Damascenone: Difference between revisions
→Biosynthesis: https://en.wikipedia.org/enwiki/w/index.php?title=Damascenone&action=submit# |
m spelling (WP:Typo Team) |
||
| (20 intermediate revisions by 14 users not shown) | |||
| Line 1: | Line 1: | ||
{{distinguish|Damascone}} |
|||
{{chembox |
{{chembox |
||
| Verifiedfields = changed |
| Verifiedfields = changed |
||
| Watchedfields = changed |
|||
| verifiedrevid = 413428485 |
| verifiedrevid = 413428485 |
||
| Name=''beta''-Damascenone |
| Name=''beta''-Damascenone |
||
| ImageFile=damascenone.png |
| ImageFile=damascenone.png |
||
| ImageSize=200px |
| ImageSize=200px |
||
| Line 20: | Line 22: | ||
| CASNo_Ref = {{cascite|correct|CAS}} |
| CASNo_Ref = {{cascite|correct|CAS}} |
||
| CASNo=23726-93-4 |
| CASNo=23726-93-4 |
||
| UNII_Ref = {{fdacite|correct|FDA}} |
|||
| UNII = U66V25TBO0 |
|||
| PubChem=5366074 |
| PubChem=5366074 |
||
| ChEBI_Ref = {{ebicite|changed|EBI}} |
| ChEBI_Ref = {{ebicite|changed|EBI}} |
||
| Line 26: | Line 30: | ||
}} |
}} |
||
|Section2={{Chembox Properties |
|Section2={{Chembox Properties |
||
| C=13 |
|||
| Formula=C<sub>13</sub>H<sub>18</sub>O |
|||
| H=18 |
|||
| MolarMass=190.28 g/mol |
|||
| O=1 |
|||
| Appearance= |
| Appearance= |
||
| Density= |
| Density= |
||
| Line 56: | Line 61: | ||
| doi = 10.1002/hlca.19730560508 }}</ref> |
| doi = 10.1002/hlca.19730560508 }}</ref> |
||
In 2008, (E)-β-damascenone was identified as a primary odorant in Kentucky |
In 2008, (E)-β-damascenone was identified as a primary odorant in [[Kentucky bourbon]].<ref>{{cite journal |
||
| title = Characterization of the Most Odor-Active Compounds in an American Bourbon Whisky by Application of the Aroma Extract Dilution Analysis |
| title = Characterization of the Most Odor-Active Compounds in an American Bourbon Whisky by Application of the Aroma Extract Dilution Analysis |
||
| author = LUIGI POISSON |
| author = LUIGI POISSON |
||
| author2 = PETER SCHIEBERLE |
| author2 = PETER SCHIEBERLE |
||
| journal = |
| journal = Journal of Agricultural and Food Chemistry |
||
| volume = 56 |
| volume = 56 |
||
| issue = 14 |
| issue = 14 |
||
| pages = 5813–5819 |
| pages = 5813–5819 |
||
| year = 2008 |
| year = 2008 |
||
| |
| doi = 10.1021/jf800382m | pmid = 18570373 |
||
}}</ref> |
|||
==Biosynthesis== |
==Biosynthesis== |
||
{{Multiple issues|section=yes| |
|||
{{Technical|section|date=May 2016}} |
{{Technical|section|date=May 2016}} |
||
| ⚫ | The biosynthesis for β-damascenone begins with [[farnesyl pyrophosphate]] (FPP) and [[isopentenyl pyrophosphate]] (IPP) reacting to produce [[geranylgeranyl pyrophosphate]] (GGPP) Figure 1. [[File:GGPP Synthesis.svg|thumb|Figure 1: GGPP Synthesis]]Next two molecules of GGPP are condensed together to produce [[phytoene]] by removal of diphosphate and a proton shift catalyzed by the enzyme [[phytoene synthase]] (PSY). Phytoene then goes through a series of desaturation reactions using the enzyme [[Phytoene desaturase (neurosporene-forming)|phytoene desaturase]] (PDS) to produce [[phytofluene]] then [[zeta-Carotene|ζ-carotene]]. Other enzymes have been found to catalyze this reaction including CrtI and CrtP.<ref>{{cite journal |
||
{{Very long|section|date=May 2016}} |
|||
| title = BCarotenoids and their cleavage products: Biosynthesis and functions |
|||
}} |
|||
| ⚫ | |||
| ⚫ | The biosynthesis for β- |
||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| year = 2011 |
|||
| doi = 10.1039/c0np00036a | pmid = 21321752 |
|||
}}</ref> |
|||
The next series of desaturation reactions is catalyzed by the enzyme [[9,9'-dicis-zeta-carotene desaturase|ζ-carotene desaturase]] (ZDS) to produce [[neurosporene]] followed by [[lycopene]]. Other enzymes that are able to catalyze this reaction include CtrI and CrtQ. Next lycopene goes through two cyclization reactions with the use of the enzyme [[Lycopene beta-cyclase|lycopene β-cyclase]] first producing [[γ-carotene]] followed by the second cyclization producing [[β-carotene]] as shown in Figure 2.[[File:Beta Carotene Synthesis.svg|thumb|Figure 2: Beta Carotene Synthesis]] The mechanism for the cyclization of lycopene to β-carotene is shown in Scheme 2. |
|||
[[File:Beta-Carotene Mechanism.svg|thumb|Scheme 1: Beta-Carotene Mechanism]] Next β-carotene reacts with O2 and the enzyme β-carotene ring hydroxylase producing [[zeaxanthin]].<ref>{{cite journal |
[[File:Beta-Carotene Mechanism.svg|thumb|Scheme 1: Beta-Carotene Mechanism]] Next β-carotene reacts with O2 and the enzyme β-carotene ring hydroxylase producing [[zeaxanthin]].<ref>{{cite journal |
||
| title = The lycopene β-cyclase plays a significant role in provitamin A biosynthesis in wheat endosperm |
| title = The lycopene β-cyclase plays a significant role in provitamin A biosynthesis in wheat endosperm |
||
| Line 94: | Line 107: | ||
| volume = 15 |
| volume = 15 |
||
| issue = 112 |
| issue = 112 |
||
| pages = |
| pages =112 |
||
| year = 2015 |
| year = 2015 |
||
| |
| doi = 10.1186/s12870-015-0514-5 | pmid = 25943989 |
||
| pmc = 4433027 |
|||
| doi = 10.1186/s12870-015-0514-5 }}</ref> |
|||
| doi-access = free |
|||
| ⚫ | Zeaxanthin then reacts with O2, NADPH (H+), and reduced ferredoxin [iron-sulfur] cluster in the presence of the enzyme [[zeaxanthin epoxidase]] (ZE) to produce antheraxanthin which |
||
}}</ref> |
|||
| ⚫ | Zeaxanthin then reacts with O2, NADPH (H+), and reduced ferredoxin [iron-sulfur] cluster in the presence of the enzyme [[zeaxanthin epoxidase]] (ZE) to produce antheraxanthin which reacts in a similar fashion to produce [[violaxanthin]]. Violaxanthin then reacts with the enzyme [[neoxanthin synthase]] to form [[neoxanthin]] the main precursor for β-damascenone as shown in Figure 3.[[File:Neoxanthin Synthesis.svg|thumb|Figure 3: Neoxanthin synthesis]]<ref>{{cite journal |
||
| title = Biosynthetic Pathway and Health Benefits of Fucoxanthin, an Algae-Specific Xanthophyll in Brown Seaweeds |
| title = Biosynthetic Pathway and Health Benefits of Fucoxanthin, an Algae-Specific Xanthophyll in Brown Seaweeds |
||
| author = Koji Mikami |
| author = Koji Mikami |
||
| Line 105: | Line 120: | ||
| volume = 14 |
| volume = 14 |
||
| issue = 7 |
| issue = 7 |
||
| pages = |
| pages = 13763–13781 |
||
| year = 2013 |
| year = 2013 |
||
| pmc = 3742216 |
|||
| url = http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3742216/ |
|||
| doi = 10.3390/ijms140713763 |
| doi = 10.3390/ijms140713763 |
||
| pmid=23820585| doi-access = free |
|||
| ⚫ | In order to generate β-damascenone from neoxanthin there are a few more modifications needed. First neoxanthin undergoes an oxidative cleavage to create the grasshopper ketone. The grasshopper ketone then undergoes a reduction to generate the allenic triol. At this stage there are two main pathways the allenic triol can take to produce the final product. The allenic triol can undergo a dehydration reaction to generate either the acetylenic diol or the allenic diol. Finally one last dehydration reaction of either the acetylenic diol or the allenic diol produces the final product β-damascenone as shown in Figure 4.[[File:Beta-Damascenone Synthesis.svg|thumb|Figure 4: Beta- |
||
}}</ref> |
|||
| ⚫ | |||
| ⚫ | In order to generate β-damascenone from neoxanthin there are a few more modifications needed. First neoxanthin undergoes an oxidative cleavage to create the grasshopper ketone. The grasshopper ketone then undergoes a reduction to generate the allenic triol. At this stage there are two main pathways the allenic triol can take to produce the final product. The allenic triol can undergo a dehydration reaction to generate either the acetylenic diol or the allenic diol. Finally one last dehydration reaction of either the acetylenic diol or the allenic diol produces the final product β-damascenone as shown in Figure 4.[[File:Beta-Damascenone Synthesis.svg|thumb|Figure 4: Beta-damascenone synthesis]]<ref>{{cite journal |
||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
|author2=Itzhak Bilkis |
|||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
|author7=Michael Naim |
|||
| ⚫ | |||
|journal= Journal of Agricultural and Food Chemistry |
|||
| ⚫ | |||
|volume=53 |
|||
| ⚫ | |||
| |
|issue=23 |
||
|pages=9199–9206 |
|||
| url = http://www.ncbi.nih.gov.pubmed/16277423 |
|||
|year=2005 |
|||
| ⚫ | |||
|pmid=16277423 |
|||
| ⚫ | |||
| ⚫ | |||
}}</ref><ref>{{cite book |
|||
| ⚫ | |||
| author = Peter Winterhalter |
| author = Peter Winterhalter |
||
| author2 = Recep Gök |
| author2 = Recep Gök |
||
| ⚫ | |||
| volume = 1134 |
| volume = 1134 |
||
| |
| pages =125–137 |
||
| pages =125-137 |
|||
| year = 2013 |
| year = 2013 |
||
| |
| doi = 10.1021/bk-2013-1134.ch011 | series = ACS Symposium Series |
||
| isbn = 978-0-8412-2778-1 |
|||
| ⚫ | |||
| ⚫ | |||
| ⚫ | }}</ref> The proposed mechanism for the conversion of the allenic triol to the acetylenic diol is shown in Scheme 3.[[File:Acetylenic Diol Mechanism.svg|thumb|Scheme 3: Acetylenic diol mechanism]] The proposed mechanism for the conversion of the acetylenic diol to the final product is shown in Scheme 4.[[File:Beta-Damascenone Mechanism.svg|thumb|Scheme 4: Beta-damascenone mechanism]] This mechanism is known as a [[Meyer-Schuster rearrangement]]. |
||
==See also== |
==See also== |
||
| Line 143: | Line 161: | ||
[[Category:Carotenoids]] |
[[Category:Carotenoids]] |
||
[[Category: |
[[Category:Enones]] |
||
[[Category:Perfume ingredients]] |
[[Category:Perfume ingredients]] |
||
[[Category:Cyclohexadienes]] |
[[Category:Cyclohexadienes]] |
||
{{ketone-stub}} |
|||
Latest revision as of 09:03, 13 March 2024
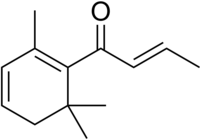
| |

| |
| Names | |
|---|---|
| IUPAC name
(E)-1-(2,6,6-Trimethyl-1-cyclohexa-1,3-dienyl)but-2-en-1-one
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.041.662 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C13H18O | |
| Molar mass | 190.286 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Damascenones are a series of closely related chemical compounds that are components of a variety of essential oils. The damascenones belong to a family of chemicals known as rose ketones, which also includes damascones and ionones. beta-Damascenone is a major contributor to the aroma of roses, despite its very low concentration, and is an important fragrance chemical used in perfumery.[1]
The damascenones are derived from the degradation of carotenoids.[2]
In 2008, (E)-β-damascenone was identified as a primary odorant in Kentucky bourbon.[3]
Biosynthesis
[edit]This section may be too technical for most readers to understand. (May 2016) |
The biosynthesis for β-damascenone begins with farnesyl pyrophosphate (FPP) and isopentenyl pyrophosphate (IPP) reacting to produce geranylgeranyl pyrophosphate (GGPP) Figure 1.

Next two molecules of GGPP are condensed together to produce phytoene by removal of diphosphate and a proton shift catalyzed by the enzyme phytoene synthase (PSY). Phytoene then goes through a series of desaturation reactions using the enzyme phytoene desaturase (PDS) to produce phytofluene then ζ-carotene. Other enzymes have been found to catalyze this reaction including CrtI and CrtP.[4] The next series of desaturation reactions is catalyzed by the enzyme ζ-carotene desaturase (ZDS) to produce neurosporene followed by lycopene. Other enzymes that are able to catalyze this reaction include CtrI and CrtQ. Next lycopene goes through two cyclization reactions with the use of the enzyme lycopene β-cyclase first producing γ-carotene followed by the second cyclization producing β-carotene as shown in Figure 2.

The mechanism for the cyclization of lycopene to β-carotene is shown in Scheme 2.

Next β-carotene reacts with O2 and the enzyme β-carotene ring hydroxylase producing zeaxanthin.[5] Zeaxanthin then reacts with O2, NADPH (H+), and reduced ferredoxin [iron-sulfur] cluster in the presence of the enzyme zeaxanthin epoxidase (ZE) to produce antheraxanthin which reacts in a similar fashion to produce violaxanthin. Violaxanthin then reacts with the enzyme neoxanthin synthase to form neoxanthin the main precursor for β-damascenone as shown in Figure 3.

[6] In order to generate β-damascenone from neoxanthin there are a few more modifications needed. First neoxanthin undergoes an oxidative cleavage to create the grasshopper ketone. The grasshopper ketone then undergoes a reduction to generate the allenic triol. At this stage there are two main pathways the allenic triol can take to produce the final product. The allenic triol can undergo a dehydration reaction to generate either the acetylenic diol or the allenic diol. Finally one last dehydration reaction of either the acetylenic diol or the allenic diol produces the final product β-damascenone as shown in Figure 4.

[7][8] The proposed mechanism for the conversion of the allenic triol to the acetylenic diol is shown in Scheme 3.
The proposed mechanism for the conversion of the acetylenic diol to the final product is shown in Scheme 4.

This mechanism is known as a Meyer-Schuster rearrangement.
See also
[edit]References
[edit]- ^ Rose (Rosa damascena), John C. Leffingwell
- ^ Sachihiko Isoe; Shigeo Katsumura; Takeo Sakan (1973). "The Synthesis of Damascenone and beta-Damascone and the possible mechanism of their formation from carotenoids". Helvetica Chimica Acta. 56 (5): 1514–1516. doi:10.1002/hlca.19730560508.
- ^ LUIGI POISSON; PETER SCHIEBERLE (2008). "Characterization of the Most Odor-Active Compounds in an American Bourbon Whisky by Application of the Aroma Extract Dilution Analysis". Journal of Agricultural and Food Chemistry. 56 (14): 5813–5819. doi:10.1021/jf800382m. PMID 18570373.
- ^ Michael H. Walter; Dieter Strack (2011). "BCarotenoids and their cleavage products: Biosynthesis and functions". Nat. Prod. Rep. 28 (4): 663–692. doi:10.1039/c0np00036a. PMID 21321752.
- ^ Jian Zeng; Cheng Wang; Xi Chen; Mingli Zang; Cuihong Yuan; Xiatian Wang; Qiong Wang; Miao Li; Xiaoyan Li; Ling Chen; Kexiu Li; Junli Chang; Yuesheng Wang; Guangxia Yang; Guangyuan He (2015). "The lycopene β-cyclase plays a significant role in provitamin A biosynthesis in wheat endosperm". BMC Plant Biology. 15 (112): 112. doi:10.1186/s12870-015-0514-5. PMC 4433027. PMID 25943989.
- ^ Koji Mikami; Masashi Hosokawa (2013). "Biosynthetic Pathway and Health Benefits of Fucoxanthin, an Algae-Specific Xanthophyll in Brown Seaweeds". Int. J. Mol. Sci. 14 (7): 13763–13781. doi:10.3390/ijms140713763. PMC 3742216. PMID 23820585.
- ^ Yair Bezman; Itzhak Bilkis; Peter Winterhalter; Peter Fleischmann; Russell L. Rouseff; Susanne Baldermann; Michael Naim (2005). "Thermal Oxidation of 9'-cis-Neoxanthin in a Model System Containing Peroxyacetic Acid Leads to Potent Odorant β-Damascenone". Journal of Agricultural and Food Chemistry. 53 (23): 9199–9206. doi:10.1021/jf051330b. PMID 16277423.
- ^ Peter Winterhalter; Recep Gök (2013). "TDN and β-Damascenone: Two Important Carotenoid Metabolites in Wine". Carotenoid Cleavage Products. ACS Symposium Series. Vol. 1134. pp. 125–137. doi:10.1021/bk-2013-1134.ch011. ISBN 978-0-8412-2778-1.
