examples include a CH<sub>2</sub> group flanked by two carbonyls or nitriles (see for example the [[Knoevenagel condensation]] and the first steps of the [[Malonic ester synthesis]]). |
|||
The aldol reaction unites two relatively simple [[molecules]] into a more complex one. Increased complexity arises because each end of the new bond may become a [[stereocenter]]. Modern methodology has not only developed high-[[Yield (chemistry)|yielding]] aldol reactions, but also completely controls both the relative and [[absolute configuration]] of these new stereocenters.<ref name="March" /> |
|||
To describe relative stereochemistry at the α- and β-carbon, older papers use saccharide chemistry's ''[[Diastereomer#Erythro / threo|erythro/threo]]'' nomenclature; more modern papers use the following ''syn''/''anti'' convention. When propionate (or higher order) nucleophiles add to aldehydes, the reader visualizes the ''R'' group of the ketone and the ''R''' group of the aldehyde aligned in a "zig zag" pattern on the paper (or screen). The disposition of the formed stereocenters is deemed ''syn'' or ''anti'', depending if they are on the same or opposite sides of the main chain: |
|||
===Order of addition=== |
|||
[[File:Aldol_syn-anti.svg|center|500x500px|Syn and anti products from an aldol (addition) reaction]] |
|||
One common solution is to form the enolate of one partner first, and then add the other partner under [[kinetic reaction control|kinetic control]].<ref name=OS1985>Bal, B.; Buse, C. T.; Smith, K.; Heathcock, C. H., [http://www.orgsyn.org/orgsyn/prep.asp?prep=cv7p0185 (2SR,3RS)-2,4-Dimethyl-3-Hydroxypentanoic Acid], ''[[Org. Syn.]]'', Coll. Vol. 7, p.185 (1990); Vol. 63, p.89 (1985).</ref> Kinetic control means that the forward aldol addition reaction must be significantly faster than the reverse retro-aldol reaction. For this approach to succeed, two other conditions must also be satisfied; it must be possible to quantitatively form the enolate of one partner, and the forward aldol reaction must be significantly faster than the transfer of the enolate from one partner to another. Common kinetic control conditions involve the formation of the enolate of a ketone with [[Lithium diisopropylamide|LDA]] at −78 °C, followed by the slow addition of an aldehyde. |
|||
The principal factor determining an aldol reaction's [[stereoselectivity]] is the enolizing metal [[counterion]]. Shorter metal-oxygen bonds "tighten" the [[transition state]] and effects greater stereoselection.<ref name="JACS1981_30992">{{cite journal |last=Evans |first=D. A. |author-link=David A. Evans |author2=Nelson J. V. |author3=Vogel E. |author4=Taber T. R. |year=1981 |title=Stereoselective aldol condensations via boron enolates |journal=[[Journal of the American Chemical Society]] |volume=103 |issue=11 |pages=3099–3111 |doi=10.1021/ja00401a031}}</ref> [[Boron]] is often used<ref>Cowden, C. J.; Paterson, I. ''[[Org. React.]]'' '''1997''', ''51'', 1.</ref><ref>{{cite book |last1=Cowden |first1=C. J. |title=Asymmetric Aldol Reactions Using Boron Enolates |last2=Paterson |first2=I. |year=2004 |isbn=978-0471264187 |series=Organic Reactions |pages=1–200 |doi=10.1002/0471264180.or051.01}}</ref> because its [[Bond length|bond lengths]] are significantly shorter than other cheap metals ([[lithium]], [[aluminium]], or [[magnesium]]). The following reaction gives a ''syn:anti'' ratio of 80:20 using a lithium enolate compared to 97:3 using a bibutylboron enolate. |
|||
[[File:Enolate_metal_ion.svg|center|510x510px]] |
|||
Where the counterion determines [[Asymmetric induction|stereoinduction]] strength, the [[Cis–trans isomerism|enolate isomer]] determines its [[Chirality (chemistry)|direction]]. [[E–Z notation|''E'' isomers]] give ''anti'' products and ''Z'' give ''syn'':<ref name="Brown19892">{{cite journal |last1=Brown |first1=H. C. |author-link=Herbert C. Brown |last2=Dhar |first2=R. K. |last3=Bakshi |first3=R. K. |last4=Pandiarajan |first4=P. K. |last5=Singaram |first5=B. |year=1989 |title=Major effect of the leaving group in dialkylboron chlorides and triflates in controlling the stereospecific conversion of ketones into either E- or Z-enol borinates |journal=[[Journal of the American Chemical Society]] |volume=111 |issue=9 |pages=3441–3442 |doi=10.1021/ja00191a058}}</ref> |
|||
[[File:Stereoselective_E-enolate_formation_of_esters_in_aldol_addtion_reactions.svg|center|500x500px|Anti-aldol formation through E-enolate]] |
|||
[[File:Stereoselective_Z-enolate_formation_of_esters_in_aldol_addtion_reactions.svg|center|700x700px|Syn-aldol formation through Z-enolate]] |
|||
===Zimmermann-Traxler model=== |
|||
==Enolates== |
|||
If the two reactants have carbonyls adjacent to a pre-existing stereocenter, then the new stereocenters may [[Stereoinduction|form at a fixed orientation relative to the old]]. This "substrate-based stereocontrol" has seen extensive study and examples pervade the literature. In many cases, a stylized [[transition state]], called the '''Zimmerman–Traxler model''', can predict the new orientation from the [[Cyclohexane conformation|configuration of a 6-membered ring]].<ref name="Zimmerman1920">{{cite journal |author=Zimmerman, H. E. |author2=Traxler, M. D. |year=1957 |title=The Stereochemistry of the Ivanov and Reformatsky Reactions. I |journal=[[Journal of the American Chemical Society]] |volume=79 |issue=8 |pages=1920–1923 |doi=10.1021/ja01565a041}}</ref> |
|||
===Formation=== |
|||
The enolate may be formed by using a strong base ("hard conditions") or using a [[Lewis acid]] and a weak base ("soft conditions"): |
|||
==== On the enol ==== |
|||
[[File:aldol scheme 3a.svg|center]] |
|||
If the enol has an adjacent stereocenter, then the two stereocenters flanking the carbonyl in the product are naturally ''syn'':<ref name="JACS1991_10472">{{cite journal |last=Evans |first=D. A. |author-link=David A. Evans |author2=Rieger D. L. |author3=Bilodeau M. T. |author4=Urpi F. |year=1991 |title=Stereoselective aldol reactions of chlorotitanium enolates. An efficient method for the assemblage of polypropionate-related synthons |journal=[[Journal of the American Chemical Society]] |volume=113 |issue=3 |pages=1047–1049 |doi=10.1021/ja00003a051}}</ref> |
|||
In this diagram, B: represents the base which takes the proton. The [[dibutylboron triflate]] actually becomes attached to the oxygen only during the reaction. The second product on the right (formed from the [[N,N-diisopropylethylamine]]) should be ''i''-Pr<sub>2</sub>EtNH<sup>+</sup> OTf<sup> −</sup>. |
|||
[[File:Enolate_alpha_center_eg.svg|alt=Aldol reaction with enolate-based stereocontrol|center]] |
|||
The underlying mechanistic reason depends on the enol isomer. For an ''E'' enolate, the stereoinduction is necessary to avoid 1,3-[[allylic strain]], while a ''Z'' enolate instead seeks to avoid 1,3-[[Diaxial strain|diaxial interactions]]:<ref name="Heathcock1980">{{cite journal |last1=Heathcock |first1=C. H. |author-link=Clayton Heathcock |last2=Buse |first2=C. T. |last3=Kleschnick |first3=W. A. |last4=Pirrung |first4=M. C. |last5=Sohn |first5=J. E. |last6=Lampe |first6=J. |year=1980 |title=Acyclic stereoselection. 7. Stereoselective synthesis of 2-alkyl-3-hydroxy carbonyl compounds by aldol condensation |journal=[[Journal of Organic Chemistry]] |volume=45 |issue=6 |pages=1066–1081 |doi=10.1021/jo01294a030}}</ref> |
|||
[[File:Enolate_alpha_center_model.svg|alt=General model of the aldol reaction with enolate-based stereocontrol|center|thumb|625x625px|For clarity, the stereocenter on the enolate has been [[Epimer|epimerized]]; in reality, the opposite diastereoface of the aldehyde would have been attacked.]] |
|||
However, [[Fráter–Seebach alkylation|Fráter & Seebach showed]] that a chelating [[Lewis base|Lewis basic]] moiety adjacent to the enol will instead cause ''anti'' addition. |
|||
For [[deprotonation]] to occur, the stereoelectronic requirement is that the alpha-C-H [[sigma bond]] must be able to overlap with the pi* orbital of the [[carbonyl]]: |
|||
==== On the electrophile ==== |
|||
[[File:scheme3c.gif|center|Stereoelectronic deprotonation requirements]] |
|||
''E'' enolates exhibit [[Felkin model|Felkin diastereoface selection]], while ''Z'' enolates exhibit anti-Felkin selectivity. The general model is presented below:<ref name="Evans1982TS">{{cite book|last=Evans|first= D. A. |first2 = J. V.|last2= Nelson|first3= T. R. |last3= Taber |title=Topics in Stereochemistry |year=1982|volume=13|pages= 1–115|chapter=Stereoselective Aldol Condensations|isbn = 9780471056805}}</ref><ref name="Roush1991">{{cite journal |author=Roush W. R. |year=1991 |title=Concerning the diastereofacial selectivity of the aldol reactions of .alpha.-methyl chiral aldehydes and lithium and boron propionate enolates |journal=[[Journal of Organic Chemistry]] |volume=56 |issue=13 |pages=4151–4157 |doi=10.1021/jo00013a015}}</ref> |
|||
===Geometry=== |
|||
Extensive studies have been performed on the formation of enolates under many different conditions. It is now possible to generate, in most cases, the desired enolate geometry:<ref name=Brown1989>{{cite journal |
|||
| title = Major effect of the leaving group in dialkylboron chlorides and triflates in controlling the stereospecific conversion of ketones into either E- or Z-enol borinates |
|||
| authorlink = Herbert C. Brown |
|||
| last1 = Brown | first1 = H. C. |
|||
| last2 = Dhar | first2 = R. K. |
|||
| last3 = Bakshi | first3 = R. K. |
|||
| last4 = Pandiarajan | first4 = P. K. |
|||
| last5 = Singaram | first5 = B. |
|||
| doi = 10.1021/ja00191a058 |
|||
| journal = [[Journal of the American Chemical Society]] |
|||
| year =1989 |
|||
| volume =111 |
|||
| pages =3441–3442 |
|||
| issue = 9 |
|||
}}</ref> |
|||
[[File:aldol scheme 3.svg|center|Stereoselective enolate generation]] |
|||
For ketones, most enolization conditions give ''Z'' enolates. For [[ester]]s, most enolization conditions give ''E'' enolates. The addition of [[Hexamethylphosphoramide|HMPA]] is known to reverse the [[stereoselectivity]] of deprotonation. |
|||
[[File:LDA enolate HMPA effect.svg|364px|center|Effect of HMPA addition]] |
|||
The stereoselective formation of enolates has been rationalized with the '''Ireland model''',<ref name = Ireland1975>{{cite journal |
|||
| title = The stereoselective generation of ester enolates |
|||
| last = Ireland | first = R. E. |
|||
|author2=Willard, A. K. |
|||
| doi = 10.1016/S0040-4039(00)91213-9 |
|||
| journal = [[Tetrahedron Letters]] |
|||
| year = 1975 |
|||
| volume = 16 |
|||
| issue = 46 |
|||
| pages = 3975–3978}}</ref><ref name = Narula1981>{{cite journal |
|||
| title = An analysis of the diastereomeric transition state interactions for the kinetic deprotonation of acyclic carbonyl derivatives with lithium diisopropylamide |
|||
| last = Narula | first = A. S. |
|||
| doi = 10.1016/S0040-4039(01)82081-5 |
|||
| journal = [[Tetrahedron Letters]] |
|||
| year = 1981 |
|||
| volume = 22 |
|||
| issue = 41 |
|||
| pages = 4119–4122}}</ref><ref name = Ireland1991>{{cite journal |
|||
| title = Stereochemical control in the ester enolate Claisen rearrangement. 1. Stereoselectivity in silyl ketene acetal formation |
|||
| last1 = Ireland | first1 = RE |
|||
| last2 = Wipf | first2 = P |
|||
| last3 = Armstrong | first3 = JD |
|||
| doi = 10.1021/jo00002a030 |
|||
| journal = [[Journal of Organic Chemistry]] |
|||
| year = 1991 |
|||
| volume = 56 |
|||
| issue = 2 |
|||
| pages = 650–657}}</ref><ref name = Xie1997>{{cite journal |
|||
| title = Highly Stereoselective Kinetic Enolate Formation: Steric vs Electronic Effects |
|||
| last1 = Xie | first1 = L |
|||
| last2 = Isenberger | first2 = KM |
|||
| last3 = Held | first3 = G |
|||
| last4 = Dahl | first4 = LM |
|||
| doi = 10.1021/jo971260a |
|||
| journal = [[Journal of Organic Chemistry]] |
|||
|date=October 1997 |
|||
| volume = 62 |
|||
| issue = 21 |
|||
| pages = 7516–7519 |
|||
| pmid = 11671880 }}</ref> although its validity is somewhat questionable. In most cases, it is not known which, if any, intermediates are [[monomer]]ic or [[oligomer]]ic in nature; nonetheless, the Ireland model remains a useful tool for understanding enolates. |
|||
[[Image:Enolate_Ireland_model.svg|center|The Ireland model]] |
|||
In the Ireland model, the deprotonation is assumed to proceed by a six-membered or cyclic<ref>[http://pharmaxchange.info/press/2011/08/directed-aldol-synthesis-part-1-formation-of-e-enolate-z-enolate/ Directed Aldol Synthesis – Formation of E-enolate and Z-enolate]</ref> monomeric transition state. The larger of the two substituents on the electrophile (in the case above, methyl is larger than proton) adopts an equatorial disposition in the favored transition state, leading to a preference for E enolates. The model clearly fails in many cases; for example, if the solvent mixture is changed from THF to 23% HMPA-THF (as seen above), the enolate geometry is reversed, which is inconsistent with this model and its cyclic transition state. |
|||
===Regiochemistry=== |
|||
If an unsymmetrical ketone is subjected to base, it has the potential to form two regioisomeric enolates (ignoring enolate geometry). For example: |
|||
[[File:Enolate regio 1.svg|center|Kinetic and thermodynamic enolates]] |
|||
The trisubstituted enolate is considered the [[thermodynamic reaction control|kinetic]] enolate, while the tetrasubstituted enolate is considered the thermodynamic enolate. The alpha hydrogen deprotonated to form the kinetic enolate is less hindered, and therefore deprotonated more quickly. In general, tetrasubstituted olefins are more stable than trisubstituted olefins due to hyperconjugative stabilization. The ratio of enolate regioisomers is heavily influenced by the choice of base. For the above example, kinetic control may be established with LDA at −78 °C, giving 99:1 selectivity of kinetic: thermodynamic enolate, while thermodynamic control may be established with [[organolithium reagent|triphenylmethyllithium]] at [[room temperature]], giving 10:90 selectivity. |
|||
In general, kinetic enolates are favored by cold temperatures, conditions that give relatively ionic metal–oxygen bonding, and rapid deprotonation using a slight excess of a strong, sterically hindered base. The large base only deprotonates the more accessible hydrogen, and the low temperatures and excess base help avoid equilibration to the more stable alternate enolate after initial enolate formation. Thermodynamic enolates are favored by longer equilibration times at higher temperatures, conditions that give relatively covalent metal–oxygen bonding, and use of a slight sub-stoichiometric amount of strong base. By using insufficient base to deprotonate all of the carbonyl molecules, the enolates and carbonyls can exchange protons with each other and equilibrate to their more stable isomer. Using various metals and solvents can provide control over the amount of ionic character in the metal–oxygen bond. |
|||
==Stereoselectivity== |
|||
The aldol reaction is particularly useful because two new stereogenic centers are generated in one reaction. Extensive research has been performed to understand the reaction mechanism and improve the selectivity observed under many different conditions. The ''syn''/''anti'' convention is commonly used to denote the relative stereochemistry at the α- and β-carbon. |
|||
[[File:Aldol_syn-anti.svg|500px|Syn and anti products from an aldol reaction|center]] |
|||
The convention applies when propionate (or higher order) nucleophiles are added to aldehydes. The ''R'' group of the ketone and the ''R''' group of the aldehyde are aligned in a "zig zag" pattern in the plane of the paper (or screen), and the disposition of the formed stereocenters is deemed ''syn'' or ''anti'', depending if they are on the same or opposite sides of the main chain. |
|||
Older papers use the ''[[Diastereomer#Erythro / threo|erythro/threo]]'' nomenclature familiar from carbohydrate chemistry. |
|||
===Enolate geometry=== |
|||
There is no significant difference between the level of [[stereoinduction]] observed with [[E-Z notation|''E'' and ''Z'']] enolates. Each alkene geometry leads primarily to one specific relative stereochemistry in the product, ''E'' giving ''anti'' and ''Z'' giving ''syn'':<ref name=Brown1989 /> |
|||
[[File:E vs Z stereoselectivity.svg|center|Anti-aldol formation via E-enolate]] |
|||
[[File:E vs Z stereoselectivity 2.svg|center|Syn-aldol formation via Z-enolate]] |
|||
===Metal ion=== |
|||
The enolate metal cation may play a large role in determining the level of stereoselectivity in the aldol reaction. [[Boron]] is often used<ref>Cowden, C. J.; Paterson, I. ''[[Org. React.]]'' '''1997''', ''51'', 1.</ref><ref> |
|||
{{cite book |
|||
| last1 = Cowden | first1 = C. J. |
|||
| last2 = Paterson | first2 = I. |
|||
| journal = Organic Reactions |
|||
| doi = 10.1002/0471264180.or051.01 |
|||
| title = Asymmetric Aldol Reactions Using Boron Enolates |
|||
| year = 2004 |
|||
}}</ref> because its [[bond length]]s are significantly shorter than that of other metals such as [[lithium]], [[aluminium]], or [[magnesium]]. |
|||
[[File:Enolate metal ion.svg|510px|center]] |
|||
For example, boron–carbon and boron–oxygen bonds are 1.4–1.5 [[Ångstrom|Å]] and 1.5–1.6 Å in length, respectively, whereas typical metal-carbon and metal-oxygen bonds are 1.9–2.2 Å and 2.0–2.2 Å in length, respectively. The use of boron rather than a metal "tightens" the [[transition state]] and gives greater stereoselectivity in the reaction.<ref name=JACS1981_3099>{{cite journal |
|||
| title = Stereoselective aldol condensations via boron enolates |
|||
| authorlink = David A. Evans |
|||
| last = Evans |
|||
| first = D. A. |
|||
|author2=Nelson J. V. |author3=Vogel E. |author4=Taber T. R. |
|||
| doi = 10.1021/ja00401a031 |
|||
| journal = [[Journal of the American Chemical Society]] |
|||
| year =1981 |
|||
| volume =103 |
|||
| pages =3099–3111 |
|||
| issue = 11}}</ref> Thus the above reaction gives a ''syn:anti'' ratio of 80:20 using a lithium enolate compared to 97:3 using a bibutylboron enolate. |
|||
===Alpha stereocenter on the enolate=== |
|||
The aldol reaction may exhibit "substrate-based stereocontrol", in which existing [[Chirality (chemistry)|chirality]] on either reactant influences the stereochemical outcome of the reaction. This has been extensively studied, and in many cases, one can predict the sense of [[asymmetric induction]], if not the absolute level of [[diastereoselectivity]]. If the enolate contains a [[stereocenter]] in the alpha position, excellent stereocontrol may be realized. |
|||
[[File:enolate alpha center.svg|center|Aldol reaction with enolate-based stereocontrol]] |
|||
In the case of an E enolate, the dominant control element is [[allylic strain|allylic 1,3-strain]] whereas in the case of a Z enolate, the dominant control element is the avoidance of 1,3-diaxial interactions. The general model is presented below: |
|||
[[File:enolate alpha center model.svg|center|General model of the aldol reaction with enolate-based stereocontrol]] |
|||
For clarity, the stereocenter on the enolate has been [[epimer]]ized; in reality, the opposite diastereoface of the aldehyde would have been attacked. In both cases, the 1,3-syn diastereomer is favored. There are many examples of this type of stereocontrol:<ref name=JACS1991_1047>{{cite journal |
|||
| title = Stereoselective aldol reactions of chlorotitanium enolates. An efficient method for the assemblage of polypropionate-related synthons |
|||
| authorlink = David A. Evans |
|||
| last = Evans |
|||
| first = D. A. |
|||
|author2=Rieger D. L. |author3=Bilodeau M. T. |author4=Urpi F. |
|||
| doi = 10.1021/ja00003a051 |
|||
| journal = [[Journal of the American Chemical Society]] |
|||
| year =1991|volume =113 |
|||
| pages =1047–1049 |
|||
| issue = 3}}</ref> |
|||
[[File:enolate alpha center eg.svg|center|Aldol reaction with enolate-based stereocontrol]] |
|||
===Alpha stereocenter on the electrophile=== |
|||
When enolates attacks aldehydes with an alpha stereocenter, excellent stereocontrol is also possible. The general observation is that ''E'' enolates exhibit [[Felkin model|Felkin]] diastereoface selection, while ''Z'' enolates exhibit anti-Felkin selectivity. The general model<ref name=Evans1982TS>Evans D. A. ''et al.'' ''Top. Stereochem.'' '''1982''', ''13'', 1–115. (Review)</ref><ref name=Roush1991>{{cite journal |
|||
| title = Concerning the diastereofacial selectivity of the aldol reactions of .alpha.-methyl chiral aldehydes and lithium and boron propionate enolates |
|||
| author = Roush W. R. |
|||
| journal = [[Journal of Organic Chemistry]] |
|||
| year = 1991 |
|||
| volume = 56 |
|||
| pages = 4151–4157 |
|||
| doi = 10.1021/jo00013a015 |
|||
| issue = 13}}</ref> is presented below: |
|||
[[File:Aldehydealphamodel.png|center|The general model of the aldol reaction with carbonyl-based stereocontrol]] |
[[File:Aldehydealphamodel.png|center|The general model of the aldol reaction with carbonyl-based stereocontrol]] |
||
Since ''Z'' enolates must |
Since the [[transition state]] for ''Z'' enolates must contain either a destabilizing ''syn''-pentane interaction or an anti-Felkin [[rotamer]], ''Z''-enolates are less diastereoselective:<ref name="JACS1982_5526">{{cite journal |
||
| title = Aldol strategy: coordination of the lithium cation with an alkoxy substituent |
| title = Aldol strategy: coordination of the lithium cation with an alkoxy substituent |
||
| author = Masamune S. |
| author = Masamune S. |
||
|author2=Ellingboe J. W. |author3=Choy W. |
|author2=Ellingboe J. W. |author3=Choy W. |
||
| doi = 10.1021/ja00384a062 |
|||
| journal = [[Journal of the American Chemical Society]] |
| journal = [[Journal of the American Chemical Society]] |
||
| year =1982 |
| year =1982 |
||
| volume =104 |
| volume =104 |
||
| pages = 1047–1049 |
| pages = 1047–1049 |
||
| issue = 20}}</ref><ref name=JACS1995_9073>{{cite journal |
| issue = 20}}</ref><ref name="JACS1995_9073">{{cite journal |
||
|title = Double Stereodifferentiating Aldol Reactions. The Documentation of "Partially Matched" Aldol Bond Constructions in the Assemblage of Polypropionate Systems |
|title = Double Stereodifferentiating Aldol Reactions. The Documentation of "Partially Matched" Aldol Bond Constructions in the Assemblage of Polypropionate Systems |
||
| |
| author-link = David A. Evans |
||
| last = Evans |
| last = Evans |
||
| first = D. A. |
| first = D. A. |
||
|author2=Dart M. J. |author3=Duffy J. L. |author4=Rieger D. L. |
|author2=Dart M. J. |author3=Duffy J. L. |author4=Rieger D. L. |
||
| doi = 10.1021/ja00140a027 |
|||
| journal = [[Journal of the American Chemical Society]] |
| journal = [[Journal of the American Chemical Society]] |
||
| year =1995 |
| year =1995 |
||
| volume =117 |
| volume =117 |
||
| pages =9073–9074 |
| pages =9073–9074 |
||
|issue = 35}}</ref> [[File:Aldehydealphaeg.png|center|Examples of the aldol reaction with carbonyl-based stereocontrol]] |
|||
|issue = 35}}</ref> |
|||
==== On both ==== |
|||
[[File:Aldehydealphaeg.png|center|Examples of the aldol reaction with carbonyl-based stereocontrol]] |
|||
If both the enolate and the aldehyde contain pre-existing chirality, then the outcome of the "double stereodifferentiating" aldol reaction may be predicted using a merged stereochemical model that takes into account all the effects discussed above.<ref name="Masamune1985">{{cite journal |
|||
===Unified model of stereoinduction=== |
|||
If both the enolate and the aldehyde both contain pre-existing chirality, then the outcome of the "double stereodifferentiating" aldol reaction may be predicted using a merged stereochemical model that takes into account the enolate facial bias, enolate geometry, and aldehyde facial bias.<ref name=Masamune1985>{{cite journal |
|||
| title = Double Asymmetric Synthesis and a New Strategy for Stereochemical Control in Organic Synthesis |
| title = Double Asymmetric Synthesis and a New Strategy for Stereochemical Control in Organic Synthesis |
||
| author = Masamune S. |
| author = Masamune S. |
||
|author2=Choy W. |author3=Petersen J. S. |author4=Sita L. R. |
|author2=Choy W. |author3=Petersen J. S. |author4=Sita L. R. |
||
| doi = 10.1002/anie.198500013 |
|||
| journal =[[Angew. Chem. Int. Ed. Engl.]] |
| journal =[[Angew. Chem. Int. Ed. Engl.]] |
||
| year =1985 |
| year =1985 |
||
| volume =24 |
| volume =24 |
||
| pages =1–30}}</ref> Several examples |
| pages =1–30}}</ref> Several examples are as follows:<ref name="JACS1995_9073" /> |
||
[[File:Mergedmodel.gif|center]] |
[[File:Mergedmodel.gif|center]] |
||
==Oxazolidinone chiral auxiliaries== |
|||
==Evans' oxazolidinone chemistry== |
|||
In the late 1970s and 1980s, [[David A. Evans]] and coworkers developed a technique for stereoselection in the aldol syntheses of aldehydes and [[Carboxylic acid|carboxylic acids]].<ref name="Evans1982AldrichActa">{{cite journal |last=Evans |first=D. A. |year=1982 |title=Studies in Asymmetric Synthesis: The Development of Practical Chiral Enolate Synthons |url=https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/marketing/global/documents/296/074/acta-vol15.pdf |journal=[[Aldrichimica Acta]] |volume=15 |page=23}}</ref><ref name="OS1990">Gage J. R.; Evans D. A., [http://www.orgsyn.org/orgsyn/prep.asp?prep=cv8p0339 Diastereoselective Aldol Condensation Using A Chiral Oxazolidinone Auxiliary: (2S*,3S*)-3-Hydroxy-3-Phenyl-2-Methylpropanoic Acid] {{Webarchive|url=https://web.archive.org/web/20120929193419/http://www.orgsyn.org/orgsyn/prep.asp?prep=cv8p0339|date=2012-09-29}}, [[Organic Syntheses]], Coll. Vol. 8, p.339 (1993); Vol. 68, p.83 (1990).</ref> The method works by temporarily appending a chiral [[oxazolidinone]] [[chiral auxiliary|auxiliary]] to create a chiral enolate. The pre-existing chirality from the auxiliary is then transferred to the aldol adduct through Zimmermann-Traxler methods, and then the oxazolidinone cleaved away. |
|||
Modern organic syntheses now require the synthesis of compounds in [[enantiopure]] form. Since the aldol addition reaction creates two new stereocenters, up to four stereoisomers may result. |
|||
[[File:Evansaldol1.gif|center|Aldol reaction creates stereoisomers]] |
[[File:Evansaldol1.gif|center|Aldol reaction creates stereoisomers]] |
||
Many methods which control both relative stereochemistry (i.e., syn or anti, as discussed above) and absolute [[stereochemistry]] (i.e., ''R'' or ''S'') have been developed. |
|||
[[File:Evansaldol2.gif|center|Four possible stereoisomers of the aldol reaction]] |
[[File:Evansaldol2.gif|center|Four possible stereoisomers of the aldol reaction]] |
||
A widely used method is the Evans' [[acyl]] [[oxazolidinone]] method.<ref name=Evans1982AldrichActa>Evans D. A. ''[[Aldrichimica Acta]]'' '''1982''', ''15'', 23. (Review)</ref><ref name=OS1990>Gage J. R.; Evans D. A., [http://www.orgsyn.org/orgsyn/prep.asp?prep=cv8p0339 Diastereoselective Aldol Condensation Using A Chiral Oxazolidinone Auxiliary: (2S*,3S*)-3-Hydroxy-3-Phenyl-2-Methylpropanoic Acid], [[Organic Syntheses]], Coll. Vol. 8, p.339 (1993); Vol. 68, p.83 (1990).</ref> Developed in the late 1970s and 1980s by [[David A. Evans]] and coworkers, the method works by temporarily creating a chiral enolate by appending a [[chiral auxiliary]]. The pre-existing chirality from the auxiliary is then transferred to the aldol adduct by performing a diastereoselective aldol reaction. Upon subsequent removal of the auxiliary, the desired aldol stereoisomer is revealed. |
|||
[[File:Evansaldol3.gif|center]] |
[[File:Evansaldol3.gif|center]] |
||
Commercial oxazolidinones are relatively expensive, but derive in 2 synthetic steps from comparatively inexpensive amino acids. (Economical large-scale syntheses prepare the auxiliary in-house.) First, a borohydride reduces the acid [[Moiety (chemistry)|moiety]]. Then the resulting amino alcohol dehydratively cyclises with a simple carbonate ester, such as diethylcarbonate. |
|||
[[File:Evansaldol4.gif|center]] |
[[File:Evansaldol4.gif|center]] |
||
The [[acylation]] of an oxazolidinone |
The [[acylation]] of an oxazolidinone is informally referred to as "loading done". |
||
''Anti'' adducts, which require an ''E'' enolate, cannot be obtained reliably with the Evans method. However, ''Z'' enolates, leading to ''syn'' adducts, can be reliably formed using boron-mediated soft enolization:<ref name="Bartroli1981">{{cite journal |
|||
| title = Enantioselective aldol condensations. 2. Erythro-selective chiral aldol condensations via boron enolates |
| title = Enantioselective aldol condensations. 2. Erythro-selective chiral aldol condensations via boron enolates |
||
| |
| author-link = David A. Evans |
||
| last = Evans |
| last = Evans |
||
| first = D. A. |
| first = D. A. |
||
|author2=Bartroli J. |author3=Shih T. L. |
|author2=Bartroli J. |author3=Shih T. L. |
||
| doi = 10.1021/ja00398a058 |
|||
| journal = [[Journal of the American Chemical Society]] |
| journal = [[Journal of the American Chemical Society]] |
||
| year =1981 |
| year =1981 |
||
| Line 456: | Line 164: | ||
[[File:Evansaldol5.gif|center]] |
[[File:Evansaldol5.gif|center]] |
||
Often, a single [[diastereomer]] may be obtained by one [[crystallization]] of the aldol adduct. |
Often, a single [[diastereomer]] may be obtained by one [[crystallization]] of the aldol adduct. |
||
Many methods cleave the auxiliary:<ref name="JACS882506">{{cite journal |
|||
| title = The total synthesis of the polyether antibiotic X-206 |
| title = The total synthesis of the polyether antibiotic X-206 |
||
| |
| author-link = David A. Evans |
||
| last = Evans |
| last = Evans |
||
| first = D. A. |
| first = D. A. |
||
|author2=Bender S. L. |author3=Morris J. |
|author2=Bender S. L. |author3=Morris J. |
||
| doi = 10.1021/ja00216a026 |
|||
| journal = [[Journal of the American Chemical Society]] |
| journal = [[Journal of the American Chemical Society]] |
||
| year =1988 |
| year =1988 |
||
| Line 471: | Line 181: | ||
[[File:Evansaldol6.gif|center|Evans' chiral oxazolidinone cleavage]] |
[[File:Evansaldol6.gif|center|Evans' chiral oxazolidinone cleavage]] |
||
==Variations== |
|||
Upon construction of the imide, both syn- and anti-selective aldol addition reactions may be performed, allowing the assemblage of three of the four possible stereoarrays: syn selective:<ref name=JACS90866>{{cite journal |
|||
A common additional chiral auxiliary is a [[thioether]] group:<ref name=JACS882506 />{{Efn|In this reaction the nucleophile is a boron enolate derived from reaction with [[dibutylboron triflate]] (nBu<sub>2</sub>BOTf), the base is [[N,N-diisopropylethylamine]]. The thioether is removed in step 2 by [[Raney Nickel]] / hydrogen [[organic reduction|reduction]]}} |
|||
| title = Diastereoselective aldol reactions using .beta.-keto imide derived enolates. A versatile approach to the assemblage of polypropionate systems |
|||
| authorlink = David A. Evans |
|||
| last = Evans |
|||
| first = D. A. |
|||
|author2=Clark J.S. |author3=Metternich R. |author4=Sheppard G.S. |
|||
| doi = 10.1021/ja00158a056 |
|||
| journal = [[Journal of the American Chemical Society]] |
|||
| year =1990 |
|||
| volume =112 |
|||
| pages =866–868 |
|||
| issue = 2}}</ref> and anti selective:<ref name=JACS922127>{{cite journal |
|||
|title = Diastereoselective anti aldol reactions of chiral ethyl ketones. Enantioselective processes for the synthesis of polypropionate natural products |
|||
| authorlink = David A. Evans |
|||
| last1 = Evans| first1 = D. A. |
|||
| last2 = Ng |first2=H.P. |
|||
| last3=Clark|first3= J.S. |
|||
| last4=Rieger|first4= D.L. |
|||
| journal = Tetrahedron |
|||
| year = 1992|volume = 48 |
|||
| pages = 2127–2142 |
|||
| doi =10.1016/S0040-4020(01)88879-7 |
|||
|issue = 11}}</ref> |
|||
[[File:Evans aldol 7.svg|center]] |
|||
In the syn-selective reactions, both enolization methods give the ''Z'' enolate, as expected; however, the stereochemical outcome of the reaction is controlled by the methyl stereocenter, rather than the chirality of the oxazolidinone. The methods described allow the stereoselective assembly of [[polyketide]]s, a class of natural products that often feature the aldol retron. |
|||
==Modern variations and methods== |
|||
Recent{{when|date=April 2011}} methodology now allows a much wider variety of aldol reactions to be conducted, often with a catalytic amount of [[chiral ligand]]. When reactions employ small amounts of [[enantiopure|enantiomerically]] pure ligands to induce the formation of enantiomerically pure products, the reactions are typically termed "catalytic, asymmetric"; for example, many different catalytic, [[asymmetric synthesis|asymmetric]] aldol reactions are now available. |
|||
===Acetate aldol reactions=== |
|||
A key limitation to the [[chiral auxiliary]] approach described previously is the failure of N-acetyl [[imide]]s to react selectively. An early approach was to use a temporary [[thioether]] group:<ref name=JACS882506 /><ref>In this reaction the nucleophile is a boron enolate derived from reaction with [[dibutylboron triflate]] (nBu<sub>2</sub>BOTf), the base is [[N,N-Diisopropylethylamine]]. The thioether is removed in step 2 by [[Raney Nickel]] / hydrogen [[organic reduction|reduction]]</ref> |
|||
[[File:Acetatealdol1.gif|center]] |
[[File:Acetatealdol1.gif|center]] |
||
===Crimmins thiazolidinethione aldol=== |
|||
===Mukaiyama aldol reaction===<ref> |
|||
In the '''Crimmins thiazolidinethione''' approach,<ref name="Crimmins1997">{{cite journal |author=Crimmins M. T. |author2=King B. W. |author3=Tabet A. E. |year=1997 |title= Asymmetric Aldol Additions with Titanium Enolates of Acyloxazolidinethiones: Dependence of Selectivity on Amine Base and Lewis Acid Stoichiometry |journal= Journal of the American Chemical Society |volume=119 |issue=33 |pages=7883–7884 |doi=10.1021/ja9716721}}</ref><ref name="Crimmins2000">{{cite journal |author= Crimmins M. T. |author2=Chaudhary K. |year=2000 |title=Titanium enolates of thiazolidinethione chiral auxiliaries: Versatile tools for asymmetric aldol additions |journal= Organic Letters |volume=2 |issue=6 |pages=775–777 |doi=10.1021/ol9913901 |pmid=10754681}}</ref> a thiazolidinethione is the chiral auxiliary<ref>{{cite journal |last1=Crimmins |first1=Michael T. |last2=Shamszad |first2=Mariam |year=2007 |title=Highly Selective Acetate Aldol Additions Using Mesityl-Substituted Chiral Auxiliaries |journal=Org. Lett. |volume=9 |issue=1 |pages=149–152 |doi=10.1021/ol062688b |pmid=17192107 |ref=1}}</ref> and can produce the "Evans syn" or "non-Evans syn" adducts by simply varying the amount of [[sparteine|(−)-sparteine]]. The reaction is believed to proceed via six-membered, titanium-bound [[transition state]]s, analogous to the proposed transition states for the Evans auxiliary. |
|||
{{cite journal |
|||
| title = The Impact of the Mukaiyama Aldol Reaction in Total Synthesis |
|||
| author = S. B. Jennifer Kan |
|||
|author2=Kenneth K.-H. Ng|author3=Ian Paterson |
|||
| journal = [[Angewandte Chemie International Edition]] |
|||
| volume = 52 |
|||
| issue = |
|||
| pages = 9097-9108 |
|||
| year = 2013 |
|||
| url = |
|||
| doi = 10.1002/anie.201303914 }} |
|||
</ref> |
|||
{{Main|Mukaiyama aldol reaction}} |
|||
The [[Mukaiyama aldol reaction]] is the [[nucleophilic addition]] of [[silyl enol ether]]s to [[aldehyde]]s catalyzed by a [[Lewis acid]] such as [[boron trifluoride]] or [[titanium tetrachloride]].<ref> |
|||
{{cite journal |
|||
| title = Reactions of silyl enol ethers with carbonyl compounds activated by titanium tetrachloride |
|||
| author = Teruaki Mukaiyama |
|||
|author2=Kazuo Banno|author3=Koichi Narasaka |
|||
| journal = [[Journal of the American Chemical Society]] |
|||
| volume = 96 |
|||
| issue = 24 |
|||
| pages = 7503–7509 |
|||
| year = 1974 |
|||
| url = |
|||
| doi = 10.1021/ja00831a019 }}</ref><ref>[http://www.orgsynth.org/orgsyn/pdfs/CV8P0323.pdf 3-Hydroxy-3-Methyl-1-Phenyl-1-Butanone by Crossed Aldol Reaction] Teruaki Mukaiyama and Koichi Narasaka [[Organic Syntheses]], Coll. Vol. 8, p.323 ('''1993'''); Vol. 65, p.6 ('''1987''')</ref> The Mukaiyama aldol reaction does not follow the Zimmerman-Traxler model. Carreira has described particularly useful asymmetric methodology with silyl ketene acetals, noteworthy for its high levels of enantioselectivity and wide substrate scope.<ref name=carreira1994>{{cite journal |
|||
| title = Catalytic, enantioselective aldol additions with methyl and ethyl acetate ''O''-silyl enolates — a chira; tridentate chelate as a ligand for titanium(IV) |
|||
| author = Carreira E.M. |
|||
|author2=Singer R.A. |author3=Lee W.S. |
|||
| doi = 10.1021/ja00098a065 |
|||
| journal = [[Journal of the American Chemical Society]] |
|||
| year =1994 |
|||
| volume =116 |
|||
| pages =8837–8 |
|||
| issue = 19}}</ref> |
|||
[[File:Crimminsaldol1.png|center|frame|NOTE: the structure of [[sparteine]] is missing an N atom]] |
|||
The method works on [[Branching (chemistry)|unbranched]] aliphatic aldehydes, which are often poor [[electrophile]]s for catalytic, asymmetric processes. This may be due to poor electronic and steric differentiation between their [[enantioface]]s. |
|||
[[File:Acetatealdol2.gif|center]] |
|||
The analogous [[vinylogous]] Mukaiyama aldol process can also be rendered catalytic and asymmetric. The example shown below works efficiently for aromatic (but not aliphatic) aldehydes and the mechanism is believed to involve a chiral, metal-bound dienolate.<ref name=Carreira1998>{{cite journal |
|||
| title = Apparent catalytic generation of chiral metal enolates: Enantioselective dienolate additions to aldehydes mediated by Tol-BINAP center Cu(II) fluoride complexes |
|||
| author = Kruger J. |
|||
|author2=Carreira E.M. |
|||
| doi = 10.1021/ja973331t |
|||
| journal = [[Journal of the American Chemical Society]] |
|||
| year =1998 |
|||
| volume =120 |
|||
| pages =837–8 |
|||
| issue = 4}}</ref><ref name=Carreira1998-2>{{cite journal |
|||
| title = Mechanistic insights into Cu-catalyzed asymmetric aldol reactions: Chemical and spectroscopic evidence for a metalloenolate intermediate |
|||
| author = Pagenkopf B.L. |
|||
|author2=Kruger J. |author3=Stojanovic A. |author4=Carreira E.M. |
|||
| doi = 10.1002/(SICI)1521-3773(19981204)37:22<3124::AID-ANIE3124>3.0.CO;2-1 |
|||
| journal = Angew. Chem. Intl. Ed. |
|||
| year =1998 |
|||
| volume =37 |
|||
| pages =3124–6 |
|||
| issue = 22 |
|||
}}</ref> |
|||
[[File:Acetatealdol3.gif|center]] |
|||
===Crimmins thiazolidinethione aldol=== |
|||
A more recent{{when|date=April 2011}} version of the Evans' auxiliary is the '''Crimmins thiazolidinethione'''.<ref name=Crimmins1997>{{cite journal |
|||
| title = Asymmetric Aldol Additions with Titanium Enolates of Acyloxazolidinethiones: Dependence of Selectivity on Amine Base and Lewis Acid Stoichiometry |
|||
| author = Crimmins M. T. |
|||
|author2=King B. W. |author3=Tabet A. E. |
|||
| doi = 10.1021/ja9716721 |
|||
| journal = Journal of the American Chemical Society |
|||
| year = 1997 |
|||
| volume =119 |
|||
| issue = 33 |
|||
| pages =7883–7884}}</ref><ref name=Crimmins2000>{{cite journal |
|||
| title = Titanium enolates of thiazolidinethione chiral auxiliaries: Versatile tools for asymmetric aldol additions |
|||
| author = Crimmins M. T. |
|||
|author2=Chaudhary K. |
|||
| doi = 10.1021/ol9913901 |
|||
| journal = Organic Letters |
|||
| year = 2000 |
|||
| volume =2 |
|||
| issue = 6 |
|||
| pages =775–777 |
|||
| pmid = 10754681}}</ref> |
|||
The [[chemical yield|yields]], [[diastereoselectivity|diastereoselectivities]], and enantioselectivities of the reaction are, in general, high, although not as high as in comparable Evans cases. Unlike the Evans auxiliary, however, the thiazoldinethione can perform acetate aldol reactions (ref: Crimmins, Org. Lett. 2007, 9(1), 149–152.) and can produce the "Evans syn" or "non-Evans syn" adducts by simply varying the amount of [[sparteine|(−)-sparteine]]. The reaction is believed to proceed via six-membered, titanium-bound [[transition state]]s, analogous to the proposed transition states for the Evans auxiliary. NOTE: the structure of sparteine shown below is missing a N atom. |
|||
=== "Masked" enols === |
|||
[[File:crimminsaldol1.gif|center]] |
|||
A common modification of the aldol reaction uses other, similar functional groups as ''ersatz'' enols. In the [[Mukaiyama aldol reaction]],<ref>{{cite journal| title = The Impact of the Mukaiyama Aldol Reaction in Total Synthesis| author = S. B. Jennifer Kan| author2=Kenneth K.-H. Ng|author3=Ian Paterson| journal = [[Angewandte Chemie International Edition]]| volume = 52| issue = 35| pages = 9097–9108| year = 2013| doi = 10.1002/anie.201303914| pmid = 23893491}}</ref> [[silyl enol ether]]s add to carbonyls in the presence of a [[Lewis acid]] catalyst, such as [[boron trifluoride]] (as [[boron trifluoride etherate]]) or [[titanium tetrachloride]].<ref>{{cite journal| title = Reactions of silyl enol ethers with carbonyl compounds activated by titanium tetrachloride| author = Teruaki Mukaiyama| author2=Kazuo Banno|author3=Koichi Narasaka| journal = [[Journal of the American Chemical Society]]| volume = 96| issue = 24| pages = 7503–7509| year = 1974| doi = 10.1021/ja00831a019}}</ref><ref>[http://www.orgsynth.org/orgsyn/pdfs/CV8P0323.pdf 3-Hydroxy-3-Methyl-1-Phenyl-1-Butanone by Crossed Aldol Reaction] Teruaki Mukaiyama and Koichi Narasaka [[Organic Syntheses]], Coll. Vol. 8, p.323 ('''1993'''); Vol. 65, p.6 ('''1987''')</ref> |
|||
In the [[Stork enamine alkylation]], secondary amines form [[enamine]]s when exposed to ketones. These enamines then react (possibly enantio­selectively<ref>{{cite book |
|||
===Organocatalysis=== |
|||
A more recent{{when|date=April 2011}} development is the use of chiral secondary [[amine]] catalysts. These secondary amines form transient [[enamine]]s when exposed to ketones, which may react enantioselectively<ref>{{cite journal |
|||
| first= E. M. |
| first= E. M. |
||
| last= Carreira |
| last= Carreira |
||
|author2= Fettes, A.|author3= Martl, C. |
|author2= Fettes, A.|author3= Martl, C. |
||
| |
| series= [[Org. React.]] |
||
| year= 2006 |
| year= 2006 |
||
| volume= 67 |
| volume= 67 |
||
| pages= 1–216 |
| pages= 1–216 |
||
| doi= 10.1002/0471264180.or067.01 |
| doi= 10.1002/0471264180.or067.01 |
||
| title= Catalytic Enantioselective Aldol Addition Reactions | isbn= 978-0471264187 |
|||
| title= Catalytic Enantioselective Aldol Addition Reactions }}</ref> with suitable aldehyde electrophiles. The amine reacts with the carbonyl to form an enamine, the enamine acts as an enol-like nucleophile, and then the amine is released from the product all—the amine itself is a catalyst. This enamine catalysis method is a type of [[organocatalysis]], since the catalyst is entirely based on a small organic molecule. In a seminal example, [[proline]] efficiently catalyzed the cyclization of a triketone: |
|||
}}</ref>) with suitable electrophiles. This strategy offers simple enantioselection without transition metals. In contrast to the preference for ''syn'' adducts typically observed in enolate-based aldol additions, these aldol additions are ''anti''-selective. |
|||
In aqueous solution, the enamine can then be hydrolyzed from the product, making it a [[organocatalysis|small organic molecule catalyst]]. In a seminal example, [[proline]] efficiently catalyzed the cyclization of a triketone: |
|||
[[File:organocatalytic1.gif|center]] |
[[File:organocatalytic1.gif|center]] |
||
This combination is the [[Hajos-Parrish reaction]]<ref>Z. G. Hajos, D. R. Parrish, German Patent DE 2102623 '''1971'''</ref><ref>{{cite journal |
|||
This reaction is known as the [[Hajos-Parrish reaction]]<ref>Z. G. Hajos, D. R. Parrish, German Patent DE 2102623 '''1971'''</ref><ref>{{cite journal |
|||
| title = Asymmetric synthesis of bicyclic intermediates of natural product chemistry |
| title = Asymmetric synthesis of bicyclic intermediates of natural product chemistry |
||
| first = Zoltan G. | last = Hajos |
| first = Zoltan G. | last = Hajos |
||
| Line 618: | Line 217: | ||
| issue = 12 |
| issue = 12 |
||
| pages =1615–1621 |
| pages =1615–1621 |
||
| doi = 10.1021/jo00925a003 }}</ref> |
| doi = 10.1021/jo00925a003 }}</ref><ref>{{cite journal |
||
| title = New Type of Asymmetric Cyclization to Optically Active Steroid CD Partial Structures |
| title = New Type of Asymmetric Cyclization to Optically Active Steroid CD Partial Structures |
||
| first = Ulrich | last = Eder |
| first = Ulrich | last = Eder |
||
| Line 627: | Line 226: | ||
| issue = 7 |
| issue = 7 |
||
| pages =1615–1621 |
| pages =1615–1621 |
||
| doi = 10.1002/anie.197104961 }}</ref> Under |
| doi = 10.1002/anie.197104961 }}</ref> Under Hajos-Parrish conditions only a catalytic amount of proline is necessary (3 mol%). There is no danger of an achiral background reaction because the transient enamine intermediates are much more nucleophilic than their parent ketone enols. |
||
A Stork-type strategy also allows the otherwise challenging cross-reactions between two aldehydes. In many cases, the conditions are mild enough to avoid polymerization:<ref>{{cite journal |last=Northrup |first=Alan B. |author2=MacMillan David W. C. |year=2002 |title=The First Direct and Enantioselective Cross-Aldol Reaction of Aldehydes |url=https://authors.library.caltech.edu/76965/2/ja0262378_s.pdf |journal=[[Journal of the American Chemical Society]] |volume=124 |issue=24 |pages=6798–6799 |doi=10.1021/ja0262378 |pmid=12059180}}</ref> [[File:organocatalytic3.gif|center]] However, selectivity requires the slow syringe-pump controlled addition of the desired electrophilic partner because both reacting partners typically have enolizable protons. If one aldehyde has no enolizable protons or alpha- or beta-branching, additional control can be achieved. |
|||
It is interesting to note that proline-catalyzed aldol reactions do not show any non-linear effects (the enantioselectivity of the products is directly proportional to the enantiopurity of the catalyst). Combined with [[isotopic labelling]] evidence and [[computational chemistry|computational studies]], the proposed [[reaction mechanism]] for proline-catalyzed aldol reactions is as follows:<ref>{{cite journal |
|||
| title = The ying and yang of asymmetric aminocatalysis |
|||
| first = Benjamin | last = List |
|||
| journal = [[Chemical Communications]] |
|||
| year =2006 |
|||
| volume = |
|||
| issue = 8 |
|||
| pages =819–824 |
|||
| doi = 10.1039/b514296m |
|||
| pmid = 16479280 }}</ref> |
|||
==="Direct" aldol additions=== |
|||
[[File:organocatalytic2.gif|center]] |
|||
In the usual aldol addition, a carbonyl compound is deprotonated to form the enolate. The enolate is added to an aldehyde or ketone, which forms an alkoxide, which is then protonated on workup. A superior method, in principle, would avoid the requirement for a multistep sequence in favor of a "direct" reaction that could be done in a single process step. |
|||
This strategy allows the otherwise challenging cross-aldol reaction between two aldehydes. In general, cross-aldol reactions between aldehydes are typically challenging because they can [[polymerization|polymerize]] easily or react unselectively to give a statistical mixture of products. The first example is shown below:<ref>{{cite journal |
|||
| title = The First Direct and Enantioselective Cross-Aldol Reaction of Aldehydes |
|||
| first = Alan B. | last = Northrup |
|||
|author2=MacMillan David W. C. |
|||
| journal = [[Journal of the American Chemical Society]] |
|||
| year =2002 |
|||
| volume = 124 |
|||
| issue = 24 |
|||
| pages =6798–6799 |
|||
| pmid = 12059180 |
|||
| doi = 10.1021/ja0262378 }}</ref> |
|||
If one coupling partner preferentially enolizes, then the general problem is that the addition generates an alkoxide, which is much more basic than the starting materials. This product binds tightly to the enolizing agent, preventing it from catalyzing additional reactants: [[File:Aldol alkoxide product.png|360px|center]] |
|||
[[File:organocatalytic3.gif|center]] |
|||
One approach, demonstrated by Evans, is to silylate the aldol adduct.<ref>{{cite journal |last=Evans |first=D. A. |author-link=David A. Evans |author2=Tedrow, J. S. |author3=Shaw, J. T. |author4=Downey, C. W. |year=2002 |title=Diastereoselective Magnesium Halide-Catalyzed anti-Aldol Reactions of Chiral N-Acyloxazolidinones |journal=[[Journal of the American Chemical Society]] |volume=124 |issue=3 |pages=392–393 |doi=10.1021/ja0119548 |pmid=11792206}}</ref><ref>{{cite journal |last=Evans |first=David A. |author-link=David A. Evans |author2=Downey, C. Wade |author3=Shaw, Jared T. |author4=Tedrow, Jason S. |year=2002 |title=Magnesium Halide-Catalyzed Anti-Aldol Reactions of Chiral N-Acylthiazolidinethiones |journal=[[Organic Letters]] |volume=4 |issue=7 |pages=1127–1130 |doi=10.1021/ol025553o |pmid=11922799}}</ref> A silicon reagent such as [[TMSCl]] is added in the reaction, which replaces the metal on the alkoxide, allowing [[Turnover number|turnover]] of the metal catalyst: [[File:Directaldol2.png|center]] |
|||
In contrast to the preference for syn adducts typically observed in enolate-based aldol additions, these organocatalyzed aldol additions are anti-selective. In many cases, the organocatalytic conditions are mild enough to avoid polymerization. However, selectivity requires the slow syringe-pump controlled addition of the desired electrophilic partner because both reacting partners typically have enolizable protons. If one aldehyde has no enolizable protons or alpha- or beta-branching, additional control can be achieved. |
|||
== Use in carbohydrate synthesis == |
|||
An elegant demonstration of the power of asymmetric organocatalytic aldol reactions was disclosed by MacMillan and coworkers in 2004 in their synthesis of differentially protected [[carbohydrate]]s. While traditional synthetic methods accomplish the synthesis of [[hexose]]s using variations of iterative [[protective group|protection-deprotection]] strategies, requiring 8–14 steps, organocatalysis can access many of the same substrates using an efficient two-step protocol involving the proline-catalyzed dimerization of alpha-oxyaldehydes followed by tandem Mukaiyama aldol cyclization. |
|||
Traditional syntheses of [[hexose]]s use variations of iterative [[protective group|protection-deprotection]] strategies, requiring 8–14 steps, organocatalysis can access many of the same substrates by a two-step protocol involving the proline-catalyzed dimerization of alpha-oxyaldehydes followed by tandem Mukaiyama aldol cyclization. |
|||
[[File:organocatalytic4.gif|center]] |
[[File:organocatalytic4.gif|center]] |
||
| Line 672: | Line 252: | ||
| year = 2004 |
| year = 2004 |
||
| doi = 10.1002/anie.200453716 |
| doi = 10.1002/anie.200453716 |
||
| pmid = 15083470 |
| pmid = 15083470| doi-access = free |
||
}} |
|||
</ref> |
</ref> |
||
Earlier studies revealed that aldehydes bearing alpha-alkyloxy or alpha-[[silyloxy]] [[substituent]]s were suitable for this reaction, while aldehydes bearing [[Electron-withdrawing group]]s such as [[acetoxy]] were unreactive. The protected [[erythrose]] product could then be converted to four possible sugars via Mukaiyama aldol addition followed by [[lactol]] formation. This requires appropriate diastereocontrol in the Mukaiyama aldol addition and the product [[carbenium ion|silyloxycarbenium ion]] to preferentially cyclize, rather than undergo further aldol reaction. In the end, [[glucose]], [[mannose]], and [[allose]] were synthesized: |
Earlier studies revealed that aldehydes bearing alpha-alkyloxy or alpha-[[silyloxy]] [[substituent]]s were suitable for this reaction, while aldehydes bearing [[Electron-withdrawing group]]s such as [[acetoxy]] were unreactive. The protected [[erythrose]] product could then be converted to four possible sugars via Mukaiyama aldol addition followed by [[lactol]] formation. This requires appropriate diastereocontrol in the Mukaiyama aldol addition and the product [[carbenium ion|silyloxycarbenium ion]] to preferentially cyclize, rather than undergo further aldol reaction. In the end, [[glucose]], [[mannose]], and [[allose]] were synthesized: |
||
[[File:OrganocatalyticTop (cropped).gif|center]] |
|||
[[File:Organocatalytic5Bottom (cropped).gif|center]] |
|||
==Biological aldol reactions== |
|||
[[File:organocatalytic5.gif|center]] |
|||
Examples of aldol reactions in biochemistry include the splitting of [[fructose-1,6-bisphosphate]] into [[dihydroxyacetone]] and [[glyceraldehyde-3-phosphate]] in the fourth stage of [[glycolysis]], which is an example of a reverse ("retro") aldol reaction catalyzed by the enzyme [[aldolase A]] (also known as fructose-1,6-bisphosphate aldolase). |
|||
In the [[glyoxylate cycle]] of plants and some prokaryotes, [[isocitrate lyase]] produces [[glyoxylate]] and [[succinate]] from [[isocitrate]]. Following deprotonation of the OH group, isocitrate lyase cleaves isocitrate into the four-carbon succinate and the two-carbon glyoxylate by an aldol cleavage reaction. This cleavage is similar mechanistically to the aldolase A reaction of glycolysis. |
|||
==="Direct" aldol additions=== |
|||
In the usual aldol addition, a carbonyl compound is deprotonated to form the enolate. The enolate is added to an aldehyde or ketone, which forms an alkoxide, which is then protonated on workup. A superior method, in principle, would avoid the requirement for a multistep sequence in favor of a "direct" reaction that could be done in a single process step. One idea is to generate the enolate using a metal [[catalyst]] that is released after the aldol addition mechanism. The general problem is that the addition generates an alkoxide, which is much more basic than the starting materials. This product binds tightly to the metal, preventing it from reacting with additional carbonyl reactants. |
|||
==History== |
|||
[[File:Aldol alkoxide product.png|360px|center]] |
|||
The aldol reaction was discovered independently by the Russian chemist (and Romantic composer) [[Alexander Borodin]] in 1869<ref>Borodin reported on the condensation of [[pentanal]] (''Valerianaldehyd'') with [[heptanal]] (''Oenanthaldehyd'') in: von Richter, V. (1869) [https://babel.hathitrust.org/cgi/pt?id=hvd.cl1hyq;view=1up;seq=600 "V. von Richter, aus St. Petersburg am 17. October 1869"] (V. von Richter [reporting] from St. Petersburg on 17. October 1869), ''[[Berichte der deutschen chemischen Gesellschaft]]'' (in German), '''2''' : 552-553. |
|||
* English version of Richter's report: (Staff) (December 10, 1869) [https://books.google.com/books?id=e1dKAAAAYAAJ&pg=PA286 "Chemical notices from foreign sources: Berichte der Deutschen Chemischen Gesellschaft zu Berlin, no. 16, 1869: Valerian aldehyde and Oenanth aldehyde – M. Borodin,"] ''[[Chemical News|The Chemical News and Journal of Industrial Science]]'', '''20''' : 286.</ref><ref>Garner, Susan Amy (2007) "Hydrogen-mediated carbon-carbon bond formations: Applied to reductive aldol and Mannich reactions," Ph.D. dissertation, University of Texas (Austin), [https://books.google.com/books?id=wc8dTz820AIC&pg=PA4 pp. 4] and [https://books.google.com/books?id=wc8dTz820AIC&pg=PA51 51.]</ref><ref>Borodin, A. (1873) [https://babel.hathitrust.org/cgi/pt?id=uc1.b3481752;view=1up;seq=116 "Ueber einen neuen Abkömmling des Valerals"] (On a new derivative of valerian aldehyde), ''[[Berichte der deutschen chemischen Gesellschaft]]'' (in German), '''6''' : 982–985.</ref> and by the French chemist [[Charles-Adolphe Wurtz]] in 1872, which originally used aldehydes to perform the reaction.<ref name=Wurtz1872>{{cite journal| last = Wurtz | first = C. A.| author-link = Charles-Adolphe Wurtz| journal = [[Bulletin de la Société Chimique de Paris]]| year = 1872| title = Sur un aldéhyde-alcool | trans-title = On an aldehyde alcohol | language = fr| series = 2nd series| volume = 17| pages = 436–442| url = https://babel.hathitrust.org/cgi/pt?id=iau.31858003147356;view=1up;seq=446}}</ref><ref name=Wurtz1872b>{{cite journal | last = Wurtz | first = C. A.| author-link = Charles-Adolphe Wurtz| journal = [[Journal für Praktische Chemie]]| year = 1872| title = Ueber einen Aldehyd-Alkohol | trans-title = About an aldehyde alcohol | language = de| volume = 5| issue = 1| pages = 457–464| url = https://babel.hathitrust.org/cgi/pt?id=njp.32101076786381;view=1up;seq=471| doi = 10.1002/prac.18720050148}}</ref><ref name=Wurtz1872c>{{cite journal| last = Wurtz | first = C. A.| author-link = Charles-Adolphe Wurtz| journal = [[Comptes rendus de l'Académie des sciences]]| year = 1872| title = Sur un aldéhyde-alcool | trans-title = On an aldehyde alcohol | language = fr| volume = 74| pages =1361| url = http://gallica.bnf.fr/ark:/12148/bpt6k3031q/f1361.table }}</ref> |
|||
[[Howard Zimmerman]] and Marjorie D. Traxler proposed their model for stereoinduction in a 1957 paper.<ref name="Zimmerman1920" /> |
|||
One approach, demonstrated by Evans, is to silylate the aldol adduct.<ref>{{cite journal |
|||
| authorlink = David A. Evans |
|||
| last = Evans |
|||
| first = D. A. |
|||
|author2= Tedrow, J. S.|author3= Shaw, J. T.|author4= Downey, C. W. |
|||
| title = Diastereoselective Magnesium Halide-Catalyzed anti-Aldol Reactions of Chiral N-Acyloxazolidinones |
|||
| journal = [[Journal of the American Chemical Society]] |
|||
| volume = 124 |
|||
| issue = 3 |
|||
| pages = 392–393 |
|||
| year = 2002 |
|||
| doi = 10.1021/ja0119548 |
|||
| pmid = 11792206 }}</ref><ref>{{cite journal |
|||
| authorlink = David A. Evans |
|||
| last = Evans |
|||
| first = David A. |
|||
|author2= Downey, C. Wade|author3= Shaw, Jared T.|author4= Tedrow, Jason S. |
|||
| title = Magnesium Halide-Catalyzed Anti-Aldol Reactions of Chiral N-Acylthiazolidinethiones |
|||
| journal = [[Organic Letters]] |
|||
| volume = 4 |
|||
| issue = 7 |
|||
| pages = 1127–1130 |
|||
| year = 2002 |
|||
| doi = 10.1021/ol025553o |
|||
| pmid = 11922799 }}</ref> A silicon reagent such as [[TMSCl]] is added in the reaction, which replaces the metal on the alkoxide, allowing [[Turnover number|turnover]] of the metal catalyst. Minimizing the number of reaction steps and amount of reactive chemicals used leads to a cost-effective and industrially useful reaction. |
|||
[[File:directaldol2.gif|center]] |
|||
A more recent{{when|date=April 2011}} [[biomimetic]] approach by Shair uses beta-thio[[ketoacid]]s as the nucleophile.<ref>{{cite journal |
|||
| last = Magdziak |
|||
| first = D. |
|||
|author2=Lalic, G.|author3= Lee, H. M.|author4= Fortner, K. C.|author5= Aloise, A. D.|author6= Shair, M. D. |
|||
| title = Catalytic Enantioselective Thioester Aldol Reactions That Are Compatible with Protic Functional Groups |
|||
| journal = [[Journal of the American Chemical Society]] |
|||
| volume = 127 |
|||
| issue = 20 |
|||
| pages = 7284–7285 |
|||
| year = 2005 |
|||
| doi = 10.1021/ja051759j |
|||
| pmid = 15898756}}</ref> The ketoacid moiety is [[decarboxylation|decarboxylated]] ''[[in situ]]''. The process is similar to the way [[malonyl-CoA]] is used by [[Polyketide synthase]]s. The [[chiral ligand]] is case is a [[bisoxazoline ligand|bisoxazoline]]). Interestingly, aromatic and branched aliphatic aldehydes are typically poor substrates. |
|||
[[File:directaldol3.gif|center]] |
|||
==Biological aldol reactions== |
|||
Examples of aldol reactions in biochemistry include the splitting of [[fructose-1,6-bisphosphate]] into [[dihydroxyacetone]] and [[glyceraldehyde-3-phosphate]] in the second stage of [[glycolysis]], which is an example of a reverse ("retro") aldol reaction catalyzed by the enzyme [[aldolase A]] (also known as fructose-1,6-bisphosphate aldolase). |
|||
In the [[glyoxylate cycle]] of plants and some prokaryotes, [[isocitrate lyase]] produces [[glyoxylate]] and [[succinate]] from [[isocitrate]]. Following deprotonation of the OH group, isocitrate lyase cleaves isocitrate into the four-carbon succinate and the two-carbon glyoxylate via an aldol cleavage reaction. This cleavage is very similar mechanistically to the aldolase A reaction of glycolysis. |
|||
==See also== |
==See also== |
||
| Line 737: | Line 276: | ||
* [[Ivanov reaction]] |
* [[Ivanov reaction]] |
||
* [[Reformatsky reaction]] |
* [[Reformatsky reaction]] |
||
* [[Claisen-Schmidt condensation]] |
|||
== Notes == |
|||
{{notelist}} |
|||
==References== |
===References=== |
||
{{Reflist|colwidth=30em}} |
{{Reflist|colwidth=30em}} |
||
== |
===Further reading=== |
||
* [[iarchive:EvansD.A.HarvardsAdvancedOrganicChemistry2003|Chem 206, 215 Lecture Notes (2003, 2006)]] by [[David A. Evans|D. A. Evans]], [[A. G. Myers]], ''et al.'', Harvard University (pp. 345, 936) |
|||
* [http://isites.harvard.edu/fs/docs/icb.topic1146159.files/JJB%20Aldol%20Lecture%2021%20Final.pdf Chem 106 Aldol Reaction Notes] |
|||
{{Organic reactions}} |
|||
[[Category:Addition reactions]] |
[[Category:Addition reactions]] |
||
[[Category:Carbon-carbon bond forming reactions]] |
[[Category:Carbon-carbon bond forming reactions]] |
||
[[Category:Alexander Borodin]] |
|||
Latest revision as of 09:58, 30 November 2024
| Aldol Addition | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Reaction type | Coupling reaction | ||||||||
| Reaction | |||||||||
| |||||||||
| Conditions | |||||||||
| Temperature | -Δ, ~-70°C[a]
| ||||||||
| Catalyst | -OH or H+
| ||||||||
| Identifiers | |||||||||
| Organic Chemistry Portal | aldol-addition | ||||||||
| RSC ontology ID | RXNO:0000016 | ||||||||
The aldol reaction (aldol addition) is a reaction in organic chemistry that combines two carbonyl compounds (e.g. aldehydes or ketones) to form a new β-hydroxy carbonyl compound. Its simplest form might involve the nucleophilic addition of an enolized ketone to another:

These products are known as aldols, from the aldehyde + alcohol, a structural motif seen in many of the products. The use of aldehyde in the name comes from its history: aldehydes are more reactive than ketones, so that the reaction was discovered first with them.[2][3][4]
The aldol reaction is paradigmatic in organic chemistry and one of the most common means of forming carbon–carbon bonds in organic chemistry.[5][6][7] It lends its name to the family of aldol reactions and similar techniques analyze a whole family of carbonyl α-substitution reactions, as well as the diketone condensations.
Scope
[edit]Aldol structural units are found in many important molecules, whether naturally occurring or synthetic.[8][9] The reaction is well used on an industrial scale, notably of pentaerythritol,[10] trimethylolpropane, the plasticizer precursor 2-ethylhexanol, and the drug Lipitor (atorvastatin, calcium salt).[11] For many of the commodity applications, the stereochemistry of the aldol reaction is unimportant, but the topic is of intense interest for the synthesis of many specialty chemicals.
Aldol dimerization
[edit]In its simplest implementation, base induces conversion of an aldehyde or a ketone to the aldol product. One example involves the aldol condensation of propionaldehyde:
- 2 CH3CH2CHO → CH3CH2CH(OH)CH(CH3)CHO
Featuring the RCH(OH)CHR'C(O)R" grouping, the product is an aldol. In this case . Such reactions are called aldol aldol dimerization.
Cross-aldol
[edit]With a mixture of carbonyl precursors, complicated mixtures can occur. Addition of base to a mixture of propionaldehyde and acetaldehyde, one obtains four products:
- CH3CH2CH(OH)CH(CH3)CHO (from two propionaldehydes), CH3CH(OH)CH2CHO (from two acetaldehydes, and both CH3CH2CH(OH)CH2CHO and CH3CH(OH)CH(CH3)CHO
The first two products are the result of aldol dimerization but the latter two result from a crossed aldol reaction. Complicated mixtures from cross aldol reactions can be avoided by using one component that cannot form an enolate, examples being formaldehyde and benzaldehyde. This approach is used in one stage in the production of trimethylolethane, which entails crossed aldol condensation of butyraldehyde and formaldehyde:
- CH3CH2CH2CHO + 2 CH2O → CH3CH2C(CH2OH)2CHO
Reactions of aldols
[edit]Aldols dehydrate:
- CH3CH2CH(OH)CH(CH3)CHO → CH3CH2CH=C(CH3)CHO + H2O
Because this conversion is facile, it is sometimes assumed. It is for this reason that the aldol reaction is sometimes called the aldol condensation.
Mechanisms
[edit]
The flask on the right is a solution of lithium diisopropylamide (LDA) in tetrahydrofuran (THF). The flask on the left is a solution of the lithium enolate of tert-butyl propionate (formed by addition of LDA to tert-butyl propionate). An aldehyde can then be added to the enolate flask to initiate an aldol addition reaction.
Both flasks are submerged in a dry ice/acetone cooling bath (−78 °C) the temperature of which is being monitored by a thermocouple (the wire on the left).
The aldol reaction has one underlying mechanism: a carbanion-like nucleophile attacks a carbonyl center.[12]
If the base is of only moderate strength such as hydroxide ion or an alkoxide, the aldol reaction occurs via nucleophilic attack by the resonance-stabilized enolate on the carbonyl group of another molecule. The product is the alkoxide salt of the aldol product. The aldol itself is then formed, and it may then undergo dehydration to give the unsaturated carbonyl compound. The scheme shows a simple mechanism for the base-catalyzed aldol reaction of an aldehyde with itself.

Although only a catalytic amount of base is required in some cases, the more usual procedure is to use a stoichiometric amount of a strong base such as LDA or NaHMDS. In this case, enolate formation is irreversible, and the aldol product is not formed until the metal alkoxide of the aldol product is protonated in a separate workup step.
When an acid catalyst is used, the initial step in the reaction mechanism involves acid-catalyzed tautomerization of the carbonyl compound to the enol. The acid also serves to activate the carbonyl group of another molecule by protonation, rendering it highly electrophilic. The enol is nucleophilic at the α-carbon, allowing it to attack the protonated carbonyl compound, leading to the aldol after deprotonation. Some may also dehydrate past the intended product to give the unsaturated carbonyl compound through aldol condensation.

Crossed-aldol reactant control
[edit]Despite the attractiveness of the aldol manifold, there are several problems that need to be addressed to render the process effective. The first problem is a thermodynamic one: most aldol reactions are reversible. Furthermore, the equilibrium is also just barely on the side of the products in the case of simple aldehyde–ketone aldol reactions.[13] If the conditions are particularly harsh (e.g.: NaOMe/MeOH/reflux), condensation may occur, but this can usually be avoided with mild reagents and low temperatures (e.g., LDA (a strong base), THF, −78 °C). Although the aldol addition usually proceeds to near completion under irreversible conditions, the isolated aldol adducts are sensitive to base-induced retro-aldol cleavage to return starting materials. In contrast, retro-aldol condensations are rare, but possible.[14] This is the basis of the catalytic strategy of class I aldolases in nature, as well as numerous small-molecule amine catalysts.[15]
When a mixture of unsymmetrical ketones are reacted, four crossed-aldol (addition) products can be anticipated:

Thus, if one wishes to obtain only one of the cross-products, one must control which carbonyl becomes the nucleophilic enol/enolate and which remains in its electrophilic carbonyl form. The simplest control is if only one of the reactants has acidic protons, and only this molecule forms the enolate. For example, the addition of diethyl malonate into benzaldehyde produces only one product:
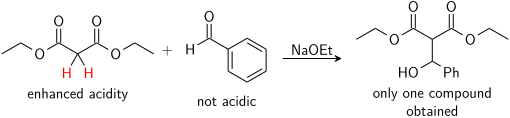
If one group is considerably more acidic than the other, the most acidic proton is abstracted by the base and an enolate is formed at that carbonyl while the less-acidic carbonyl remains electrophilic. This type of control works only if the difference in acidity is large enough and base is the limiting reactant. A typical substrate for this situation is when the deprotonatable position is activated by more than one carbonyl-like group. Common examples include a CH2 group flanked by two carbonyls or nitriles (see for example the Knoevenagel condensation and the first steps of the malonic ester synthesis and acetoacetic ester synthesis).
Otherwise, the most acidic carbonyls are typically also the most active electrophiles: first aldehydes, then ketones, then esters, and finally amides. Thus cross-aldehyde reactions are typically most challenging because they can polymerize easily or react unselectively to give a statistical mixture of products.[16]
One common solution is to form the enolate of one partner first, and then add the other partner under kinetic control.[17] Kinetic control means that the forward aldol addition reaction must be significantly faster than the reverse retro-aldol reaction. For this approach to succeed, two other conditions must also be satisfied; it must be possible to quantitatively form the enolate of one partner, and the forward aldol reaction must be significantly faster than the transfer of the enolate from one partner to another. Common kinetic control conditions involve the formation of the enolate of a ketone with LDA at −78 °C, followed by the slow addition of an aldehyde.
Stereoselectivity
[edit]The aldol reaction unites two relatively simple molecules into a more complex one. Increased complexity arises because each end of the new bond may become a stereocenter. Modern methodology has not only developed high-yielding aldol reactions, but also completely controls both the relative and absolute configuration of these new stereocenters.[6]
To describe relative stereochemistry at the α- and β-carbon, older papers use saccharide chemistry's erythro/threo nomenclature; more modern papers use the following syn/anti convention. When propionate (or higher order) nucleophiles add to aldehydes, the reader visualizes the R group of the ketone and the R' group of the aldehyde aligned in a "zig zag" pattern on the paper (or screen). The disposition of the formed stereocenters is deemed syn or anti, depending if they are on the same or opposite sides of the main chain:

The principal factor determining an aldol reaction's stereoselectivity is the enolizing metal counterion. Shorter metal-oxygen bonds "tighten" the transition state and effects greater stereoselection.[18] Boron is often used[19][20] because its bond lengths are significantly shorter than other cheap metals (lithium, aluminium, or magnesium). The following reaction gives a syn:anti ratio of 80:20 using a lithium enolate compared to 97:3 using a bibutylboron enolate.

Where the counterion determines stereoinduction strength, the enolate isomer determines its direction. E isomers give anti products and Z give syn:[21]


Zimmermann-Traxler model
[edit]If the two reactants have carbonyls adjacent to a pre-existing stereocenter, then the new stereocenters may form at a fixed orientation relative to the old. This "substrate-based stereocontrol" has seen extensive study and examples pervade the literature. In many cases, a stylized transition state, called the Zimmerman–Traxler model, can predict the new orientation from the configuration of a 6-membered ring.[22]
On the enol
[edit]If the enol has an adjacent stereocenter, then the two stereocenters flanking the carbonyl in the product are naturally syn:[23]
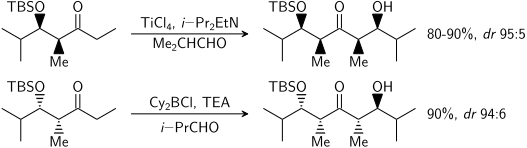
The underlying mechanistic reason depends on the enol isomer. For an E enolate, the stereoinduction is necessary to avoid 1,3-allylic strain, while a Z enolate instead seeks to avoid 1,3-diaxial interactions:[24]
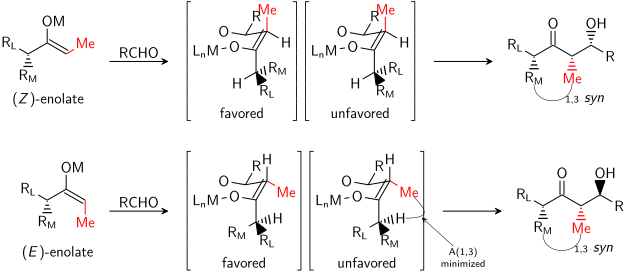
However, Fráter & Seebach showed that a chelating Lewis basic moiety adjacent to the enol will instead cause anti addition.
On the electrophile
[edit]E enolates exhibit Felkin diastereoface selection, while Z enolates exhibit anti-Felkin selectivity. The general model is presented below:[25][26]

Since the transition state for Z enolates must contain either a destabilizing syn-pentane interaction or an anti-Felkin rotamer, Z-enolates are less diastereoselective:[27][28]

On both
[edit]If both the enolate and the aldehyde contain pre-existing chirality, then the outcome of the "double stereodifferentiating" aldol reaction may be predicted using a merged stereochemical model that takes into account all the effects discussed above.[29] Several examples are as follows:[28]

Oxazolidinone chiral auxiliaries
[edit]In the late 1970s and 1980s, David A. Evans and coworkers developed a technique for stereoselection in the aldol syntheses of aldehydes and carboxylic acids.[30][31] The method works by temporarily appending a chiral oxazolidinone auxiliary to create a chiral enolate. The pre-existing chirality from the auxiliary is then transferred to the aldol adduct through Zimmermann-Traxler methods, and then the oxazolidinone cleaved away.

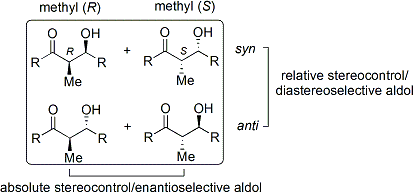

Commercial oxazolidinones are relatively expensive, but derive in 2 synthetic steps from comparatively inexpensive amino acids. (Economical large-scale syntheses prepare the auxiliary in-house.) First, a borohydride reduces the acid moiety. Then the resulting amino alcohol dehydratively cyclises with a simple carbonate ester, such as diethylcarbonate.

The acylation of an oxazolidinone is informally referred to as "loading done".
Anti adducts, which require an E enolate, cannot be obtained reliably with the Evans method. However, Z enolates, leading to syn adducts, can be reliably formed using boron-mediated soft enolization:[32]

Often, a single diastereomer may be obtained by one crystallization of the aldol adduct.
Many methods cleave the auxiliary:[33]

Variations
[edit]A common additional chiral auxiliary is a thioether group:[33][b]

Crimmins thiazolidinethione aldol
[edit]In the Crimmins thiazolidinethione approach,[34][35] a thiazolidinethione is the chiral auxiliary[36] and can produce the "Evans syn" or "non-Evans syn" adducts by simply varying the amount of (−)-sparteine. The reaction is believed to proceed via six-membered, titanium-bound transition states, analogous to the proposed transition states for the Evans auxiliary.

"Masked" enols
[edit]A common modification of the aldol reaction uses other, similar functional groups as ersatz enols. In the Mukaiyama aldol reaction,[37] silyl enol ethers add to carbonyls in the presence of a Lewis acid catalyst, such as boron trifluoride (as boron trifluoride etherate) or titanium tetrachloride.[38][39]
In the Stork enamine alkylation, secondary amines form enamines when exposed to ketones. These enamines then react (possibly enantioselectively[40]) with suitable electrophiles. This strategy offers simple enantioselection without transition metals. In contrast to the preference for syn adducts typically observed in enolate-based aldol additions, these aldol additions are anti-selective.
In aqueous solution, the enamine can then be hydrolyzed from the product, making it a small organic molecule catalyst. In a seminal example, proline efficiently catalyzed the cyclization of a triketone:

This combination is the Hajos-Parrish reaction[41][42][43] Under Hajos-Parrish conditions only a catalytic amount of proline is necessary (3 mol%). There is no danger of an achiral background reaction because the transient enamine intermediates are much more nucleophilic than their parent ketone enols.
A Stork-type strategy also allows the otherwise challenging cross-reactions between two aldehydes. In many cases, the conditions are mild enough to avoid polymerization:[44]

However, selectivity requires the slow syringe-pump controlled addition of the desired electrophilic partner because both reacting partners typically have enolizable protons. If one aldehyde has no enolizable protons or alpha- or beta-branching, additional control can be achieved.
"Direct" aldol additions
[edit]In the usual aldol addition, a carbonyl compound is deprotonated to form the enolate. The enolate is added to an aldehyde or ketone, which forms an alkoxide, which is then protonated on workup. A superior method, in principle, would avoid the requirement for a multistep sequence in favor of a "direct" reaction that could be done in a single process step.
If one coupling partner preferentially enolizes, then the general problem is that the addition generates an alkoxide, which is much more basic than the starting materials. This product binds tightly to the enolizing agent, preventing it from catalyzing additional reactants:

One approach, demonstrated by Evans, is to silylate the aldol adduct.[45][46] A silicon reagent such as TMSCl is added in the reaction, which replaces the metal on the alkoxide, allowing turnover of the metal catalyst:

Use in carbohydrate synthesis
[edit]Traditional syntheses of hexoses use variations of iterative protection-deprotection strategies, requiring 8–14 steps, organocatalysis can access many of the same substrates by a two-step protocol involving the proline-catalyzed dimerization of alpha-oxyaldehydes followed by tandem Mukaiyama aldol cyclization.

The aldol dimerization of alpha-oxyaldehydes requires that the aldol adduct, itself an aldehyde, be inert to further aldol reactions.[47] Earlier studies revealed that aldehydes bearing alpha-alkyloxy or alpha-silyloxy substituents were suitable for this reaction, while aldehydes bearing Electron-withdrawing groups such as acetoxy were unreactive. The protected erythrose product could then be converted to four possible sugars via Mukaiyama aldol addition followed by lactol formation. This requires appropriate diastereocontrol in the Mukaiyama aldol addition and the product silyloxycarbenium ion to preferentially cyclize, rather than undergo further aldol reaction. In the end, glucose, mannose, and allose were synthesized:


Biological aldol reactions
[edit]Examples of aldol reactions in biochemistry include the splitting of fructose-1,6-bisphosphate into dihydroxyacetone and glyceraldehyde-3-phosphate in the fourth stage of glycolysis, which is an example of a reverse ("retro") aldol reaction catalyzed by the enzyme aldolase A (also known as fructose-1,6-bisphosphate aldolase).
In the glyoxylate cycle of plants and some prokaryotes, isocitrate lyase produces glyoxylate and succinate from isocitrate. Following deprotonation of the OH group, isocitrate lyase cleaves isocitrate into the four-carbon succinate and the two-carbon glyoxylate by an aldol cleavage reaction. This cleavage is similar mechanistically to the aldolase A reaction of glycolysis.
History
[edit]The aldol reaction was discovered independently by the Russian chemist (and Romantic composer) Alexander Borodin in 1869[48][49][50] and by the French chemist Charles-Adolphe Wurtz in 1872, which originally used aldehydes to perform the reaction.[2][3][4]
Howard Zimmerman and Marjorie D. Traxler proposed their model for stereoinduction in a 1957 paper.[22]
See also
[edit]- Aldol–Tishchenko reaction
- Baylis–Hillman reaction
- Ivanov reaction
- Reformatsky reaction
- Claisen-Schmidt condensation
Notes
[edit]- ^ It is typically best to minimize heat for this reaction. As removal of water from excess heat risks shifting the equilibrium in favor of a dehydration reaction, leading to the aldol condensation product.
By avoiding heat, it can help avoid dehydration so that the majority of product produced is the aldol addition product.[1] - ^ In this reaction the nucleophile is a boron enolate derived from reaction with dibutylboron triflate (nBu2BOTf), the base is N,N-diisopropylethylamine. The thioether is removed in step 2 by Raney Nickel / hydrogen reduction
References
[edit]- ^ Klein, David R. (December 22, 2020). Organic chemistry (4th ed.). Hoboken, NJ: Wiley. p. 1014. ISBN 978-1-119-65959-4. OCLC 1201694230.
- ^ a b Wurtz, C. A. (1872). "Sur un aldéhyde-alcool" [On an aldehyde alcohol]. Bulletin de la Société Chimique de Paris. 2nd series (in French). 17: 436–442.
- ^ a b Wurtz, C. A. (1872). "Ueber einen Aldehyd-Alkohol" [About an aldehyde alcohol]. Journal für Praktische Chemie (in German). 5 (1): 457–464. doi:10.1002/prac.18720050148.
- ^ a b Wurtz, C. A. (1872). "Sur un aldéhyde-alcool" [On an aldehyde alcohol]. Comptes rendus de l'Académie des sciences (in French). 74: 1361.
- ^ Wade, L. G. (2005). Organic Chemistry (6th ed.). Upper Saddle River, New Jersey: Prentice Hall. pp. 1056–66. ISBN 978-0-13-236731-8.
- ^ a b Smith, Michael B.; March, Jerry (2006). March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure. doi:10.1002/0470084960. ISBN 9780470084960.
- ^ Mahrwald, R. (2004). Modern Aldol Reactions, Volumes 1 and 2. Weinheim, Germany: Wiley-VCH Verlag GmbH & Co. KGaA. pp. 1218–23. ISBN 978-3-527-30714-2.
- ^ Heathcock, C. H. (1991). "The Aldol Reaction: Acid and General Base Catalysis". In Trost, B. M.; Fleming, I. (eds.). Comprehensive Organic Synthesis. Vol. 2. Elsevier Science. pp. 133–179. doi:10.1016/B978-0-08-052349-1.00027-5. ISBN 978-0-08-052349-1.
- ^ Paterson, I. (1988). "New Asymmetric Aldol Methodology Using Boron Enolates". Chem. Ind. 12: 390–394.
- ^ Mestres R. (2004). "A green look at the aldol reaction". Green Chemistry. 6 (12): 583–603. doi:10.1039/b409143b.
- ^ Jie Jack Li; et al. (2004). Contemporary Drug Synthesis. Wiley-Interscience. pp. 118–. ISBN 978-0-471-21480-9.
- ^ Grossmann, Robert B. (Jan 2002). The Art of Writing Reasonable Organic Reaction Mechanisms (2nd ed.). New York: Springer. p. 133. ISBN 0-387-95468-6.
- ^ Molander, G. A., ed. (2011). Stereoselective Synthesis 2: Stereoselective Reactions of Carbonyl and Imino Groups (1 ed.). Stuttgart: Georg Thieme Verlag. doi:10.1055/sos-sd-202-00331. ISBN 978-3-13-154121-5.
- ^ Guthrie, J.P.; Cooper, K.J.; Cossar, J.; Dawson, B.A.; Taylor, K.F. (1984). "The retroaldol reaction of cinnamaldehyde". Can. J. Chem. 62 (8): 1441–1445. doi:10.1139/v84-243.
- ^ Molander, ed. (2011). Stereoselective Synthesis 2: Stereoselective Reactions of Carbonyl and Imino Groups (1 ed.). Stuttgart: Georg Thieme Verlag. doi:10.1055/sos-sd-202-00331. ISBN 978-3-13-154121-5.
- ^ Warren, Stuart; Wyatt, Paul (2008). Organic synthesis: the disconnection approach (2nd ed.). Wiley. ISBN 978-0-470-71236-8.
- ^ Bal, B.; Buse, C. T.; Smith, K.; Heathcock, C. H., (2SR,3RS)-2,4-Dimethyl-3-Hydroxypentanoic Acid Archived 2011-06-06 at the Wayback Machine, Org. Synth., Coll. Vol. 7, p.185 (1990); Vol. 63, p.89 (1985).
- ^ Evans, D. A.; Nelson J. V.; Vogel E.; Taber T. R. (1981). "Stereoselective aldol condensations via boron enolates". Journal of the American Chemical Society. 103 (11): 3099–3111. doi:10.1021/ja00401a031.
- ^ Cowden, C. J.; Paterson, I. Org. React. 1997, 51, 1.
- ^ Cowden, C. J.; Paterson, I. (2004). Asymmetric Aldol Reactions Using Boron Enolates. Organic Reactions. pp. 1–200. doi:10.1002/0471264180.or051.01. ISBN 978-0471264187.
- ^ Brown, H. C.; Dhar, R. K.; Bakshi, R. K.; Pandiarajan, P. K.; Singaram, B. (1989). "Major effect of the leaving group in dialkylboron chlorides and triflates in controlling the stereospecific conversion of ketones into either E- or Z-enol borinates". Journal of the American Chemical Society. 111 (9): 3441–3442. doi:10.1021/ja00191a058.
- ^ a b Zimmerman, H. E.; Traxler, M. D. (1957). "The Stereochemistry of the Ivanov and Reformatsky Reactions. I". Journal of the American Chemical Society. 79 (8): 1920–1923. doi:10.1021/ja01565a041.
- ^ Evans, D. A.; Rieger D. L.; Bilodeau M. T.; Urpi F. (1991). "Stereoselective aldol reactions of chlorotitanium enolates. An efficient method for the assemblage of polypropionate-related synthons". Journal of the American Chemical Society. 113 (3): 1047–1049. doi:10.1021/ja00003a051.
- ^ Heathcock, C. H.; Buse, C. T.; Kleschnick, W. A.; Pirrung, M. C.; Sohn, J. E.; Lampe, J. (1980). "Acyclic stereoselection. 7. Stereoselective synthesis of 2-alkyl-3-hydroxy carbonyl compounds by aldol condensation". Journal of Organic Chemistry. 45 (6): 1066–1081. doi:10.1021/jo01294a030.
- ^ Evans, D. A.; Nelson, J. V.; Taber, T. R. (1982). "Stereoselective Aldol Condensations". Topics in Stereochemistry. Vol. 13. pp. 1–115. ISBN 9780471056805.
- ^ Roush W. R. (1991). "Concerning the diastereofacial selectivity of the aldol reactions of .alpha.-methyl chiral aldehydes and lithium and boron propionate enolates". Journal of Organic Chemistry. 56 (13): 4151–4157. doi:10.1021/jo00013a015.
- ^ Masamune S.; Ellingboe J. W.; Choy W. (1982). "Aldol strategy: coordination of the lithium cation with an alkoxy substituent". Journal of the American Chemical Society. 104 (20): 1047–1049. doi:10.1021/ja00384a062.
- ^ a b Evans, D. A.; Dart M. J.; Duffy J. L.; Rieger D. L. (1995). "Double Stereodifferentiating Aldol Reactions. The Documentation of "Partially Matched" Aldol Bond Constructions in the Assemblage of Polypropionate Systems". Journal of the American Chemical Society. 117 (35): 9073–9074. doi:10.1021/ja00140a027.
- ^ Masamune S.; Choy W.; Petersen J. S.; Sita L. R. (1985). "Double Asymmetric Synthesis and a New Strategy for Stereochemical Control in Organic Synthesis". Angew. Chem. Int. Ed. Engl. 24: 1–30. doi:10.1002/anie.198500013.
- ^ Evans, D. A. (1982). "Studies in Asymmetric Synthesis: The Development of Practical Chiral Enolate Synthons" (PDF). Aldrichimica Acta. 15: 23.
- ^ Gage J. R.; Evans D. A., Diastereoselective Aldol Condensation Using A Chiral Oxazolidinone Auxiliary: (2S*,3S*)-3-Hydroxy-3-Phenyl-2-Methylpropanoic Acid Archived 2012-09-29 at the Wayback Machine, Organic Syntheses, Coll. Vol. 8, p.339 (1993); Vol. 68, p.83 (1990).
- ^ Evans, D. A.; Bartroli J.; Shih T. L. (1981). "Enantioselective aldol condensations. 2. Erythro-selective chiral aldol condensations via boron enolates". Journal of the American Chemical Society. 103 (8): 2127–2129. doi:10.1021/ja00398a058.
- ^ a b Evans, D. A.; Bender S. L.; Morris J. (1988). "The total synthesis of the polyether antibiotic X-206". Journal of the American Chemical Society. 110 (8): 2506–2526. doi:10.1021/ja00216a026.
- ^ Crimmins M. T.; King B. W.; Tabet A. E. (1997). "Asymmetric Aldol Additions with Titanium Enolates of Acyloxazolidinethiones: Dependence of Selectivity on Amine Base and Lewis Acid Stoichiometry". Journal of the American Chemical Society. 119 (33): 7883–7884. doi:10.1021/ja9716721.
- ^ Crimmins M. T.; Chaudhary K. (2000). "Titanium enolates of thiazolidinethione chiral auxiliaries: Versatile tools for asymmetric aldol additions". Organic Letters. 2 (6): 775–777. doi:10.1021/ol9913901. PMID 10754681.
- ^ Crimmins, Michael T.; Shamszad, Mariam (2007). "Highly Selective Acetate Aldol Additions Using Mesityl-Substituted Chiral Auxiliaries". Org. Lett. 9 (1): 149–152. doi:10.1021/ol062688b. PMID 17192107.
- ^ S. B. Jennifer Kan; Kenneth K.-H. Ng; Ian Paterson (2013). "The Impact of the Mukaiyama Aldol Reaction in Total Synthesis". Angewandte Chemie International Edition. 52 (35): 9097–9108. doi:10.1002/anie.201303914. PMID 23893491.
- ^ Teruaki Mukaiyama; Kazuo Banno; Koichi Narasaka (1974). "Reactions of silyl enol ethers with carbonyl compounds activated by titanium tetrachloride". Journal of the American Chemical Society. 96 (24): 7503–7509. doi:10.1021/ja00831a019.
- ^ 3-Hydroxy-3-Methyl-1-Phenyl-1-Butanone by Crossed Aldol Reaction Teruaki Mukaiyama and Koichi Narasaka Organic Syntheses, Coll. Vol. 8, p.323 (1993); Vol. 65, p.6 (1987)
- ^ Carreira, E. M.; Fettes, A.; Martl, C. (2006). Catalytic Enantioselective Aldol Addition Reactions. Org. React. Vol. 67. pp. 1–216. doi:10.1002/0471264180.or067.01. ISBN 978-0471264187.
- ^ Z. G. Hajos, D. R. Parrish, German Patent DE 2102623 1971
- ^ Hajos, Zoltan G.; Parrish, David R. (1974). "Asymmetric synthesis of bicyclic intermediates of natural product chemistry". Journal of Organic Chemistry. 39 (12): 1615–1621. doi:10.1021/jo00925a003.
- ^ Eder, Ulrich; Sauer, Gerhard; Wiechert, Rudolf (1971). "New Type of Asymmetric Cyclization to Optically Active Steroid CD Partial Structures". Angewandte Chemie International Edition in English. 10 (7): 1615–1621. doi:10.1002/anie.197104961.
- ^ Northrup, Alan B.; MacMillan David W. C. (2002). "The First Direct and Enantioselective Cross-Aldol Reaction of Aldehydes" (PDF). Journal of the American Chemical Society. 124 (24): 6798–6799. doi:10.1021/ja0262378. PMID 12059180.
- ^ Evans, D. A.; Tedrow, J. S.; Shaw, J. T.; Downey, C. W. (2002). "Diastereoselective Magnesium Halide-Catalyzed anti-Aldol Reactions of Chiral N-Acyloxazolidinones". Journal of the American Chemical Society. 124 (3): 392–393. doi:10.1021/ja0119548. PMID 11792206.
- ^ Evans, David A.; Downey, C. Wade; Shaw, Jared T.; Tedrow, Jason S. (2002). "Magnesium Halide-Catalyzed Anti-Aldol Reactions of Chiral N-Acylthiazolidinethiones". Organic Letters. 4 (7): 1127–1130. doi:10.1021/ol025553o. PMID 11922799.
- ^ Northrup A. B.; Mangion I. K.; Hettche F.; MacMillan D. W. C. (2004). "Enantioselective Organocatalytic Direct Aldol Reactions of -Oxyaldehydes: Step One in a Two-Step Synthesis of Carbohydrates". Angewandte Chemie International Edition in English. 43 (16): 2152–2154. doi:10.1002/anie.200453716. PMID 15083470.
- ^ Borodin reported on the condensation of pentanal (Valerianaldehyd) with heptanal (Oenanthaldehyd) in: von Richter, V. (1869) "V. von Richter, aus St. Petersburg am 17. October 1869" (V. von Richter [reporting] from St. Petersburg on 17. October 1869), Berichte der deutschen chemischen Gesellschaft (in German), 2 : 552-553.
- English version of Richter's report: (Staff) (December 10, 1869) "Chemical notices from foreign sources: Berichte der Deutschen Chemischen Gesellschaft zu Berlin, no. 16, 1869: Valerian aldehyde and Oenanth aldehyde – M. Borodin," The Chemical News and Journal of Industrial Science, 20 : 286.
- ^ Garner, Susan Amy (2007) "Hydrogen-mediated carbon-carbon bond formations: Applied to reductive aldol and Mannich reactions," Ph.D. dissertation, University of Texas (Austin), pp. 4 and 51.
- ^ Borodin, A. (1873) "Ueber einen neuen Abkömmling des Valerals" (On a new derivative of valerian aldehyde), Berichte der deutschen chemischen Gesellschaft (in German), 6 : 982–985.
Further reading
[edit]- Chem 206, 215 Lecture Notes (2003, 2006) by D. A. Evans, A. G. Myers, et al., Harvard University (pp. 345, 936)
