User:GomezChristian/sandbox: Difference between revisions
Charles Cole (talk | contribs) No edit summary |
mNo edit summary |
||
| (34 intermediate revisions by 4 users not shown) | |||
| Line 2: | Line 2: | ||
Note: Group's draft space -[[User:GomezChristian|GomezChristian]] ([[User talk:GomezChristian|talk]]) 21:10, 21 February 2017 (UTC) |
Note: Group's draft space -[[User:GomezChristian|GomezChristian]] ([[User talk:GomezChristian|talk]]) 21:10, 21 February 2017 (UTC) |
||
Evaluation of Chorismate Mutase Article: |
<u>Evaluation of Chorismate Mutase Article:</u> |
||
1.Elaboration could be made on conformational trapping of the substrate in the enzyme active site. |
1.Elaboration could be made on conformational trapping of the substrate in the enzyme active site. |
||
| Line 12: | Line 12: | ||
4. Figure for reaction has a misspelling of chorismate (chromismate). |
4. Figure for reaction has a misspelling of chorismate (chromismate). |
||
[[User:Charles Cole|Charles Cole]] ([[User talk:Charles Cole|talk]]) 21:41, 21 February 2017 (UTC) |
[[User:Charles Cole|Charles Cole]] ([[User talk:Charles Cole|talk]]) 21:41, 21 February 2017 (UTC) |
||
= Chorismate Mutase = |
|||
In [[enzymology]], a '''chorismate mutase''' ({{EC number|5.4.99.5}}) is an [[enzyme]] that [[Catalysis|catalyzes]] the [[chemical reaction]] for the conversion of [[chorismate]] to [[prephenate]] in the [[Shikimate pathway|pathway]] to the production of [[phenylalanine]] and [[tyrosine]], also known as the [[shikimate]] pathway.{{enzyme|Name=chorismate mutase|EC_number=5.4.99.5|CAS_number=9068-30-8|IUBMB_EC_number=5/4/99/5|GO_code=0004106|image=Chorismate_mutase_with_TSA_bound_4.jpg|width=|caption=Crystal Structure of Chorismate Mutase with Bound Transition State Analog|name=Chorismate Mutase}} |
|||
Hence, this enzyme has one [[Substrate (biochemistry)|substrate]], [[chorismate]], and one [[Product (chemistry)|product]], [[prephenate]]. Chorismate mutase is found at a branch point in the pathway. The enzyme channels chorismate to the [[biosynthesis]] of tyrosine and phenylalanine and away from [[tryptophan]].<ref name="Qamra_2006">{{cite journal|date=June 2006|title=The 2.15 A crystal structure of Mycobacterium tuberculosis chorismate mutase reveals an unexpected gene duplication and suggests a role in host-pathogen interactions|journal=Biochemistry|volume=45|issue=23|pages=6997–7005|doi=10.1021/bi0606445|pmid=16752890|vauthors=Qamra R, Prakash P, Aruna B, Hasnain SE, Mande SC}}</ref> The enzyme's role in maintaining the balance of these aromatic amino acids in the cell is vital.<ref name="Kast_2000">{{cite journal|date=November 2000|title=A strategically positioned cation is crucial for efficient catalysis by chorismate mutase|journal=The Journal of Biological Chemistry|volume=275|issue=47|pages=36832–8|doi=10.1074/jbc.M006351200|pmid=10960481|vauthors=Kast P, Grisostomi C, Chen IA, Li S, Krengel U, Xue Y, Hilvert D}}</ref> This is the single known example of a naturally occurring enzyme catalyzing a [[pericyclic reaction]].<ref name="Kast_2000" />{{#tag:ref|Dimethylallyltryptophan synthase has been proposed to catalyze a [[Cope rearrangement]], but this has yet to be proven definitively<ref name="Luk_2011">{{cite journal | vauthors = Luk LY, Qian Q, Tanner ME | title = A cope rearrangement in the reaction catalyzed by dimethylallyltryptophan synthase? | journal = Journal of the American Chemical Society | volume = 133 | issue = 32 | pages = 12342–5 | date = August 2011 | pmid = 21766851 | pmc = | doi = 10.1021/ja2034969 }}</ref>|group="nb"}} Chorismate mutase is only found in fungi, bacteria, and higher plants. This protein may use the [[morpheein]] model of [[allosteric regulation]].<ref name="pmid22182754">{{cite journal|last-author-amp=yes|date=March 2012|title=Dynamic dissociating homo-oligomers and the control of protein function|url=http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=22182754|journal=Archives of Biochemistry and Biophysics|volume=519|issue=2|pages=131–43|doi=10.1016/j.abb.2011.11.020|pmc=3298769|pmid=22182754|vauthors=Selwood T, Jaffe EK}}</ref> |
|||
== Protein family == |
|||
[[File:Chorismate-mutase-pdb-2CHS.png|link=https://en.wikipedia.org/wiki/File:Chorismate-mutase-pdb-2CHS.png|alt=chorismate mutase|left|thumb|216x216px|'''Chorismate mutase'''. Rendered from PDB [http://www.rcsb.org/pdb/explore/explore.do?structureId=2CHS 2CHS].]] |
|||
This enzyme belongs to the family of [[Isomerase|isomerases]], specifically those intramolecular [[Transferase|transferases]] transferring other groups. The [[List of enzymes|systematic name]] of this enzyme class is '''chorismate pyruvatemutase'''. Chorismate mutase, also known as '''hydroxyphenylpyruvate synthase''', participates in phenylalanine, tyrosine and tryptophan biosynthesis.<ref name="Qamra_2006" /> The structures of chorismate mutases vary in different organisms, but the majority belong to the AroQ family and are characterized by an intertwined homodimer of 3-helical subunits. Most chorismate mutases in this family look similar to that of ''[[Escherichia coli]]''. For example, the secondary structure of the chorismate mutase of [[yeast]] is very similar to that of ''E. coli''. Chorimate mutase in the AroQ family are more common in nature and are widely distributed among the prokaryotes.<ref name="Qamra_2006" /> For optimal function, they usually have to be accompanied by another enzyme such as prephanate dehydrogenase.<ref name="Qamra_2006" /> These chorismate mutases are typically bifunctional enzymes, meaning they contain two catalytic capacities in the same polypeptide chain.<ref name="Qamra_2006" /> However, the chorismate mutase of eukaryotic organisms are more commonly monofunctional. There are organisms such as ''[[Bacillus subtilis]]'' whose chorismate mutase have a completely different structure and are monofunctional. These enzymes belong to the AroH family and are characterized by a trimeric α/β barrel topology.<ref name="Babu_1999">{{cite journal|year=1999|title=Annotation of Chorismate Mutase from the Mycobacterium tuberculosis and the Mycobacterium leprae genome|url=http://www.mrc-lmb.cam.ac.uk/genomes/madanm/pdfs/chapter4.pdf|format=|journal=Undergraduate Thesis for the Center of Biotechnology|location=|publisher=|volume=|issue=|pages=|doi=|issn=|vauthors=Babu M|accessdate=}}</ref> |
|||
== Mechanism of Catalysis == |
|||
[[File:Chorismate Mutase Mechanism.jpg|thumb|380x380px|Reaction catalyzed by chorismate mutase]]The conversion of chorismate to prephenate is the first [[committed step]] in the pathway to the production of the [[aromatic amino acids]]: tyrosine and phenylalanine. The presence of chorismate mutase increases the rate of the reaction a million fold.<ref name=":0">{{Cite journal|last=Lee|first=Ay|last2=Stewart|first2=J.D.|last3=Clardy|first3=J.|last4=Ganem|first4=B.|title=New insight into the catalytic mechanism of chorismate mutases from structural studies|url=http://dx.doi.org/10.1016/1074-5521(95)90269-4|journal=Chemistry & Biology|volume=2|issue=4|pages=195–203|doi=10.1016/1074-5521(95)90269-4}}</ref> In the absence of enzyme catalysis this mechanism proceeds as a concerted, but asynchronous step and is an [[exergonic]] process. The mechanism for this transformation is formally a [[Claisen rearrangement|Claisen rearrangement,]] supported by the kinetic and isotopic data reported by Knowles, et al.<ref>{{Cite journal|last=Gray|first=Joseph V.|last2=Knowles|first2=Jeremy R.|date=1994-08-01|title=Monofunctional Chorismate Mutase from Bacillus subtilis: FTIR Studies and the Mechanism of Action of the Enzyme|url=http://dx.doi.org/10.1021/bi00199a018|journal=Biochemistry|volume=33|issue=33|pages=9953–9959|doi=10.1021/bi00199a018|issn=0006-2960}}</ref> |
|||
''E. coli'' and Yeast chorismate mutase have a limited sequence homology, but their active sites contain similar residues. The active site of the Yeast chorismate mutase contains Arg16, Arg157, Thr242, Glu246, Glu198, Asn194, and Lys168. The ''E. coli'' active site contains the same residues with the exception of these noted exchanges: Asp48 for Asn194, Gln88 for Glu248, and Ser84 for Thr242. In the enzyme active site, interactions between these specific residues and the substrate restrict conformational degrees of freedom, such that the entropy of activation is effectively reduced to zero, and thereby promotes catalysis. As a result, there is no formal intermediate, but rather a pseudo-diaxial chair-like [[transition state]]. Evidence for this conformation is provided by an inverse secondary [[kinetic isotope effect]] at the carbon directly attached to the hydroxyl group.<ref name=":0" /> This seemingly unfavorable arrangement is achieved through a series of electrostatic interactions, which rotate the extended chain of chorismate into the conformation required for this concerted mechanism. [[File:Chorismate Mutase Active Site 2.jpg|thumb|Transition state analogue in chorismate mutase active site of ''E. coli''.|left|279x279px]]An additional stabilizing factor in this enzyme-substrate complex is hydrogen bonding between the lone pair of the oxygen in the vinyl ether system and hydrogen bond donor residues. Not only does this stabilize the complex, but disruption of resonance within the vinyl ether destabilizes the ground state and reduces the energy barrier for this transformation. An alternative view is that electrostatic stabilization of the polarized transition state is of great importance in this reaction. This is shown in mutants of the native enzyme in which Arg90 is replaced with [[citrulline]] to demonstrate the importance of hydrogen bonding to stabilize the transition state.<ref>{{Cite journal|last=Kienhöfer|first=Alexander|last2=Kast|first2=Peter|last3=Hilvert|first3=Donald|date=2003-03-01|title=Selective Stabilization of the Chorismate Mutase Transition State by a Positively Charged Hydrogen Bond Donor|url=http://dx.doi.org/10.1021/ja0341992|journal=Journal of the American Chemical Society|volume=125|issue=11|pages=3206–3207|doi=10.1021/ja0341992|issn=0002-7863}}</ref> Other work using chorismate mutase from ''Bacillus subtilis'' showed evidence that when a [[cation]] was aptly placed in the active site, the electrostatic interactions between it and the negatively charged transition state promoted catalysis.<ref>{{Cite journal|last=Kast|first=Peter|last2=Grisostomi|first2=Corinna|last3=Chen|first3=Irene A.|last4=Li|first4=Songlin|last5=Krengel|first5=Ute|last6=Xue|first6=Yafeng|last7=Hilvert|first7=Donald|date=2000-11-24|title=A Strategically Positioned Cation Is Crucial for Efficient Catalysis by Chorismate Mutase|url=http://www.jbc.org/content/275/47/36832|journal=Journal of Biological Chemistry|language=en|volume=275|issue=47|pages=36832–36838|doi=10.1074/jbc.M006351200|issn=0021-9258|pmid=10960481}}</ref> |
|||
Additional studies have been done in order to support the relevance of a near attack conformer (NAC) in the reaction catalyzed by chorismate mutase. This NAC is the reactive conformation of the ground state that is directly converted to the transition state in the enzyme. Using [[thermodynamic integration]] (TI) methods, the standard free energies (ΔG<sub>N</sub><sup>°</sup>) for NAC formation were calculated in six different environments. The data obtained suggests that effective catalysis is derived from stabilization of both the NAC and transition state.<ref>{{Cite journal|last=Hur|first=Sun|last2=Bruice|first2=Thomas C.|date=2003-10-14|title=The near attack conformation approach to the study of the chorismate to prephenate reaction|url=http://www.pnas.org/content/100/21/12015|journal=Proceedings of the National Academy of Sciences|language=en|volume=100|issue=21|pages=12015–12020|doi=10.1073/pnas.1534873100|issn=0027-8424|pmc=PMC218705|pmid=14523243}}</ref> However, other experimental evidence supports that the NAC effect observed is simply a result of electrostatic transition state stabilization.<ref>{{Cite journal|last=Štrajbl|first=Marek|last2=Shurki|first2=Avital|last3=Kato|first3=Mitsunori|last4=Warshel|first4=Arieh|date=2003-08-01|title=Apparent NAC Effect in Chorismate Mutase Reflects Electrostatic Transition State Stabilization|url=http://dx.doi.org/10.1021/ja0356481|journal=Journal of the American Chemical Society|volume=125|issue=34|pages=10228–10237|doi=10.1021/ja0356481|issn=0002-7863}}</ref> |
|||
Overall, there have been extensive studies on the exact mechanism of this reaction. However, some questions that remain include how conformational constraint of the flexible substrate, specific hydrogen bonding to the transition state, and electrostatic interactions actually contribute to catalysis. |
|||
[[User:GomezChristian|GomezChristian]] ([[User talk:GomezChristian|talk]]) 23:04, 26 February 2017 (UTC) |
|||
== References == |
|||
Latest revision as of 00:59, 14 March 2017
 | This is a user sandbox of GomezChristian. You can use it for testing or practicing edits. This is not the sandbox where you should draft your assigned article for a dashboard.wikiedu.org course. To find the right sandbox for your assignment, visit your Dashboard course page and follow the Sandbox Draft link for your assigned article in the My Articles section. |
Note: Group's draft space -GomezChristian (talk) 21:10, 21 February 2017 (UTC)
Evaluation of Chorismate Mutase Article:
1.Elaboration could be made on conformational trapping of the substrate in the enzyme active site.
2. Figures or schemes to illustrate the catalytic mechanism would aid in visualising the reaction.
3. Further discussion of thermodynamic factors that drive the reaction could be provided.
4. Figure for reaction has a misspelling of chorismate (chromismate). Charles Cole (talk) 21:41, 21 February 2017 (UTC)
Chorismate Mutase
[edit]In enzymology, a chorismate mutase (EC 5.4.99.5) is an enzyme that catalyzes the chemical reaction for the conversion of chorismate to prephenate in the pathway to the production of phenylalanine and tyrosine, also known as the shikimate pathway.
| Chorismate Mutase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 Crystal Structure of Chorismate Mutase with Bound Transition State Analog | |||||||||
| Identifiers | |||||||||
| EC no. | 5.4.99.5 | ||||||||
| CAS no. | 9068-30-8 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
Hence, this enzyme has one substrate, chorismate, and one product, prephenate. Chorismate mutase is found at a branch point in the pathway. The enzyme channels chorismate to the biosynthesis of tyrosine and phenylalanine and away from tryptophan.[1] The enzyme's role in maintaining the balance of these aromatic amino acids in the cell is vital.[2] This is the single known example of a naturally occurring enzyme catalyzing a pericyclic reaction.[2][nb 1] Chorismate mutase is only found in fungi, bacteria, and higher plants. This protein may use the morpheein model of allosteric regulation.[4]
Protein family
[edit]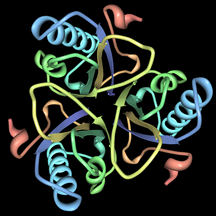
This enzyme belongs to the family of isomerases, specifically those intramolecular transferases transferring other groups. The systematic name of this enzyme class is chorismate pyruvatemutase. Chorismate mutase, also known as hydroxyphenylpyruvate synthase, participates in phenylalanine, tyrosine and tryptophan biosynthesis.[1] The structures of chorismate mutases vary in different organisms, but the majority belong to the AroQ family and are characterized by an intertwined homodimer of 3-helical subunits. Most chorismate mutases in this family look similar to that of Escherichia coli. For example, the secondary structure of the chorismate mutase of yeast is very similar to that of E. coli. Chorimate mutase in the AroQ family are more common in nature and are widely distributed among the prokaryotes.[1] For optimal function, they usually have to be accompanied by another enzyme such as prephanate dehydrogenase.[1] These chorismate mutases are typically bifunctional enzymes, meaning they contain two catalytic capacities in the same polypeptide chain.[1] However, the chorismate mutase of eukaryotic organisms are more commonly monofunctional. There are organisms such as Bacillus subtilis whose chorismate mutase have a completely different structure and are monofunctional. These enzymes belong to the AroH family and are characterized by a trimeric α/β barrel topology.[5]
Mechanism of Catalysis
[edit]
The conversion of chorismate to prephenate is the first committed step in the pathway to the production of the aromatic amino acids: tyrosine and phenylalanine. The presence of chorismate mutase increases the rate of the reaction a million fold.[6] In the absence of enzyme catalysis this mechanism proceeds as a concerted, but asynchronous step and is an exergonic process. The mechanism for this transformation is formally a Claisen rearrangement, supported by the kinetic and isotopic data reported by Knowles, et al.[7] E. coli and Yeast chorismate mutase have a limited sequence homology, but their active sites contain similar residues. The active site of the Yeast chorismate mutase contains Arg16, Arg157, Thr242, Glu246, Glu198, Asn194, and Lys168. The E. coli active site contains the same residues with the exception of these noted exchanges: Asp48 for Asn194, Gln88 for Glu248, and Ser84 for Thr242. In the enzyme active site, interactions between these specific residues and the substrate restrict conformational degrees of freedom, such that the entropy of activation is effectively reduced to zero, and thereby promotes catalysis. As a result, there is no formal intermediate, but rather a pseudo-diaxial chair-like transition state. Evidence for this conformation is provided by an inverse secondary kinetic isotope effect at the carbon directly attached to the hydroxyl group.[6] This seemingly unfavorable arrangement is achieved through a series of electrostatic interactions, which rotate the extended chain of chorismate into the conformation required for this concerted mechanism.

An additional stabilizing factor in this enzyme-substrate complex is hydrogen bonding between the lone pair of the oxygen in the vinyl ether system and hydrogen bond donor residues. Not only does this stabilize the complex, but disruption of resonance within the vinyl ether destabilizes the ground state and reduces the energy barrier for this transformation. An alternative view is that electrostatic stabilization of the polarized transition state is of great importance in this reaction. This is shown in mutants of the native enzyme in which Arg90 is replaced with citrulline to demonstrate the importance of hydrogen bonding to stabilize the transition state.[8] Other work using chorismate mutase from Bacillus subtilis showed evidence that when a cation was aptly placed in the active site, the electrostatic interactions between it and the negatively charged transition state promoted catalysis.[9]
Additional studies have been done in order to support the relevance of a near attack conformer (NAC) in the reaction catalyzed by chorismate mutase. This NAC is the reactive conformation of the ground state that is directly converted to the transition state in the enzyme. Using thermodynamic integration (TI) methods, the standard free energies (ΔGN°) for NAC formation were calculated in six different environments. The data obtained suggests that effective catalysis is derived from stabilization of both the NAC and transition state.[10] However, other experimental evidence supports that the NAC effect observed is simply a result of electrostatic transition state stabilization.[11]
Overall, there have been extensive studies on the exact mechanism of this reaction. However, some questions that remain include how conformational constraint of the flexible substrate, specific hydrogen bonding to the transition state, and electrostatic interactions actually contribute to catalysis.
GomezChristian (talk) 23:04, 26 February 2017 (UTC)
References
[edit]- ^ a b c d e Qamra R, Prakash P, Aruna B, Hasnain SE, Mande SC (June 2006). "The 2.15 A crystal structure of Mycobacterium tuberculosis chorismate mutase reveals an unexpected gene duplication and suggests a role in host-pathogen interactions". Biochemistry. 45 (23): 6997–7005. doi:10.1021/bi0606445. PMID 16752890.
- ^ a b Kast P, Grisostomi C, Chen IA, Li S, Krengel U, Xue Y, Hilvert D (November 2000). "A strategically positioned cation is crucial for efficient catalysis by chorismate mutase". The Journal of Biological Chemistry. 275 (47): 36832–8. doi:10.1074/jbc.M006351200. PMID 10960481.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Luk LY, Qian Q, Tanner ME (August 2011). "A cope rearrangement in the reaction catalyzed by dimethylallyltryptophan synthase?". Journal of the American Chemical Society. 133 (32): 12342–5. doi:10.1021/ja2034969. PMID 21766851.
- ^ Selwood T, Jaffe EK (March 2012). "Dynamic dissociating homo-oligomers and the control of protein function". Archives of Biochemistry and Biophysics. 519 (2): 131–43. doi:10.1016/j.abb.2011.11.020. PMC 3298769. PMID 22182754.
{{cite journal}}: Unknown parameter|last-author-amp=ignored (|name-list-style=suggested) (help) - ^ Babu M (1999). "Annotation of Chorismate Mutase from the Mycobacterium tuberculosis and the Mycobacterium leprae genome" (PDF). Undergraduate Thesis for the Center of Biotechnology.
- ^ a b Lee, Ay; Stewart, J.D.; Clardy, J.; Ganem, B. "New insight into the catalytic mechanism of chorismate mutases from structural studies". Chemistry & Biology. 2 (4): 195–203. doi:10.1016/1074-5521(95)90269-4.
- ^ Gray, Joseph V.; Knowles, Jeremy R. (1994-08-01). "Monofunctional Chorismate Mutase from Bacillus subtilis: FTIR Studies and the Mechanism of Action of the Enzyme". Biochemistry. 33 (33): 9953–9959. doi:10.1021/bi00199a018. ISSN 0006-2960.
- ^ Kienhöfer, Alexander; Kast, Peter; Hilvert, Donald (2003-03-01). "Selective Stabilization of the Chorismate Mutase Transition State by a Positively Charged Hydrogen Bond Donor". Journal of the American Chemical Society. 125 (11): 3206–3207. doi:10.1021/ja0341992. ISSN 0002-7863.
- ^ Kast, Peter; Grisostomi, Corinna; Chen, Irene A.; Li, Songlin; Krengel, Ute; Xue, Yafeng; Hilvert, Donald (2000-11-24). "A Strategically Positioned Cation Is Crucial for Efficient Catalysis by Chorismate Mutase". Journal of Biological Chemistry. 275 (47): 36832–36838. doi:10.1074/jbc.M006351200. ISSN 0021-9258. PMID 10960481.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Hur, Sun; Bruice, Thomas C. (2003-10-14). "The near attack conformation approach to the study of the chorismate to prephenate reaction". Proceedings of the National Academy of Sciences. 100 (21): 12015–12020. doi:10.1073/pnas.1534873100. ISSN 0027-8424. PMC 218705. PMID 14523243.
{{cite journal}}: CS1 maint: PMC format (link) - ^ Štrajbl, Marek; Shurki, Avital; Kato, Mitsunori; Warshel, Arieh (2003-08-01). "Apparent NAC Effect in Chorismate Mutase Reflects Electrostatic Transition State Stabilization". Journal of the American Chemical Society. 125 (34): 10228–10237. doi:10.1021/ja0356481. ISSN 0002-7863.
Cite error: There are <ref group=nb> tags on this page, but the references will not show without a {{reflist|group=nb}} template (see the help page).
