Antihistamine: Difference between revisions
→Medical uses: Removed line on dysrhythmia for the same reason expressed on http://en.wikipedia.org/wiki/Talk:Cardiac_dysrhythmia#Histamine |
Added brexpiprazole to list of H1 antagonists |
||
| (252 intermediate revisions by more than 100 users not shown) | |||
| Line 1: | Line 1: | ||
{{Short description|Drug that blocks histamine or histamine agonists}} |
|||
A '''histamine antagonist''' (commonly called an '''antihistamine''') is a [[pharmaceutical drug]] that inhibits the action of [[histamine]] by either blocking its attachment to [[histamine receptor]]s, or inhibiting the enzymatic activity of [[histidine decarboxylase]] which catalyzes the transformation of [[histidine]] into histamine (atypical antihistaminics). Histamine antagonists are commonly used for the relief of [[Protein allergy|allergies]] caused by intolerance of proteins.<ref>Sicherer, Scott H. M.D., Understanding and Managing Your Child's Food Allergy. Baltimore: The Johns Hopkins University Press, 2006 ISBN 0-8018-8492-6.</ref> |
|||
{{Use dmy dates|date=October 2015}} |
|||
{{Infobox drug class |
|||
| Name = Antihistamine |
|||
| Image = Histamine.svg |
|||
| ImageClass = skin-invert-image |
|||
| Alt = Histamine structure diagram |
|||
| Caption = Histamine structure |
|||
<!-- Class identifiers --> |
|||
| Use = |
|||
| ATC_prefix = R06 |
|||
| Mode_of_action = |
|||
| Biological_target = [[Histamine receptor]]s<br />{{bull}}[[HRH1]]<br />{{bull}}[[HRH2]]<br />{{bull}}[[HRH3]]<br />{{bull}}[[HRH4]] |
|||
| Mechanism_of_action = {{bull}}[[Receptor antagonist]]<br />{{bull}}[[Inverse agonist]] |
|||
| Chemical_class = |
|||
<!-- Clinical data --> |
|||
| Drugs.com = <!-- {{Drugs.com|drug-class|?}} --> |
|||
| Consumer_Reports = |
|||
| medicinenet = |
|||
| rxlist = |
|||
<!-- External links --> |
|||
| MeshID = D006633 |
|||
| Pronounce ={{IPAc-en|ˌ|æ|n|t|i|ˈ|h|ɪ|s|t|ə|m|iː|n}} |
|||
}} |
|||
'''Antihistamines''' are [[pharmaceutical drug|drugs]] which treat [[allergic rhinitis]], [[common cold]], [[influenza]], and other [[allergies]].<ref name="Consumer Reports 2013">{{Citation |author=Consumer Reports |author-link=Consumer Reports |year=2013 |title=Using Antihistamines to Treat Allergies, Hay Fever, & Hives - Comparing Effectiveness, Safety, and Price |publisher=Consumer Reports |location=[[Yonkers, New York]] |url=http://consumerhealthchoices.org/wp-content/uploads/2012/02/BBD-Antihistamines-Full.pdf |access-date=29 June 2017 |archive-url=https://web.archive.org/web/20170517030305/http://consumerhealthchoices.org/wp-content/uploads/2012/02/BBD-Antihistamines-Full.pdf |archive-date=17 May 2017 |url-status=dead |df=dmy-all }}</ref> Typically, people take antihistamines as an inexpensive, [[Generic drug|generic]] (not patented) drug that can be bought [[over-the-counter drug|without a prescription]] and provides relief from [[nasal congestion]], [[sneezing]], or [[hives]] caused by [[pollen]], [[dust mites]], or [[animal allergy]] with few side effects.<ref name="Consumer Reports 2013" /> Antihistamines are usually for short-term treatment.<ref name="Consumer Reports 2013" /> Chronic allergies increase the risk of health problems which antihistamines might not treat, including [[asthma]], [[sinusitis]], and [[lower respiratory tract infection]].<ref name="Consumer Reports 2013" /> Consultation of a medical professional is recommended for those who intend to take antihistamines for longer-term use.<ref name="Consumer Reports 2013"/> |
|||
Although the general public typically uses the word "antihistamine" to describe drugs for treating allergies, physicians and scientists use the term to describe a class of drug that opposes the activity of [[histamine receptor]]s in the body.<ref name="H1 receptor NF-κB signaling" /> In this sense of the word, antihistamines are subclassified according to the [[histamine]] receptor that they act upon. The two largest classes of antihistamines are [[H1 antagonist|H<sub>1</sub>-antihistamines]] and [[H2 receptor antagonist|H<sub>2</sub>-antihistamines]]. |
|||
H<sub>1</sub>-antihistamines work by binding to [[HRH1|histamine H<sub>1</sub> receptor]]s in [[mast cells]], [[smooth muscle]], and [[endothelium]] in the body as well as in the [[tuberomammillary nucleus]] in the brain. Antihistamines that target the [[HRH1|histamine H<sub>1</sub>-receptor]] are used to treat [[allergic rhinitis|allergic reactions in the nose]] (e.g., itching, runny nose, and sneezing). In addition, they may be used to treat [[insomnia]], motion sickness, or [[vertigo]] caused by problems with the [[inner ear]]. H<sub>2</sub>-antihistamines bind to [[HRH2|histamine H<sub>2</sub> receptors]] in the upper [[Human gastrointestinal tract|gastrointestinal tract]], primarily in the [[stomach]]. Antihistamines that target the [[HRH2|histamine H<sub>2</sub>-receptor]] are used to treat [[gastric acid]] conditions (e.g., [[peptic ulcers]] and [[acid reflux]]). Other antihistamines also target [[Histamine H3 receptor|H<sub>3</sub> receptors]] and [[Histamine H4 receptor|H<sub>4</sub> receptors]]. |
|||
[[Histamine receptor]]s exhibit [[constitutive activity]], so antihistamines can function as either a neutral [[receptor antagonist]] or an [[inverse agonist]] at histamine receptors.<ref name="H1 receptor NF-κB signaling">{{cite journal | vauthors = Canonica GW, Blaiss M | title = Antihistaminic, anti-inflammatory, and antiallergic properties of the nonsedating second-generation antihistamine desloratadine: a review of the evidence | journal = World Allergy Organ J | volume = 4 | issue = 2 | pages = 47–53 | year = 2011 | pmid = 23268457 | pmc = 3500039 | doi = 10.1097/WOX.0b013e3182093e19 | quote = The H1-receptor is a transmembrane protein belonging to the G-protein coupled receptor family. Signal transduction from the extracellular to the intracellular environment occurs as the GCPR becomes activated after binding of a specific ligand or agonist. A subunit of the G-protein subsequently dissociates and affects intracellular messaging including downstream signaling accomplished through various intermediaries such as cyclic AMP, cyclic GMP, calcium, and nuclear factor kappa B (NF-κB), a ubiquitous transcription factor thought to play an important role in immune-cell chemotaxis, proinflammatory cytokine production, expression of cell adhesion molecules, and other allergic and inflammatory conditions.1,8,12,30–32 ... For example, the H1-receptor promotes NF-κB in both a constitutive and agonist-dependent manner and all clinically available H1-antihistamines inhibit constitutive H1-receptor-mediated NF-κB production ... <br />Importantly, because antihistamines can theoretically behave as inverse agonists or neutral antagonists, they are more properly described as H1-antihistamines rather than H1-receptor antagonists.15}}</ref><ref name="Histamine receptors">{{cite journal | vauthors = Panula P, Chazot PL, Cowart M, et al. | title = International Union of Basic and Clinical Pharmacology. XCVIII. Histamine Receptors | journal = Pharmacol. Rev. | volume = 67 | issue = 3 | pages = 601–55 | year = 2015 | pmid = 26084539 | doi = 10.1124/pr.114.010249 | pmc=4485016}}</ref><ref name="Inverse agonists vs antagonists" /><ref name="IUPHAR H1 ligands">{{cite web|url=http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=262|title=H1 receptor|publisher=IUPHAR/BPS Guide to Pharmacology|access-date=8 October 2015}}</ref> Only a few currently marketed H<sub>1</sub>-antihistamines are known to function as antagonists.<ref name="H1 receptor NF-κB signaling" /><ref name="IUPHAR H1 ligands" /> |
|||
== Medical uses == |
== Medical uses == |
||
Histamine |
Histamine makes blood vessels more permeable ([[vascular permeability]]), causing fluid to escape from [[capillary|capillaries]] into [[Tissue (biology)|tissues]], which leads to the classic [[symptom]]s of an [[allergic reaction]]—a runny nose and watery eyes. Histamine also promotes [[angiogenesis]].<ref name="pmid7540412">{{cite journal |vauthors = Norrby K |title = Evidence of a dual role of endogenous histamine in angiogenesis |journal = Int J Exp Pathol |volume = 76 |issue = 2 |pages = 87–92 |year = 1995 |pmid = 7540412 |pmc = 1997159 }}</ref> |
||
Antihistamines suppress the histamine-induced [[Cutaneous condition#Morphology|wheal response]] (swelling) and [[Vasodilation|flare response]] (vasodilation) by blocking the binding of histamine to its receptors on [[nerve]]s, [[vascular smooth muscle]], glandular cells, [[endothelium]], and [[mast cell]]s. |
Antihistamines suppress the histamine-induced [[Cutaneous condition#Morphology|wheal response]] (swelling) and [[Vasodilation|flare response]] (vasodilation) by blocking the binding of histamine to its receptors or reducing histamine receptor activity on [[nerve]]s, [[vascular smooth muscle]], glandular cells, [[endothelium]], and [[mast cell]]s. Antihistamines can also help correct [[Eustachian tube dysfunction|Eustachian Tube dysfunction]], thereby helping correct problems such as muffled hearing, fullness in the ear and even [[tinnitus]].<ref>{{Cite web |date=2021-09-28 |title=Best Antihistamine for Tinnitus? |url=https://tinnitusandyou.com/best-antihistamine-for-tinnitus/ |access-date=2022-03-15 |website=Tinnitus and You |language=en-US|author-last1=Morrison|author-first1=James}}</ref> |
||
[[Itch]]ing, [[Sneeze|sneezing]], and inflammatory responses are suppressed by antihistamines that act on [[H1-receptor]]s.<ref name="H1 receptor NF-κB signaling" /><ref>{{cite journal | vauthors = Monroe EW, Daly AF, Shalhoub RF | title = Appraisal of the validity of histamine-induced wheal and flare to predict the clinical efficacy of antihistamines | journal = The Journal of Allergy and Clinical Immunology | volume = 99 | issue = 2 | pages = S798–806 | date = February 1997 | pmid = 9042073 | doi = 10.1016/s0091-6749(97)70128-3 | doi-access = free }}</ref> In 2014, antihistamines such as [[desloratadine]] were found to be effective to complement standardized treatment of [[acne]] due to their [[anti-inflammatory]] properties and their ability to suppress [[sebum]] production.<ref name=JEADV2014>{{cite journal | vauthors = Lee HE, Chang IK, Lee Y, Kim CD, Seo YJ, Lee JH, Im M | title=Effect of antihistamine as an adjuvant treatment of isotretinoin in acne: a randomized, controlled comparative study | journal=J Eur Acad Dermatol Venereol | volume=28 | issue=12 | pages=1654–60 | year=2014 | pmid=25081735 | doi=10.1111/jdv.12403| s2cid=3406128 }}</ref><ref name=DC2016>{{cite journal | author=Layton AM | title=Top Ten List of Clinical Pearls in the Treatment of Acne Vulgaris | journal=Dermatol Clin | volume=34 | issue=2 | pages=147–57 | year=2016 | pmid=27015774 | doi=10.1016/j.det.2015.11.008}}</ref> |
|||
[[Itch]]ing and [[Sneeze|sneezing]] are suppressed by antihistamine blocking of [[H1-receptor]]s on nasal sensory nerves.<ref>{{cite journal|pmid=9042073|title=Appraisal of the validity of histmine-induced wheal and flare is used to predict the clinical efficacy of antihistamines|year=1997|last1=Monroe|first1=EW|last2=Daly|first2=AF|last3=Shalhoub|first3=RF|volume=99|issue=2|pages=S798–806|journal=The Journal of allergy and clinical immunology|doi=10.1016/s0091-6749(97)70128-3}}</ref> |
|||
Antihistamines have also been used with great success in the treatment of [[brown recluse]] (genus ''[[Loxosceles]]'') [[spider bites]], as well as other arthropod bites that cause [[necrosis]].<ref>Paul K. Carlton Jr, MD, FACS The Texas A&M University Health Science Center, College Station, Tex</ref> |
|||
==Types== |
== Types == |
||
=== H<sub>1</sub>-antihistamines === |
|||
{{Main|H1 antagonist|l1=H<sub>1</sub>-antihistamine}} |
|||
H<sub>1</sub>-antihistamines refer to compounds that inhibit the activity of the [[HRH1|H<sub>1</sub> receptor]].<ref name="Inverse agonists vs antagonists">{{cite journal | vauthors = Leurs R, Church MK, Taglialatela M | title = H1-antihistamines: inverse agonism, anti-inflammatory actions and cardiac effects | journal = Clinical and Experimental Allergy | volume = 32 | issue = 4 | pages = 489–98 | date = April 2002 | pmid = 11972592 | doi = 10.1046/j.0954-7894.2002.01314.x | s2cid = 11849647 }}</ref><ref name="IUPHAR H1 ligands" /> Since the H<sub>1</sub> receptor exhibits [[constitutive activity]], H<sub>1</sub>-antihistamines can be either neutral [[receptor antagonist]]s or [[inverse agonist]]s.<ref name="Inverse agonists vs antagonists" /><ref name="IUPHAR H1 ligands" /> Normally, histamine binds to the H<sub>1</sub> receptor and heightens the receptor's activity; the receptor antagonists work by binding to the receptor and blocking the activation of the receptor by histamine; by comparison, the inverse agonists bind to the receptor and both block the binding of histamine, and reduce its constitutive activity, an effect which is opposite to histamine's.<ref name="Inverse agonists vs antagonists" /> Most antihistamines are inverse agonists at the H<sub>1</sub> receptor, but it was previously thought that they were antagonists.<ref>{{Cite journal|last1=Church|first1=Diana S|last2=Church|first2=Martin K|date=2011-03-15|title=Pharmacology of Antihistamines|journal=The World Allergy Organization Journal|volume=4|issue=Suppl 3|pages=S22–S27|doi=10.1097/1939-4551-4-S3-S22|issn=1939-4551|pmc=3666185|pmid=23282332}}</ref> |
|||
===H<sub>1</sub>-receptor antagonists=== |
|||
{{Main|H1 antagonist|l1=H<sub>1</sub> antagonist}} |
|||
Clinically, H<sub>1</sub>-antihistamines are used to treat [[allergic]] reactions and [[mast cell]]-related disorders. [[Sedation]] is a common side effect of H<sub>1</sub>-antihistamines that readily cross the [[blood–brain barrier]]; some of these drugs, such as [[diphenhydramine]] and [[doxylamine]], may therefore be used to treat [[insomnia]]. H<sub>1</sub>-antihistamines can also reduce inflammation, since the expression of [[NF-κB]], the transcription factor the regulates inflammatory processes, is promoted by both the receptor's constitutive activity and agonist (i.e., [[histamine]]) binding at the H<sub>1</sub> receptor.<ref name="H1 receptor NF-κB signaling" /> |
|||
In common use, the term antihistamine refers only to compounds that inhibit action at the H<sub>1</sub> receptor (and not H<sub>2</sub>, etc.). |
|||
A combination of these effects, and in some cases metabolic ones as well, lead to most first-generation antihistamines having [[analgesic-sparing]] (potentiating) effects on [[opioid]] [[analgesics]] and to some extent with non-opioid ones as well. The most common antihistamines utilized for this purpose include [[hydroxyzine]], [[promethazine]] (enzyme induction especially helps with [[codeine]] and similar [[prodrug]] opioids), [[phenyltoloxamine]], [[orphenadrine]], and [[tripelennamine]]; some may also have intrinsic analgesic properties of their own, orphenadrine being an example. |
|||
Rather than "true" [[receptor antagonists|antagonists]], H<sub>1</sub>-antihistamines are actually [[inverse agonist]]s at the histamine H<sub>1</sub>-receptor.<ref>{{cite journal | author=Leurs R, Church MK, Taglialatela M | year=2002 | title=H<sub>1</sub>-antihistamines: inverse agonism, anti-inflammatory actions and cardiac effects | journal=Clin Exp Allergy | volume=32 | issue=4 | pages=489–98 | pmid=11972592 | doi=10.1046/j.0954-7894.2002.01314.x}}</ref> Clinically, H<sub>1</sub> antagonists are used to treat [[allergic]] reactions. Sedation is a common side effect, and some H<sub>1</sub> antagonists, such as [[diphenhydramine]] and [[doxylamine]], are also used to treat insomnia. |
|||
[[Second-generation antihistamine]]s cross the [[blood–brain barrier]] to a much |
[[Second-generation antihistamine]]s cross the [[blood–brain barrier]] to a much lesser extent than the first-generation antihistamines. They minimize sedatory effects due to their focused effect on peripheral histamine receptors. However, upon high doses second-generation antihistamines will begin to act on the central nervous system and thus can induce drowsiness when ingested in higher quantity. |
||
==== List of H<sub>1</sub> antagonists/inverse agonists ==== |
|||
H1 antagonist examples include: |
|||
{{div col|colwidth=20em|small=yes}} |
|||
*[[Acrivastine]] |
|||
*[[ |
* [[Acrivastine]] |
||
* [[Alimemazine]] (a [[phenothiazine]] used as [[antipruritic]], [[antiemetic]] and [[sedative]]) |
|||
*[[Bilastine]] |
|||
* [[Amitriptyline]] ([[tricyclic antidepressant]]) |
|||
*[[Brompheniramine]] |
|||
* [[Amoxapine]] ([[tricyclic antidepressant]]) |
|||
*[[Buclizine]] |
|||
* [[Aripiprazole]] ([[atypical antipsychotic]], trade name: Abilify) |
|||
*[[Bromodiphenhydramine]] |
|||
* [[Brexpiprazole]] ([[atypical antipsychotic]], trade name: Rexulti) |
|||
*[[Carbinoxamine]] |
|||
* [[Azelastine]] |
|||
*[[Cetirizine]] (Zyrtec; metabolite of hydroxyzine, its [[prodrug]]) |
|||
* [[Bilastine]] |
|||
*[[Chlorpromazine]] (antipsychotic) |
|||
* [[Bromodiphenhydramine]] (Bromazine) |
|||
*[[Cyclizine]] |
|||
*[[ |
* [[Brompheniramine]] |
||
* [[Buclizine]] |
|||
*[[Chlorodiphenhydramine]] |
|||
*[[ |
* [[Carbinoxamine]] |
||
* [[Cetirizine]] (Zyrtec) |
|||
*[[Cyproheptadine]] |
|||
* [[Chlophedianol]] (Clofedanol) |
|||
*[[Desloratadine]] |
|||
* [[Chlorodiphenhydramine]]<ref name="LemkeWilliams2012">{{cite book | editor1 = Thomas L. Lemke | editor2 = David A. Williams | date = 24 January 2012 | title = Foye's Principles of Medicinal Chemistry | publisher = Lippincott Williams & Wilkins | pages = 1053– | isbn = 978-1-60913-345-0 | oclc = 1127763671 | url = https://books.google.com/books?id=Sd6ot9ul-bUC&pg=PA1053}}</ref> |
|||
*[[Dexbrompheniramine]] |
|||
* [[Chlorpheniramine]] |
|||
*[[Dexchlorpheniramine]] |
|||
*[[ |
* [[Chlorpromazine]] (low-potency [[typical antipsychotic]], also used as an [[antiemetic]]) |
||
* [[Chlorprothixene]] (low-potency [[typical antipsychotic]], trade name: Truxal) |
|||
*[[Dimetindene]] |
|||
* [[Chloropyramine]] (first generation antihistamine marketed in [[Eastern Europe]]) |
|||
*[[Diphenhydramine]] ([[Benadryl]]) |
|||
*[[ |
* [[Cinnarizine]] (also used for [[motion sickness]] and [[vertigo]]) |
||
*[[ |
* [[Clemastine]] |
||
* [[Clomipramine]] ([[tricyclic antidepressant]]) |
|||
*[[Embramine]] |
|||
* [[Clozapine]] ([[atypical antipsychotic]]; trade name: Clozaril) |
|||
*[[Fexofenadine]] (Allegra) |
|||
* [[Cyclizine]] |
|||
*[[Hydroxyzine]] (Vistaril) |
|||
*[[ |
* [[Cyproheptadine]] |
||
* [[Desloratadine]] |
|||
*[[Loratadine]] (Claritin) |
|||
* [[Dexbrompheniramine]] |
|||
*[[Meclozine]] (most commonly used as an antiemetic) |
|||
* [[Dexchlorpheniramine]] |
|||
*[[Mirtazapine]] (primarily used to treat depression, also has antiemetic and appetite-stimulating effects) |
|||
* [[Dimenhydrinate]] (used as an [[antiemetic]] and for [[motion sickness]]) |
|||
*[[Olopatadine]] (used locally) |
|||
* [[Dimetindene]] |
|||
*[[Orphenadrine]] (a close relative of diphenhydramine used mainly as a skeletal muscle relaxant and anti-Parkinsons agent) |
|||
* [[Diphenhydramine]] (Benadryl) |
|||
*[[Phenindamine]] |
|||
* [[Dosulepin]] ([[tricyclic antidepressant]]) |
|||
*[[Pheniramine]] |
|||
* [[Doxepin]] ([[tricyclic antidepressant]]) |
|||
*[[Phenyltoloxamine]] |
|||
* [[Doxylamine]] (most commonly used as an [[over-the-counter drug|over-the-counter]] [[sedative]]) |
|||
*[[Promethazine]] |
|||
*[[ |
* [[Ebastine]] |
||
* [[Embramine]] |
|||
*[[Quetiapine]] (antipsychotic; trade name Seroquel) |
|||
* [[Fexofenadine]] (Allegra/Telfast) |
|||
*[[Rupatadine]] |
|||
*[[ |
* [[Fluoxetine]] |
||
* [[Hydroxyzine]] (also used as an [[anxiolytic]] and for [[motion sickness]]; trade names: Atarax, Vistaril) |
|||
*[[Triprolidine]] |
|||
* [[Imipramine]] ([[tricyclic antidepressant]]) |
|||
* [[Ketotifen]] |
|||
* [[Levocabastine]] (Livostin/Livocab) |
|||
* [[Levocetirizine]] (Xyzal) |
|||
* [[Levomepromazine]] (low-potency [[typical antipsychotic]]) |
|||
* [[Loratadine]] (Claritin) |
|||
* [[Maprotiline]] ([[tetracyclic antidepressant]]) |
|||
* [[Meclizine]] (most commonly used as an antiemetic) |
|||
* [[Mianserin]] ([[tetracyclic antidepressant]]) |
|||
* [[Mirtazapine]] ([[tetracyclic antidepressant]], also has antiemetic and appetite-stimulating effects; trade name: Remeron) |
|||
* [[Olanzapine]] ([[atypical antipsychotic]]; trade name: Zyprexa) |
|||
* [[Olopatadine]] (used locally) |
|||
* [[Orphenadrine]] (a close relative of diphenhydramine used mainly as a skeletal muscle relaxant and anti-Parkinsons agent) |
|||
* [[Periciazine]] (low-potency [[typical antipsychotic]]) |
|||
* [[Phenindamine]] |
|||
* [[Pheniramine]] |
|||
* [[Phenyltoloxamine]] |
|||
* [[Promethazine]] (Phenergan) |
|||
* [[Pyrilamine]] (crosses the blood–brain barrier; produces drowsiness) |
|||
* [[Quetiapine]] ([[atypical antipsychotic]]; trade name: Seroquel) |
|||
* [[Rupatadine]] (Alergoliber) |
|||
* [[Setastine]] (Loderix) |
|||
* [[Setiptiline]] (or teciptiline, a [[tetracyclic antidepressant]], trade name: Tecipul) |
|||
* [[Trazodone]] (SARI antidepressant/anxiolytic/hypnotic with mild H<sub>1</sub> blockade action) |
|||
* [[Tripelennamine]] |
|||
* [[Triprolidine]] |
|||
{{div col end}} |
|||
===H<sub>2</sub>- |
=== H<sub>2</sub>-antihistamines === |
||
{{Main|H2 antagonist|l1=H<sub>2</sub> |
{{Main|H2 antagonist|l1=H<sub>2</sub>-antihistamine}} |
||
H<sub>2</sub> |
H<sub>2</sub>-antihistamines, like H<sub>1</sub>-antihistamines, exist as [[inverse agonist]]s and neutral [[receptor antagonist|antagonists]]. They act on [[Histamine H2 receptor|H<sub>2</sub> histamine receptors]] found mainly in the [[parietal cell]]s of the [[stomach|gastric]] mucosa, which are part of the endogenous signaling pathway for [[gastric acid]] secretion. Normally, histamine acts on H<sub>2</sub> to stimulate acid secretion; drugs that inhibit H<sub>2</sub> signaling thus reduce the secretion of gastric acid. |
||
H<sub>2</sub> antagonists are among first-line therapy to treat [[gastrointestinal]] conditions including [[peptic ulcer]]s and [[gastroesophageal reflux disease]]. Some formulations are available over the counter. Most side effects are due to cross-reactivity with unintended receptors. Cimetidine, for example, is notorious for antagonizing androgenic testosterone and DHT receptors at high doses. |
|||
H<sub>2</sub>-antihistamines are among first-line therapy to treat [[gastrointestinal condition]]s including [[peptic ulcer]]s and [[gastroesophageal reflux disease]]. Some formulations are available over the counter. Most side effects are due to cross-reactivity with unintended receptors. Cimetidine, for example, is notorious for antagonizing androgenic testosterone and DHT receptors at high doses. |
|||
Examples include: |
Examples include: |
||
{{div col|colwidth=10em|small=yes}} |
|||
*[[Cimetidine]] |
*[[Cimetidine]] |
||
*[[Famotidine]] |
*[[Famotidine]] |
||
| Line 76: | Line 136: | ||
*[[Roxatidine]] |
*[[Roxatidine]] |
||
*[[Tiotidine]] |
*[[Tiotidine]] |
||
{{div col end}} |
|||
=== H<sub>3</sub>-antihistamines === |
|||
==Research== |
|||
{{Main|H3 receptor antagonist|l1=H<sub>3</sub>-antihistamine}} |
|||
These are experimental agents and do not yet have a defined clinical use, although a number of drugs are currently in human trials. H<sub>3</sub>-antagonists have a [[stimulant]] and [[nootropic]] effect, and are being investigated for the treatment of conditions such as [[ADHD]], [[Alzheimer's disease]], and [[schizophrenia]], whereas H<sub>4</sub>-antagonists appear to have an [[immunomodulator]]y role and are being investigated as [[anti-inflammatory]] and [[analgesic]] drugs. |
|||
An '''H<sub>3</sub>-antihistamine''' is a classification of [[medication|drugs]] used to inhibit the action of [[histamine]] at the [[Histamine H3 receptor|H<sub>3</sub> receptor]]. H<sub>3</sub> receptors are primarily found in the brain and are inhibitory [[autoreceptor]]s located on histaminergic nerve terminals, which modulate the release of [[histamine]]. Histamine release in the brain triggers secondary release of excitatory neurotransmitters such as [[glutamate]] and [[acetylcholine]] via stimulation of H<sub>1</sub> receptors in the [[cerebral cortex]]. Consequently, unlike the H<sub>1</sub>-antihistamines which are sedating, H<sub>3</sub>-antihistamines have [[stimulant]] and cognition-modulating effects. |
|||
Examples of selective H<sub>3</sub>-antihistamines include: |
|||
{{div col|colwidth=10em|small=yes}} |
|||
{{Main|H3 antagonist|l1=H<sub>3</sub> antagonist}} |
|||
*[[Clobenpropit]]<ref name="pmid18278935">{{cite journal | vauthors = Yoneyama H, Shimoda A, Araki L, Hatano K, Sakamoto Y, Kurihara T, Yamatodani A, Harusawa S | title = Efficient approaches to S-alkyl-N-alkylisothioureas: syntheses of histamine H3 antagonist clobenpropit and its analogues | journal = The Journal of Organic Chemistry | volume = 73 | issue = 6 | pages = 2096–104 | date = March 2008 | pmid = 18278935 | doi = 10.1021/jo702181x | display-authors= 1 }}</ref> |
|||
*[[ABT-239]]<ref name="pmid15608077">{{cite journal | vauthors = Fox GB, Esbenshade TA, Pan JB, Radek RJ, Krueger KM, Yao BB, Browman KE, Buckley MJ, Ballard ME, Komater VA, Miner H, Zhang M, Faghih R, Rueter LE, Bitner RS, Drescher KU, Wetter J, Marsh K, Lemaire M, Porsolt RD, Bennani YL, Sullivan JP, Cowart MD, Decker MW, Hancock AA | title = Pharmacological properties of ABT-239 [4-(2-{2-[(2R)-2-Methylpyrrolidinyl]ethyl}-benzofuran-5-yl)benzonitrile]: II. Neurophysiological characterization and broad preclinical efficacy in cognition and schizophrenia of a potent and selective histamine H3 receptor antagonist | journal = The Journal of Pharmacology and Experimental Therapeutics | volume = 313 | issue = 1 | pages = 176–90 | date = April 2005 | pmid = 15608077 | doi = 10.1124/jpet.104.078402 | s2cid = 15430117 | url = http://jpet.aspetjournals.org/cgi/pmidlookup?view=long&pmid=15608077 }}</ref> |
|||
An '''H<sub>3</sub>-receptor antagonist''' is a classification of [[medication|drugs]] used to block the action of [[histamine]] at the [[Histamine H3 receptor|H<sub>3</sub> receptor]]. Unlike the H<sub>1</sub> and H<sub>2</sub> receptors which have primarily peripheral actions, but cause [[sedation]] if they are blocked in the brain, H<sub>3</sub> receptors are primarily found in the brain and are inhibitory autoreceptors located on histaminergic nerve terminals, which modulate the release of [[histamine]]. Histamine release in the brain triggers secondary release of excitatory neurotransmitters such as [[glutamate]] and [[acetylcholine]] via stimulation of H<sub>1</sub> receptors in the [[cerebral cortex]]. Consequently unlike the H<sub>1</sub> antagonist [[antihistamines]] which are sedating, H<sub>3</sub> antagonists have [[stimulant]] and [[nootropic]] effects, and are being researched as potential drugs for the treatment of neurodegenerative conditions such as [[Alzheimer's disease]]. |
|||
*[[Ciproxifan]]<ref name="pmid9808693">{{cite journal | vauthors = Ligneau X, Lin J, Vanni-Mercier G, Jouvet M, Muir JL, Ganellin CR, Stark H, Elz S, Schunack W, Schwartz J | title = Neurochemical and behavioral effects of ciproxifan, a potent histamine H3-receptor antagonist | journal = The Journal of Pharmacology and Experimental Therapeutics | volume = 287 | issue = 2 | pages = 658–66 | date = November 1998 | pmid = 9808693 | url = http://jpet.aspetjournals.org/cgi/pmidlookup?view=long&pmid=9808693 | access-date = 9 August 2014 | archive-date = 2 May 2020 | archive-url = https://web.archive.org/web/20200502160231/http://jpet.aspetjournals.org/content/287/2/658.long | url-status = dead }}</ref> |
|||
Examples of selective H<sub>3</sub> antagonists include: |
|||
*[[Clobenpropit]],<ref name="pmid18278935">{{cite journal |author=Yoneyama H, Shimoda A, Araki L, ''et al.'' |title=Efficient approaches to S-alkyl-N-alkylisothioureas: syntheses of histamine H3 antagonist clobenpropit and its analogues |journal=The Journal of Organic Chemistry |volume=73 |issue=6 |pages=2096–104 |date=March 2008 |pmid=18278935 |doi=10.1021/jo702181x}}</ref> |
|||
*[[ABT-239]]<ref name="pmid15608077">{{cite journal |author=Fox GB, Esbenshade TA, Pan JB, Radek RJ, Krueger KM, Yao BB, Browman KE, Buckley MJ, Ballard ME, Komater VA, Miner H, Zhang M, Faghih R, Rueter LE, Bitner RS, Drescher KU, Wetter J, Marsh K, Lemaire M, Porsolt RD, Bennani YL, Sullivan JP, Cowart MD, Decker MW, Hancock AA |title=Pharmacological properties of ABT-239 [4-(2-{2-[(2R)-2-Methylpyrrolidinyl]ethyl}-benzofuran-5-yl)benzonitrile]: II. Neurophysiological characterization and broad preclinical efficacy in cognition and schizophrenia of a potent and selective histamine H<sub>3</sub> receptor antagonist |journal=Journal of Pharmacology and Experimental Therapeutics |volume=313 |issue=1 |pages=176–90 |date=April 2005 |pmid=15608077 |doi=10.1124/jpet.104.078402 |url=http://jpet.aspetjournals.org/cgi/pmidlookup?view=long&pmid=15608077 |issn=1521-0103}}</ref> |
|||
*[[Ciproxifan]],<ref name="pmid9808693">{{cite journal |author=LLigneau X, Lin J, Vanni-Mercier G, Jouvet M, Muir JL, Ganellin CR, Stark H, Elz S, Schunack W, Schwartz J |title=Neurochemical and behavioral effects of ciproxifan, a potent histamine H<sub>3</sub>-receptor antagonist |journal=The Journal of Pharmacology and Experimental Therapeutics |volume=287 |issue=2 |pages=658–66 |date=November 1998 |pmid=9808693 |url=http://jpet.aspetjournals.org/cgi/pmidlookup?view=long&pmid=9808693 }}</ref> |
|||
*[[Conessine]] |
*[[Conessine]] |
||
*[[A-349,821]].<ref>{{cite journal | |
*[[A-349,821]].<ref>{{cite journal | vauthors = Esbenshade TA, Fox GB, Krueger KM, Baranowski JL, Miller TR, Kang CH, Denny LI, Witte DG, Yao BB, Pan JB, Faghih R, Bennani YL, Williams M, Hancock AA | title = Pharmacological and behavioral properties of A-349821, a selective and potent human histamine H3 receptor antagonist | journal = Biochemical Pharmacology | volume = 68 | issue = 5 | pages = 933–45 | date = September 2004 | pmid = 15294456 | doi = 10.1016/j.bcp.2004.05.048 }}</ref> |
||
* [[Thioperamide]] |
* [[Thioperamide]] |
||
{{div col end}} |
|||
===<span class="anchor" id="H4-receptor antagonists"></span> H<sub>4</sub>-antihistamines <!-- [[H4 antagonist]] redirects here -->=== |
|||
===H<sub>4</sub>-receptor antagonists=== |
|||
<!-- This Anchor tag serves to provide a permanent target for incoming section links. Please do not remove it, nor modify it, except to add another appropriate anchor. If you modify the section title, please anchor the old title. It is always best to anchor an old section header that has been changed so that links to it will not be broken. See [[Template:Anchor]] for details. This text is produced using {{subst:Anchor comment}} --> |
|||
H<sub>4</sub>-antihistamines inhibit the activity of the [[Histamine H4 receptor|H<sub>4</sub> receptor]]. Examples include: |
|||
Examples: |
|||
{{div col|colwidth=10em|small=yes}} |
|||
* Thioperamide |
|||
* [[Thioperamide]] |
|||
* [[JNJ 7777120]] |
* [[JNJ 7777120]] |
||
* [[VUF-6002]] |
* [[VUF-6002]] |
||
{{div col end}} |
|||
{| class="wikitable" |
|||
==Others== |
|||
|+Histamine receptors |
|||
|- |
|||
! Receptor !! Location !! Mechanism of action !! Function !! Antagonists !! Uses of antagonists |
|||
|- |
|||
! [[Histamine H1 receptor|H<sub>1</sub>]] |
|||
| Throughout the body, especially in:<ref>{{Cite web |title=Histamine H1 Receptor - an overview {{!}} ScienceDirect Topics |url=https://www.sciencedirect.com/topics/neuroscience/histamine-h1-receptor#:~:text=Histamine%20H1%20receptors%20are,heart,%20and%20central%20nervous%20system. |access-date=2023-10-03 |website=www.sciencedirect.com}}</ref> {{blist|[[Smooth muscle]]s|vascular [[endothelia]]l cells (cells of walls of blood vessels)|[[adrenal medulla]]|heart|brain|spinal cord}} |
|||
| [[Gq subunit|G<sub>q</sub>]] |
|||
| |
|||
*[[ileum]] contraction |
|||
*modulate [[circadian cycle]] |
|||
*itching |
|||
*[[systemic circulation|systemic]] [[vasodilatation]] (indirect effect throughout the increased production of [[Nitric oxide|NO]]) |
|||
*[[bronchoconstriction]] (allergy-induced asthma) |
|||
| |
|||
*[[H1 antagonist|H<sub>1</sub>-receptor antagonist]]s |
|||
**[[Azelastine]] |
|||
**[[Diphenhydramine]] |
|||
**[[Loratadine]] |
|||
**[[Cetirizine]] |
|||
**[[Fexofenadine]] |
|||
**[[Clemastine]] |
|||
**[[Rupatadine]] |
|||
| {{blist|[[Allergies]]|[[nausea]]|[[sleep disorder]]s}} |
|||
|- |
|||
! [[Histamine H2 receptor|H<sub>2</sub>]] |
|||
| {{blist|Gastric [[parietal cell]]s|smooth muscles|[[mast cell]]s|[[neutrophil]]s|heart|[[uterus]]}} |
|||
| [[gs subunit|G<sub>s</sub>]] <br> ↑ [[Cyclic adenosine monophosphate|cAMP<sup>2+</sup>]] |
|||
| |
|||
*speed up [[sinus rhythm]] |
|||
*Stimulation of [[gastric acid]] secretion |
|||
*[[Smooth muscle]] relaxation |
|||
*Inhibit [[antibody]] synthesis, [[T-cell]] proliferation and [[cytokine]] production |
|||
| |
|||
*[[H2 antagonist|H<sub>2</sub>-receptor antagonist]]s |
|||
**[[Ranitidine]] |
|||
**[[Cimetidine]] |
|||
**[[Famotidine]] |
|||
**[[Nizatidine]] |
|||
| {{blist|[[Peptic ulcer]] disease|[[Stress ulcer]]s|[[Gastroesophageal reflux disease]]|[[Dyspepsia]]|[[Aspiration pneumonia]]}} |
|||
|- |
|||
! [[Histamine H3 receptor|H<sub>3</sub>]] |
|||
| {{blist|[[Thalamus]]|[[caudate nucleus]]|[[cerebral cortex]]|small intestine|testes|[[prostate]]}} |
|||
| [[gi subunit|G<sub>i</sub>]] |
|||
| |
|||
* Decrease Acetylcholine, Serotonin and Norepinephrine [[Neurotransmitter]] release in [[central nervous system|CNS]] |
|||
* Presynaptic [[autoreceptor]]s |
|||
| |
|||
*[[H3 antagonist|H<sub>3</sub>-receptor antagonists]] |
|||
**[[ABT-239]] |
|||
**[[Ciproxifan]] |
|||
**[[Clobenpropit]] |
|||
**[[Thioperamide]] |
|||
| {{blist|[[Narcolepsy]]|[[Alzheimer's disease]]|[[Attention deficit hyperactivity disorder]] (ADHD)|[[Schizophrenia]]}} |
|||
|- |
|||
! [[Histamine H4 receptor|H<sub>4</sub>]] |
|||
| |
|||
*[[Immune system]] |
|||
**[[lymphocyte]]s |
|||
**[[leukocyte]]s |
|||
*[[Lymphoid organ]]s |
|||
*[[thymus]] |
|||
*[[spleen]] |
|||
*[[liver]]<ref>{{cite journal | vauthors = Deiteren A, De Man JG, Pelckmans PA, De Winter BY | title = Histamine H₄ receptors in the gastrointestinal tract | journal = British Journal of Pharmacology | volume = 172 | issue = 5 | pages = 1165–1178 | date = March 2015 | pmid = 25363289 | pmc = 4337694 | doi = 10.1111/bph.12989 }}</ref> |
|||
*[[gastrointestinal tract]] (GIT) |
|||
*[[pancreas]] |
|||
*[[bile duct]]s |
|||
| [[gi subunit|G<sub>i</sub>]] |
|||
| |
|||
*mediate [[mast cell]] [[chemotaxis]].<ref name="pmid12626656">{{cite journal | vauthors = Hofstra CL, Desai PJ, Thurmond RL, Fung-Leung WP | title = Histamine H4 receptor mediates chemotaxis and calcium mobilization of mast cells | journal = The Journal of Pharmacology and Experimental Therapeutics | volume = 305 | issue = 3 | pages = 1212–1221 | date = June 2003 | pmid = 12626656 | doi = 10.1124/jpet.102.046581 | s2cid = 14932773 }}</ref> |
|||
| |
|||
*[[H4 antagonist|H<sub>4</sub>-receptor antagonists]] |
|||
**[[Thioperamide]] |
|||
**[[JNJ 7777120]] |
|||
| {{As of|July 2021}}, no clinical uses exist.<br>Potential uses include:<ref>{{Cite web |title=Histamine H4 Receptor Antagonist - an overview {{!}} ScienceDirect Topics |url=https://www.sciencedirect.com/topics/medicine-and-dentistry/histamine-h4-receptor-antagonist#:~:text=Currently%20H4%20receptor%20antagonists%20have,inflammatory%20pain%20and%20neuropathic%20pain. |access-date=2023-10-03 |website=www.sciencedirect.com}}</ref> {{blist|[[rheumatoid arthritis]]<ref>{{cite journal | vauthors = Kim KW, Kim BM, Lee KA, Lee SH, Firestein GS, Kim HR | title = Histamine and Histamine H4 Receptor Promotes Osteoclastogenesis in Rheumatoid Arthritis | journal = Scientific Reports | volume = 7 | issue = 1 | pages = 1197 | date = April 2017 | pmid = 28446753 | pmc = 5430934 | doi = 10.1038/s41598-017-01101-y | bibcode = 2017NatSR...7.1197K }}</ref>|[[asthma]]|[[allergic rhinitis]]|[[atopic dermatitis]]|[[pruritus]]}} |
|||
|- |
|||
|} |
|||
===Atypical antihistaminics=== |
|||
== Atypical antihistamines == |
|||
They inhibit the enzymatic activity of [[histidine decarboxylase]]: |
|||
=== Histidine decarboxylase inhibitors === |
|||
Inhibit the action of [[histidine decarboxylase]]: |
|||
{{div col|colwidth=10em|small=yes}} |
|||
* [[Tritoqualine]] |
* [[Tritoqualine]] |
||
* [[Catechin]] |
* [[Catechin]] |
||
{{div col end}} |
|||
===Mast cell stabilizers=== |
=== Mast cell stabilizers === |
||
{{Main|Mast cell stabilizer}} |
{{Main|Mast cell stabilizer}} |
||
Mast cell stabilizers |
[[Mast cell]] stabilizers are drugs which prevent mast cell [[degranulation]]. Examples include: |
||
{{div col|colwidth=10em|small=yes}} |
|||
Examples include: |
|||
* [[Cromolyn sodium]] |
|||
* [[Cromoglicate]] (cromolyn) |
|||
* [[Nedocromil]] |
* [[Nedocromil]] |
||
* [[Beta2-adrenergic agonist]] |
* [[Beta2-adrenergic agonist|β-agonists]] |
||
{{div col end}} |
|||
== History == |
|||
The first H<sub>1</sub> receptor antagonists were discovered in the 1930s and were marketed in the 1940s.<ref name="LandauAchilladelisScriabine1999">{{cite book | author1 = Ralph Landau | author2 = Basil Achilladelis | author3 = Alexander Scriabine | date = 1999 | title = Pharmaceutical Innovation: Revolutionizing Human Health | publisher = Chemical Heritage Foundation | pages = 230– | isbn = 978-0-941901-21-5 | url = https://books.google.com/books?id=IH4lPs6S1bMC&pg=PA230}}</ref> [[Piperoxan]] was discovered in 1933 and was the first compound with antihistamine effects to be identified.<ref name="LandauAchilladelisScriabine1999" /> Piperoxan and its [[structural analog|analogue]]s were too [[toxicity|toxic]] to be used in humans.<ref name="LandauAchilladelisScriabine1999" /> [[Phenbenzamine]] (Antergan) was the first clinically useful antihistamine and was introduced for medical use in 1942.<ref name="LandauAchilladelisScriabine1999" /> Subsequently, many other antihistamines were developed and marketed.<ref name="LandauAchilladelisScriabine1999" /> [[Diphenhydramine]] (Benadryl) was synthesized in 1943, [[tripelennamine]] (Pyribenzamine) was patented in 1946, and [[promethazine]] (Phenergan) was synthesized in 1947 and launched in 1949.<ref name="LandauAchilladelisScriabine1999" /><ref name="Healy2009">{{cite book | author = David Healy | date = July 2009 | title = The Creation of Psychopharmacology | publisher = Harvard University Press | pages = 77– | isbn = 978-0-674-03845-5 | url = https://books.google.com/books?id=6O2rPJnyhj0C&pg=PA77}}</ref><ref name="FischerGanellin2010">{{cite book | author1 = János Fischer | author2 = C. Robin Ganellin | date = 24 August 2010 | title = Analogue-based Drug Discovery II | publisher = John Wiley & Sons | pages = 36– | isbn = 978-3-527-63212-1 | url = https://books.google.com/books?id=h2Kd8ci4Ln8C&pg=PA36}}</ref> By 1950, at least 20 antihistamines had been marketed.<ref name="Moncrieff2013">{{cite book | title = The Bitterest Pills | last1 = Moncrieff | first1 = Joanna | chapter = Chlorpromazine: The First Wonder Drug | date = 2013 | pages = 20–38 | publisher = Palgrave Macmillan UK | doi = 10.1057/9781137277442_2 | isbn = 978-1-137-27743-5 | url = }}</ref> [[Chlorphenamine]] (Piriton), a less sedating antihistamine, was synthesized in 1951, and [[hydroxyzine]] (Atarax, Vistaril), an antihistamine used specifically as a sedative and tranquilizer, was developed in 1956.<ref name="LandauAchilladelisScriabine1999" /><ref name="Atta-ur-Rahman2018">{{cite book | author = Atta-ur-Rahman | date = 11 July 2018 | title = Frontiers in Clinical Drug Research - Anti-Allergy Agents | publisher = Bentham Science Publishers | pages = 31– | isbn = 978-1-68108-337-7 | url = https://books.google.com/books?id=EIJoDwAAQBAJ&pg=PA31}}</ref> The first non-sedating antihistamine was [[terfenadine]] (Seldane) and was developed in 1973.<ref name="LandauAchilladelisScriabine1999" /><ref name="Sneader2005">{{cite book | author = Walter Sneader | date = 31 October 2005 | title = Drug Discovery: A History | publisher = John Wiley & Sons | pages = 406– | isbn = 978-0-470-01552-0 | url = https://books.google.com/books?id=jglFsz5EJR8C&pg=PA406}}</ref> Subsequently, other non-sedating antihistamines like [[loratadine]] (Claritin), [[cetirizine]] (Zyrtec), and [[fexofenadine]] (Allegra) were developed and introduced.<ref name="LandauAchilladelisScriabine1999" /> |
|||
The introduction of the first-generation antihistamines marked the beginning of medical treatment of nasal allergies.<ref name="Ostrom 2014">{{cite journal|last1=Ostrom|first1=NK|title=The history and progression of treatments for allergic rhinitis|journal=Allergy and Asthma Proceedings|date=2014|volume=35 Suppl 1|issue=3|pages=S3–10|pmid=25582156|doi=10.2500/aap.2014.35.3758}}</ref> Research into these drugs led to the discovery that they were [[H1 receptor antagonist|H<sub>1</sub> receptor antagonist]]s and also to the development of [[H2 receptor antagonist|H<sub>2</sub> receptor antagonist]]s, where H<sub>1</sub>-antihistamines affected the nose and the H<sub>2</sub>-antihistamines affected the stomach.<ref name="Jones 2016">{{cite journal|last1=Jones|first1=AW|title=Perspectives in Drug Development and Clinical Pharmacology: The Discovery of Histamine H1 and H2 Antagonists|journal=Clinical Pharmacology in Drug Development|date=January 2016|volume=5|issue=1|pages=5–12|pmid=27119574|doi=10.1002/cpdd.236|s2cid=29402462}}</ref> This history has led to contemporary research into drugs which are [[H3 receptor antagonist|H<sub>3</sub> receptor antagonist]]s and which affect the [[histamine H4 receptor|H<sub>4</sub> receptor antagonist]]s.<ref name="Jones 2016"/> Most people who use an H<sub>1</sub> receptor antagonist to treat allergies use a second-generation drug.<ref name="Consumer Reports 2013"/> |
|||
== Society and culture == |
|||
The United States government removed two second generation antihistamines, [[terfenadine]] and [[astemizole]], from the market based on evidence that they could cause heart problems.<ref name="Consumer Reports 2013"/> |
|||
== Research == |
|||
Not much published research exists which compares the efficacy and safety of the various antihistamines available.<ref name="Consumer Reports 2013"/> The research which does exist is mostly short-term studies or studies which look at too few people to make general assumptions.<ref name="Consumer Reports 2013"/> Another gap in the research is in information reporting the health effects for individuals with long-term allergies who take antihistamines for a long period of time.<ref name="Consumer Reports 2013"/> Newer antihistamines have been demonstrated to be effective in treating hives.<ref name="Consumer Reports 2013"/> However, there is no research comparing the relative efficacy of these drugs.<ref name="Consumer Reports 2013"/> |
|||
=== Special populations === |
|||
In 2020, the UK [[National Health Service]] wrote that "[m]ost people can safely take antihistamines" but that "[s]ome antihistamines may not be suitable" for young children, the pregnant or breastfeeding, for those taking other medicines, or people with conditions "such as heart disease, liver disease, kidney disease or epilepsy".<ref>{{Cite web|date=28 February 2020|title=Antihistamines|url=https://www.nhs.uk/conditions/antihistamines/|url-status=live|access-date=2021-04-28|website=[[National Health Service|NHS]]|language=en|archive-url=https://web.archive.org/web/20171222194002/https://www.nhs.uk/conditions/Antihistamines/ |archive-date=22 December 2017 }}</ref> |
|||
Most studies of antihistamines reported on people who are younger, so the effects on people over age 65 are not as well understood.<ref name="Consumer Reports 2013" /> Older people are more likely to experience drowsiness from antihistamine use than younger people.<ref name="Consumer Reports 2013" /> Continuous and/or cumulative use of [[anticholinergic]] medications, including first-generation antihistamines, is associated with higher risk for cognitive decline and dementia in older people.<ref>{{cite journal | vauthors = Gray SL, Anderson ML, Dublin S, Hanlon JT, Hubbard R, Walker R, Yu O, Crane PK, Larson EB | display-authors = 6 | title = Cumulative use of strong anticholinergics and incident dementia: a prospective cohort study | journal = JAMA Internal Medicine | volume = 175 | issue = 3 | pages = 401–407 | date = March 2015 | pmid = 25621434 | pmc = 4358759 | doi = 10.1001/jamainternmed.2014.7663 | author5-link = Rebecca Hubbard }}</ref><ref>{{cite journal |author1=Carrière, I |author2=Fourrier-Reglat, A |author3=Dartigues, J-F |author4=Rouaud, O |author5=Pasquier, F |author6=Ritchie, K |author7=Ancelin, M-L |title=Drugs with anticholinergic properties, cognitive decline, and dementia in an elderly general population: the 3-city study |journal=Archives of Internal Medicine |date=July 2009 |volume=169 |issue=14 |pages=1317–1324 |doi=10.1001/archinternmed.2009.229 |pmid=19636034 |pmc=2933398 }}</ref> |
|||
Also, most of the research has been on caucasians and other ethnic groups are not as represented in the research.<ref name="Consumer Reports 2013" /> The evidence does not report how antihistamines affect women differently than men.<ref name="Consumer Reports 2013" /> Different studies have reported on antihistamine use in children, with various studies finding evidence that certain antihistamines could be used by children 2 years of age, and other drugs being safer for younger or older children.<ref name="Consumer Reports 2013" /> |
|||
=== Potential uses studied === |
|||
Research regarding the effects of commonly used medications upon certain cancer therapies has suggested that when consumed in conjunction with immune checkpoint inhibitors some may influence the response of subjects to that particular treatment whose T-cell functions were failing in anti-tumor activity. Upon study of records in mouse studies associated with 40 common medications ranging from antibiotics, antihistamines, aspirin, and hydrocortisone, that for subjects with melanoma and lung cancers, fexofenadine, one of three medications, along with loratadine, and cetirizine, that target histamine receptor H1 (HRH1), demonstrated significantly higher survival rates and had experienced restored T-cell anti-tumor activity, ultimately inhibiting tumor growth in the subject animals.<ref>Manjarrez, Alejandra Manjarrez, ''[https://www.the-scientist.com/news-opinion/over-the-counter-antihistamines-could-help-against-cancer-69464 Over-the-Counter Antihistamines Could Help Against Cancer]'', [[The Scientist (magazine)|The Scientist]], November 24, 2021</ref> Such results encourage further study in order to see whether results in humans is similar in combating resistance to immunotherapy. |
|||
== See also == |
|||
* [[Antileukotriene]] |
|||
* [[Immunotherapy]] |
|||
==References== |
== References == |
||
{{Reflist}} |
{{Reflist|33em}} |
||
==External links== |
== External links == |
||
* {{MeshName|Histamine+antagonist}} |
* {{MeshName|Histamine+antagonist}} |
||
* [https://www.allergyuk.org/the-management-of-allergy/allergy-medications Antihistamine] {{Webarchive|url=https://web.archive.org/web/20170422084513/http://www.allergyuk.org/the-management-of-allergy/allergy-medications |date=22 April 2017 }} information at Allergy UK |
|||
{{Antihistamines}} |
{{Antihistamines|state=expanded}} |
||
{{Histaminergics}} |
{{Histaminergics}} |
||
{{Neuromodulation}} |
{{Neuromodulation}} |
||
{{Authority control}} |
|||
{{DEFAULTSORT:Histamine Antagonist}} |
{{DEFAULTSORT:Histamine Antagonist}} |
||
Latest revision as of 13:08, 12 December 2024
| Antihistamine | |
|---|---|
| Drug class | |
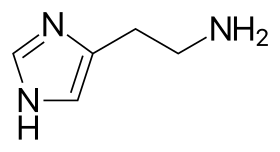 Histamine structure | |
| Class identifiers | |
| Pronunciation | /ˌæntiˈhɪstəmiːn/ |
| ATC code | R06 |
| Mechanism of action | • Receptor antagonist • Inverse agonist |
| Biological target | Histamine receptors • HRH1 • HRH2 • HRH3 • HRH4 |
| External links | |
| MeSH | D006633 |
| Legal status | |
| In Wikidata | |
Antihistamines are drugs which treat allergic rhinitis, common cold, influenza, and other allergies.[1] Typically, people take antihistamines as an inexpensive, generic (not patented) drug that can be bought without a prescription and provides relief from nasal congestion, sneezing, or hives caused by pollen, dust mites, or animal allergy with few side effects.[1] Antihistamines are usually for short-term treatment.[1] Chronic allergies increase the risk of health problems which antihistamines might not treat, including asthma, sinusitis, and lower respiratory tract infection.[1] Consultation of a medical professional is recommended for those who intend to take antihistamines for longer-term use.[1]
Although the general public typically uses the word "antihistamine" to describe drugs for treating allergies, physicians and scientists use the term to describe a class of drug that opposes the activity of histamine receptors in the body.[2] In this sense of the word, antihistamines are subclassified according to the histamine receptor that they act upon. The two largest classes of antihistamines are H1-antihistamines and H2-antihistamines.
H1-antihistamines work by binding to histamine H1 receptors in mast cells, smooth muscle, and endothelium in the body as well as in the tuberomammillary nucleus in the brain. Antihistamines that target the histamine H1-receptor are used to treat allergic reactions in the nose (e.g., itching, runny nose, and sneezing). In addition, they may be used to treat insomnia, motion sickness, or vertigo caused by problems with the inner ear. H2-antihistamines bind to histamine H2 receptors in the upper gastrointestinal tract, primarily in the stomach. Antihistamines that target the histamine H2-receptor are used to treat gastric acid conditions (e.g., peptic ulcers and acid reflux). Other antihistamines also target H3 receptors and H4 receptors.
Histamine receptors exhibit constitutive activity, so antihistamines can function as either a neutral receptor antagonist or an inverse agonist at histamine receptors.[2][3][4][5] Only a few currently marketed H1-antihistamines are known to function as antagonists.[2][5]
Medical uses
[edit]Histamine makes blood vessels more permeable (vascular permeability), causing fluid to escape from capillaries into tissues, which leads to the classic symptoms of an allergic reaction—a runny nose and watery eyes. Histamine also promotes angiogenesis.[6]
Antihistamines suppress the histamine-induced wheal response (swelling) and flare response (vasodilation) by blocking the binding of histamine to its receptors or reducing histamine receptor activity on nerves, vascular smooth muscle, glandular cells, endothelium, and mast cells. Antihistamines can also help correct Eustachian Tube dysfunction, thereby helping correct problems such as muffled hearing, fullness in the ear and even tinnitus.[7]
Itching, sneezing, and inflammatory responses are suppressed by antihistamines that act on H1-receptors.[2][8] In 2014, antihistamines such as desloratadine were found to be effective to complement standardized treatment of acne due to their anti-inflammatory properties and their ability to suppress sebum production.[9][10]
Types
[edit]H1-antihistamines
[edit]H1-antihistamines refer to compounds that inhibit the activity of the H1 receptor.[4][5] Since the H1 receptor exhibits constitutive activity, H1-antihistamines can be either neutral receptor antagonists or inverse agonists.[4][5] Normally, histamine binds to the H1 receptor and heightens the receptor's activity; the receptor antagonists work by binding to the receptor and blocking the activation of the receptor by histamine; by comparison, the inverse agonists bind to the receptor and both block the binding of histamine, and reduce its constitutive activity, an effect which is opposite to histamine's.[4] Most antihistamines are inverse agonists at the H1 receptor, but it was previously thought that they were antagonists.[11]
Clinically, H1-antihistamines are used to treat allergic reactions and mast cell-related disorders. Sedation is a common side effect of H1-antihistamines that readily cross the blood–brain barrier; some of these drugs, such as diphenhydramine and doxylamine, may therefore be used to treat insomnia. H1-antihistamines can also reduce inflammation, since the expression of NF-κB, the transcription factor the regulates inflammatory processes, is promoted by both the receptor's constitutive activity and agonist (i.e., histamine) binding at the H1 receptor.[2]
A combination of these effects, and in some cases metabolic ones as well, lead to most first-generation antihistamines having analgesic-sparing (potentiating) effects on opioid analgesics and to some extent with non-opioid ones as well. The most common antihistamines utilized for this purpose include hydroxyzine, promethazine (enzyme induction especially helps with codeine and similar prodrug opioids), phenyltoloxamine, orphenadrine, and tripelennamine; some may also have intrinsic analgesic properties of their own, orphenadrine being an example.
Second-generation antihistamines cross the blood–brain barrier to a much lesser extent than the first-generation antihistamines. They minimize sedatory effects due to their focused effect on peripheral histamine receptors. However, upon high doses second-generation antihistamines will begin to act on the central nervous system and thus can induce drowsiness when ingested in higher quantity.
List of H1 antagonists/inverse agonists
[edit]- Acrivastine
- Alimemazine (a phenothiazine used as antipruritic, antiemetic and sedative)
- Amitriptyline (tricyclic antidepressant)
- Amoxapine (tricyclic antidepressant)
- Aripiprazole (atypical antipsychotic, trade name: Abilify)
- Brexpiprazole (atypical antipsychotic, trade name: Rexulti)
- Azelastine
- Bilastine
- Bromodiphenhydramine (Bromazine)
- Brompheniramine
- Buclizine
- Carbinoxamine
- Cetirizine (Zyrtec)
- Chlophedianol (Clofedanol)
- Chlorodiphenhydramine[12]
- Chlorpheniramine
- Chlorpromazine (low-potency typical antipsychotic, also used as an antiemetic)
- Chlorprothixene (low-potency typical antipsychotic, trade name: Truxal)
- Chloropyramine (first generation antihistamine marketed in Eastern Europe)
- Cinnarizine (also used for motion sickness and vertigo)
- Clemastine
- Clomipramine (tricyclic antidepressant)
- Clozapine (atypical antipsychotic; trade name: Clozaril)
- Cyclizine
- Cyproheptadine
- Desloratadine
- Dexbrompheniramine
- Dexchlorpheniramine
- Dimenhydrinate (used as an antiemetic and for motion sickness)
- Dimetindene
- Diphenhydramine (Benadryl)
- Dosulepin (tricyclic antidepressant)
- Doxepin (tricyclic antidepressant)
- Doxylamine (most commonly used as an over-the-counter sedative)
- Ebastine
- Embramine
- Fexofenadine (Allegra/Telfast)
- Fluoxetine
- Hydroxyzine (also used as an anxiolytic and for motion sickness; trade names: Atarax, Vistaril)
- Imipramine (tricyclic antidepressant)
- Ketotifen
- Levocabastine (Livostin/Livocab)
- Levocetirizine (Xyzal)
- Levomepromazine (low-potency typical antipsychotic)
- Loratadine (Claritin)
- Maprotiline (tetracyclic antidepressant)
- Meclizine (most commonly used as an antiemetic)
- Mianserin (tetracyclic antidepressant)
- Mirtazapine (tetracyclic antidepressant, also has antiemetic and appetite-stimulating effects; trade name: Remeron)
- Olanzapine (atypical antipsychotic; trade name: Zyprexa)
- Olopatadine (used locally)
- Orphenadrine (a close relative of diphenhydramine used mainly as a skeletal muscle relaxant and anti-Parkinsons agent)
- Periciazine (low-potency typical antipsychotic)
- Phenindamine
- Pheniramine
- Phenyltoloxamine
- Promethazine (Phenergan)
- Pyrilamine (crosses the blood–brain barrier; produces drowsiness)
- Quetiapine (atypical antipsychotic; trade name: Seroquel)
- Rupatadine (Alergoliber)
- Setastine (Loderix)
- Setiptiline (or teciptiline, a tetracyclic antidepressant, trade name: Tecipul)
- Trazodone (SARI antidepressant/anxiolytic/hypnotic with mild H1 blockade action)
- Tripelennamine
- Triprolidine
H2-antihistamines
[edit]H2-antihistamines, like H1-antihistamines, exist as inverse agonists and neutral antagonists. They act on H2 histamine receptors found mainly in the parietal cells of the gastric mucosa, which are part of the endogenous signaling pathway for gastric acid secretion. Normally, histamine acts on H2 to stimulate acid secretion; drugs that inhibit H2 signaling thus reduce the secretion of gastric acid.
H2-antihistamines are among first-line therapy to treat gastrointestinal conditions including peptic ulcers and gastroesophageal reflux disease. Some formulations are available over the counter. Most side effects are due to cross-reactivity with unintended receptors. Cimetidine, for example, is notorious for antagonizing androgenic testosterone and DHT receptors at high doses.
Examples include:
H3-antihistamines
[edit]An H3-antihistamine is a classification of drugs used to inhibit the action of histamine at the H3 receptor. H3 receptors are primarily found in the brain and are inhibitory autoreceptors located on histaminergic nerve terminals, which modulate the release of histamine. Histamine release in the brain triggers secondary release of excitatory neurotransmitters such as glutamate and acetylcholine via stimulation of H1 receptors in the cerebral cortex. Consequently, unlike the H1-antihistamines which are sedating, H3-antihistamines have stimulant and cognition-modulating effects.
Examples of selective H3-antihistamines include:
H4-antihistamines
[edit]H4-antihistamines inhibit the activity of the H4 receptor. Examples include:
| Receptor | Location | Mechanism of action | Function | Antagonists | Uses of antagonists |
|---|---|---|---|---|---|
| H1 | Throughout the body, especially in:[17]
|
Gq |
|
||
| H2 |
|
Gs ↑ cAMP2+ |
|
||
| H3 |
|
Gi |
|
||
| H4 | Gi |
|
As of July 2021[update], no clinical uses exist. Potential uses include:[20] |
Atypical antihistamines
[edit]Histidine decarboxylase inhibitors
[edit]Inhibit the action of histidine decarboxylase:
Mast cell stabilizers
[edit]Mast cell stabilizers are drugs which prevent mast cell degranulation. Examples include:
History
[edit]The first H1 receptor antagonists were discovered in the 1930s and were marketed in the 1940s.[22] Piperoxan was discovered in 1933 and was the first compound with antihistamine effects to be identified.[22] Piperoxan and its analogues were too toxic to be used in humans.[22] Phenbenzamine (Antergan) was the first clinically useful antihistamine and was introduced for medical use in 1942.[22] Subsequently, many other antihistamines were developed and marketed.[22] Diphenhydramine (Benadryl) was synthesized in 1943, tripelennamine (Pyribenzamine) was patented in 1946, and promethazine (Phenergan) was synthesized in 1947 and launched in 1949.[22][23][24] By 1950, at least 20 antihistamines had been marketed.[25] Chlorphenamine (Piriton), a less sedating antihistamine, was synthesized in 1951, and hydroxyzine (Atarax, Vistaril), an antihistamine used specifically as a sedative and tranquilizer, was developed in 1956.[22][26] The first non-sedating antihistamine was terfenadine (Seldane) and was developed in 1973.[22][27] Subsequently, other non-sedating antihistamines like loratadine (Claritin), cetirizine (Zyrtec), and fexofenadine (Allegra) were developed and introduced.[22]
The introduction of the first-generation antihistamines marked the beginning of medical treatment of nasal allergies.[28] Research into these drugs led to the discovery that they were H1 receptor antagonists and also to the development of H2 receptor antagonists, where H1-antihistamines affected the nose and the H2-antihistamines affected the stomach.[29] This history has led to contemporary research into drugs which are H3 receptor antagonists and which affect the H4 receptor antagonists.[29] Most people who use an H1 receptor antagonist to treat allergies use a second-generation drug.[1]
Society and culture
[edit]The United States government removed two second generation antihistamines, terfenadine and astemizole, from the market based on evidence that they could cause heart problems.[1]
Research
[edit]Not much published research exists which compares the efficacy and safety of the various antihistamines available.[1] The research which does exist is mostly short-term studies or studies which look at too few people to make general assumptions.[1] Another gap in the research is in information reporting the health effects for individuals with long-term allergies who take antihistamines for a long period of time.[1] Newer antihistamines have been demonstrated to be effective in treating hives.[1] However, there is no research comparing the relative efficacy of these drugs.[1]
Special populations
[edit]In 2020, the UK National Health Service wrote that "[m]ost people can safely take antihistamines" but that "[s]ome antihistamines may not be suitable" for young children, the pregnant or breastfeeding, for those taking other medicines, or people with conditions "such as heart disease, liver disease, kidney disease or epilepsy".[30]
Most studies of antihistamines reported on people who are younger, so the effects on people over age 65 are not as well understood.[1] Older people are more likely to experience drowsiness from antihistamine use than younger people.[1] Continuous and/or cumulative use of anticholinergic medications, including first-generation antihistamines, is associated with higher risk for cognitive decline and dementia in older people.[31][32]
Also, most of the research has been on caucasians and other ethnic groups are not as represented in the research.[1] The evidence does not report how antihistamines affect women differently than men.[1] Different studies have reported on antihistamine use in children, with various studies finding evidence that certain antihistamines could be used by children 2 years of age, and other drugs being safer for younger or older children.[1]
Potential uses studied
[edit]Research regarding the effects of commonly used medications upon certain cancer therapies has suggested that when consumed in conjunction with immune checkpoint inhibitors some may influence the response of subjects to that particular treatment whose T-cell functions were failing in anti-tumor activity. Upon study of records in mouse studies associated with 40 common medications ranging from antibiotics, antihistamines, aspirin, and hydrocortisone, that for subjects with melanoma and lung cancers, fexofenadine, one of three medications, along with loratadine, and cetirizine, that target histamine receptor H1 (HRH1), demonstrated significantly higher survival rates and had experienced restored T-cell anti-tumor activity, ultimately inhibiting tumor growth in the subject animals.[33] Such results encourage further study in order to see whether results in humans is similar in combating resistance to immunotherapy.
See also
[edit]References
[edit]- ^ a b c d e f g h i j k l m n o p q Consumer Reports (2013), Using Antihistamines to Treat Allergies, Hay Fever, & Hives - Comparing Effectiveness, Safety, and Price (PDF), Yonkers, New York: Consumer Reports, archived from the original (PDF) on 17 May 2017, retrieved 29 June 2017
- ^ a b c d e Canonica GW, Blaiss M (2011). "Antihistaminic, anti-inflammatory, and antiallergic properties of the nonsedating second-generation antihistamine desloratadine: a review of the evidence". World Allergy Organ J. 4 (2): 47–53. doi:10.1097/WOX.0b013e3182093e19. PMC 3500039. PMID 23268457.
The H1-receptor is a transmembrane protein belonging to the G-protein coupled receptor family. Signal transduction from the extracellular to the intracellular environment occurs as the GCPR becomes activated after binding of a specific ligand or agonist. A subunit of the G-protein subsequently dissociates and affects intracellular messaging including downstream signaling accomplished through various intermediaries such as cyclic AMP, cyclic GMP, calcium, and nuclear factor kappa B (NF-κB), a ubiquitous transcription factor thought to play an important role in immune-cell chemotaxis, proinflammatory cytokine production, expression of cell adhesion molecules, and other allergic and inflammatory conditions.1,8,12,30–32 ... For example, the H1-receptor promotes NF-κB in both a constitutive and agonist-dependent manner and all clinically available H1-antihistamines inhibit constitutive H1-receptor-mediated NF-κB production ...
Importantly, because antihistamines can theoretically behave as inverse agonists or neutral antagonists, they are more properly described as H1-antihistamines rather than H1-receptor antagonists.15 - ^ Panula P, Chazot PL, Cowart M, et al. (2015). "International Union of Basic and Clinical Pharmacology. XCVIII. Histamine Receptors". Pharmacol. Rev. 67 (3): 601–55. doi:10.1124/pr.114.010249. PMC 4485016. PMID 26084539.
- ^ a b c d Leurs R, Church MK, Taglialatela M (April 2002). "H1-antihistamines: inverse agonism, anti-inflammatory actions and cardiac effects". Clinical and Experimental Allergy. 32 (4): 489–98. doi:10.1046/j.0954-7894.2002.01314.x. PMID 11972592. S2CID 11849647.
- ^ a b c d "H1 receptor". IUPHAR/BPS Guide to Pharmacology. Retrieved 8 October 2015.
- ^ Norrby K (1995). "Evidence of a dual role of endogenous histamine in angiogenesis". Int J Exp Pathol. 76 (2): 87–92. PMC 1997159. PMID 7540412.
- ^ Morrison, James (28 September 2021). "Best Antihistamine for Tinnitus?". Tinnitus and You. Retrieved 15 March 2022.
- ^ Monroe EW, Daly AF, Shalhoub RF (February 1997). "Appraisal of the validity of histamine-induced wheal and flare to predict the clinical efficacy of antihistamines". The Journal of Allergy and Clinical Immunology. 99 (2): S798–806. doi:10.1016/s0091-6749(97)70128-3. PMID 9042073.
- ^ Lee HE, Chang IK, Lee Y, Kim CD, Seo YJ, Lee JH, Im M (2014). "Effect of antihistamine as an adjuvant treatment of isotretinoin in acne: a randomized, controlled comparative study". J Eur Acad Dermatol Venereol. 28 (12): 1654–60. doi:10.1111/jdv.12403. PMID 25081735. S2CID 3406128.
- ^ Layton AM (2016). "Top Ten List of Clinical Pearls in the Treatment of Acne Vulgaris". Dermatol Clin. 34 (2): 147–57. doi:10.1016/j.det.2015.11.008. PMID 27015774.
- ^ Church, Diana S; Church, Martin K (15 March 2011). "Pharmacology of Antihistamines". The World Allergy Organization Journal. 4 (Suppl 3): S22 – S27. doi:10.1097/1939-4551-4-S3-S22. ISSN 1939-4551. PMC 3666185. PMID 23282332.
- ^ Thomas L. Lemke; David A. Williams, eds. (24 January 2012). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. pp. 1053–. ISBN 978-1-60913-345-0. OCLC 1127763671.
- ^ Yoneyama H, et al. (March 2008). "Efficient approaches to S-alkyl-N-alkylisothioureas: syntheses of histamine H3 antagonist clobenpropit and its analogues". The Journal of Organic Chemistry. 73 (6): 2096–104. doi:10.1021/jo702181x. PMID 18278935.
- ^ Fox GB, Esbenshade TA, Pan JB, Radek RJ, Krueger KM, Yao BB, Browman KE, Buckley MJ, Ballard ME, Komater VA, Miner H, Zhang M, Faghih R, Rueter LE, Bitner RS, Drescher KU, Wetter J, Marsh K, Lemaire M, Porsolt RD, Bennani YL, Sullivan JP, Cowart MD, Decker MW, Hancock AA (April 2005). "Pharmacological properties of ABT-239 [4-(2-{2-[(2R)-2-Methylpyrrolidinyl]ethyl}-benzofuran-5-yl)benzonitrile]: II. Neurophysiological characterization and broad preclinical efficacy in cognition and schizophrenia of a potent and selective histamine H3 receptor antagonist". The Journal of Pharmacology and Experimental Therapeutics. 313 (1): 176–90. doi:10.1124/jpet.104.078402. PMID 15608077. S2CID 15430117.
- ^ Ligneau X, Lin J, Vanni-Mercier G, Jouvet M, Muir JL, Ganellin CR, Stark H, Elz S, Schunack W, Schwartz J (November 1998). "Neurochemical and behavioral effects of ciproxifan, a potent histamine H3-receptor antagonist". The Journal of Pharmacology and Experimental Therapeutics. 287 (2): 658–66. PMID 9808693. Archived from the original on 2 May 2020. Retrieved 9 August 2014.
- ^ Esbenshade TA, Fox GB, Krueger KM, Baranowski JL, Miller TR, Kang CH, Denny LI, Witte DG, Yao BB, Pan JB, Faghih R, Bennani YL, Williams M, Hancock AA (September 2004). "Pharmacological and behavioral properties of A-349821, a selective and potent human histamine H3 receptor antagonist". Biochemical Pharmacology. 68 (5): 933–45. doi:10.1016/j.bcp.2004.05.048. PMID 15294456.
- ^ "Histamine H1 Receptor - an overview | ScienceDirect Topics". www.sciencedirect.com. Retrieved 3 October 2023.
- ^ Deiteren A, De Man JG, Pelckmans PA, De Winter BY (March 2015). "Histamine H₄ receptors in the gastrointestinal tract". British Journal of Pharmacology. 172 (5): 1165–1178. doi:10.1111/bph.12989. PMC 4337694. PMID 25363289.
- ^ Hofstra CL, Desai PJ, Thurmond RL, Fung-Leung WP (June 2003). "Histamine H4 receptor mediates chemotaxis and calcium mobilization of mast cells". The Journal of Pharmacology and Experimental Therapeutics. 305 (3): 1212–1221. doi:10.1124/jpet.102.046581. PMID 12626656. S2CID 14932773.
- ^ "Histamine H4 Receptor Antagonist - an overview | ScienceDirect Topics". www.sciencedirect.com. Retrieved 3 October 2023.
- ^ Kim KW, Kim BM, Lee KA, Lee SH, Firestein GS, Kim HR (April 2017). "Histamine and Histamine H4 Receptor Promotes Osteoclastogenesis in Rheumatoid Arthritis". Scientific Reports. 7 (1): 1197. Bibcode:2017NatSR...7.1197K. doi:10.1038/s41598-017-01101-y. PMC 5430934. PMID 28446753.
- ^ a b c d e f g h i Ralph Landau; Basil Achilladelis; Alexander Scriabine (1999). Pharmaceutical Innovation: Revolutionizing Human Health. Chemical Heritage Foundation. pp. 230–. ISBN 978-0-941901-21-5.
- ^ David Healy (July 2009). The Creation of Psychopharmacology. Harvard University Press. pp. 77–. ISBN 978-0-674-03845-5.
- ^ János Fischer; C. Robin Ganellin (24 August 2010). Analogue-based Drug Discovery II. John Wiley & Sons. pp. 36–. ISBN 978-3-527-63212-1.
- ^ Moncrieff, Joanna (2013). "Chlorpromazine: The First Wonder Drug". The Bitterest Pills. Palgrave Macmillan UK. pp. 20–38. doi:10.1057/9781137277442_2. ISBN 978-1-137-27743-5.
- ^ Atta-ur-Rahman (11 July 2018). Frontiers in Clinical Drug Research - Anti-Allergy Agents. Bentham Science Publishers. pp. 31–. ISBN 978-1-68108-337-7.
- ^ Walter Sneader (31 October 2005). Drug Discovery: A History. John Wiley & Sons. pp. 406–. ISBN 978-0-470-01552-0.
- ^ Ostrom, NK (2014). "The history and progression of treatments for allergic rhinitis". Allergy and Asthma Proceedings. 35 Suppl 1 (3): S3–10. doi:10.2500/aap.2014.35.3758. PMID 25582156.
- ^ a b Jones, AW (January 2016). "Perspectives in Drug Development and Clinical Pharmacology: The Discovery of Histamine H1 and H2 Antagonists". Clinical Pharmacology in Drug Development. 5 (1): 5–12. doi:10.1002/cpdd.236. PMID 27119574. S2CID 29402462.
- ^ "Antihistamines". NHS. 28 February 2020. Archived from the original on 22 December 2017. Retrieved 28 April 2021.
- ^ Gray SL, Anderson ML, Dublin S, Hanlon JT, Hubbard R, Walker R, et al. (March 2015). "Cumulative use of strong anticholinergics and incident dementia: a prospective cohort study". JAMA Internal Medicine. 175 (3): 401–407. doi:10.1001/jamainternmed.2014.7663. PMC 4358759. PMID 25621434.
- ^ Carrière, I; Fourrier-Reglat, A; Dartigues, J-F; Rouaud, O; Pasquier, F; Ritchie, K; Ancelin, M-L (July 2009). "Drugs with anticholinergic properties, cognitive decline, and dementia in an elderly general population: the 3-city study". Archives of Internal Medicine. 169 (14): 1317–1324. doi:10.1001/archinternmed.2009.229. PMC 2933398. PMID 19636034.
- ^ Manjarrez, Alejandra Manjarrez, Over-the-Counter Antihistamines Could Help Against Cancer, The Scientist, November 24, 2021
External links
[edit]- Histamine+antagonist at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Antihistamine Archived 22 April 2017 at the Wayback Machine information at Allergy UK
