Cell wall: Difference between revisions
i fixed evrything Tag: repeating characters |
Citation bot (talk | contribs) Removed parameters. | Use this bot. Report bugs. | #UCB_CommandLine |
||
| (338 intermediate revisions by more than 100 users not shown) | |||
| Line 1: | Line 1: | ||
{{Short description|Outermost layer of some cells}} |
|||
A '''cell wall''' is not a structural layer surrounding some types of [[cell (biology)|cell]]s, situated outside the [[cell membrane]]. It can be tough, flexible, and sometimes rigid. It provides the cell with both structural support and protection, and also acts as a filtering mechanism. Cell walls are present in most [[prokaryote]]s (except [[mycoplasma]] bacteria), in [[algae]], [[plant]]s and [[fungi]] but rarely in other [[eukaryote]]s including animals. A major function is to act as pressure vessels, preventing [[Cytolysis|over-expansion]] of the cell when water enters. |
|||
{{pp-vandalism|small=yes}} |
|||
{{Cell biology|plantcell=yes}} |
|||
A '''cell wall''' is a structural layer that surrounds some [[Cell type|cell types]], found immediately outside the [[cell membrane]]. It can be tough, flexible, and sometimes rigid. Primarily, it provides the cell with structural support, shape, protection, and functions as a selective barrier.<ref name="Romaniuk">{{cite journal | vauthors = Romaniuk JA, Cegelski L | title = Bacterial cell wall composition and the influence of antibiotics by cell-wall and whole-cell NMR | journal = Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences | volume = 370 | issue = 1679 | pages = 20150024 | date = October 2015 | pmid = 26370936 | pmc = 4632600 | doi = 10.1098/rstb.2015.0024 }}</ref> Another vital role of the cell wall is to help the cell withstand [[osmotic pressure]] and mechanical stress. While absent in many [[eukaryotes]], including animals, cell walls are prevalent in other organisms such as [[fungi]], [[algae]] and [[plants]], and are commonly found in most [[Prokaryote|prokaryotes]], with the exception of [[Mollicutes|mollicute]] bacteria. |
|||
The composition of cell walls varies |
The composition of cell walls varies across [[taxonomic groups]], [[species]], cell type, and the [[cell cycle]]. In [[Embryophyte|land plants]], the primary cell wall comprises [[Polysaccharide|polysaccharides]] like [[cellulose]], [[hemicellulose]]s, and [[pectin]]. Often, other [[Polymer|polymers]] such as [[lignin]], [[suberin]] or [[cutin]] are anchored to or embedded in plant cell walls. [[Algae]] exhibit cell walls composed of [[Glycoprotein|glycoproteins]] and [[Polysaccharide|polysaccharides]], such as [[carrageenan]] and [[agar]], distinct from those in land plants. Bacterial cell walls contain [[peptidoglycan]], while [[archaeal]] cell walls vary in composition, potentially consisting of glycoprotein [[S-layer]]s, [[pseudopeptidoglycan]], or polysaccharides. Fungi possess cell walls constructed from the polymer [[chitin]], specifically [[N-acetylglucosamine]]. [[Diatom|diatoms]] have a unique cell wall composed of [[biogenic silica]].<ref>{{cite book |last1=Rutledge |first1=Ryan D. |last2=Wright |first2=David W. | chapter = Biomineralization: Peptide-Mediated Synthesis of Materials | chapter-url = https://books.google.com/books?id=8y-CKE8_Bi0C&pg=RA2-PT52 | veditors = Lukehart CM, Scott RA | title=Nanomaterials: Inorganic and Bioinorganic Perspectives | publisher=Wiley | series=EIC Books | year=2013 | isbn=978-1-118-62522-4 |access-date=2016-03-14}}</ref> |
||
==History== |
==History== |
||
A plant cell wall was first observed and named (simply as a "wall") by [[Robert Hooke]] in 1665.<ref>Hooke |
A plant cell wall was first observed and named (simply as a "wall") by [[Robert Hooke]] in 1665.<ref>{{cite book | vauthors = Hooke R | date = 1665 | title = Micrographia: or, Some physiological descriptions of minute bodies made by magnifying glasses | location = London | veditors = Martyn J, Allestry J | url = http://www.gutenberg.org/ebooks/15491 }}</ref> However, "the dead excrusion product of the living protoplast" was forgotten, for almost three centuries, being the subject of scientific interest mainly as a resource for industrial processing or in relation to animal or human health.<ref name="Sattelmacher_2000">{{cite journal | vauthors = Sattelmacher B | year = 2000 | title = The apoplast and its significance for plant mineral nutrition | journal = New Phytologist | volume = 149 | issue = 2| pages = 167–192 | doi=10.1046/j.1469-8137.2001.00034.x| pmid = 33874640 | doi-access = free }}</ref> |
||
In 1804, [[Karl Rudolphi]] and [[Johann Heinrich Friedrich Link|J.H.F. Link]] proved that cells had independent |
In 1804, [[Karl Rudolphi]] and [[Johann Heinrich Friedrich Link|J.H.F. Link]] proved that cells had independent cell walls.<ref>{{cite book | vauthors = Link HF | title = Grundlehren der anatomie und physiologie der pflanzen. | publisher = Danckwerts | date = 1807 | url = http://www.mathnat.uni-rostock.de/geschichte/kalenderblatt/kalenderblatt-dezember-2013/ }}</ref><ref>{{cite journal | vauthors = Baker JR | title = The Cell-Theory: A Restatement, History, and Critique: Part III. The Cell as a Morphological Unit. | journal = Journal of Cell Science | date = June 1952 | volume = 3 | issue = 22 | pages = 157–90 | doi = 10.1242/jcs.s3-93.22.157 | url = http://paperity.org/p/46837964/the-cell-theory-a-restatement-history-and-critique-part-iii-the-cell-as-a-morphological }}</ref> Before, it had been thought that cells shared walls and that fluid passed between them this way. |
||
(Danckwerts), [https://archive.org/details/grundlehrendera01linkgoog].</ref><ref>Baker, J. R. 1952. The cell-theory: a restatement, history, and critique. Part III. The cell as a morphological unit. ''Quart. J. Microscop. Sci.'' 93: 157-190, [http://paperity.org/p/46837964/the-cell-theory-a-restatement-history-and-critique-part-iii-the-cell-as-a-morphological].</ref> Before, it had been thought that cells shared walls and that fluid passed between them this way. |
|||
The mode of formation of the cell wall was controversial in the 19th century. [[Hugo von Mohl]] (1853, 1858) advocated the idea that the cell wall grows by apposition. [[Carl Nägeli]] (1858, 1862, 1863) believed that the growth of the wall in thickness and in area was due to a process termed intussusception. Each theory was improved in the following decades: the apposition (or lamination) theory by [[Eduard Strasburger]] (1882, 1889), and the intussusception theory by [[Julius Wiesner]] (1886).<ref>Sharp |
The mode of formation of the cell wall was controversial in the 19th century. [[Hugo von Mohl]] (1853, 1858) advocated the idea that the cell wall grows by apposition. [[Carl Nägeli]] (1858, 1862, 1863) believed that the growth of the wall in thickness and in area was due to a process termed intussusception. Each theory was improved in the following decades: the apposition (or lamination) theory by [[Eduard Strasburger]] (1882, 1889), and the intussusception theory by [[Julius Wiesner]] (1886).<ref>{{cite book | vauthors = Sharp LW | date = 1921 | url = https://archive.org/details/introductiontocy032473mbp | title = Introduction To Cytology | location = New York | publisher = McGraw Hill | page = [https://archive.org/details/introductiontocy032473mbp/page/n38 25] }}</ref> |
||
In 1930, [[Ernst Münch]] coined the term ''[[apoplast]]'' in order to separate the "living" [[symplast]] from the "dead" plant region, the latter of which included the cell wall.<ref>Münch |
In 1930, [[Ernst Münch]] coined the term ''[[apoplast]]'' in order to separate the "living" [[symplast]] from the "dead" plant region, the latter of which included the cell wall.<ref>{{cite book | vauthors = Münch E | date = 1930 | title = Die Stoffbewegungen in der Pflanze | publisher = Verlag von Gustav Fischer | location = Jena }}</ref> |
||
By the 1980s, some authors suggested replacing the term "cell wall", particularly as it was used for plants, with the more precise term "[[extracellular matrix]]", as used for animal cells,<ref>Roberts |
By the 1980s, some authors suggested replacing the term "cell wall", particularly as it was used for plants, with the more precise term "[[extracellular matrix]]", as used for animal cells,<ref>{{cite journal | vauthors = Roberts K | title = The plant extracellular matrix: in a new expansive mood | journal = Current Opinion in Cell Biology | volume = 6 | issue = 5 | pages = 688–94 | date = October 1994 | pmid = 7833049 | doi = 10.1016/0955-0674(89)90074-4 }}</ref><ref name="Sattelmacher_2000" />{{rp|168}} but others preferred the older term.<ref>{{cite book | vauthors = Evert RF | date = 2006 | title = Esau's Plant Anatomy: Meristems, Cells, and Tissues of the Plant Body: Their Structure, Function, and Development | edition = 3rd | publisher = John Wiley & Sons, Inc | location = Hoboken, New Jersey | pages = 65–66 | url = https://books.google.com/books?id=0DhEBA5xgbkC | isbn = 978-0-470-04737-8 }}</ref> |
||
==Properties== |
==Properties== |
||
{{more citations needed section|date=November 2017}} |
|||
[[File:Eukaryota cell strucutre.PNG|thumb |
[[File:Eukaryota cell strucutre.PNG|thumb|right|Diagram of the plant cell, with the cell wall in green.]] |
||
Cell walls serve similar purposes in those organisms that possess them. They may give cells rigidity and strength, offering protection against mechanical stress. In multicellular organisms, they permit the organism to build and hold a definite shape |
Cell walls serve similar purposes in those organisms that possess them. They may give cells rigidity and strength, offering protection against mechanical stress. The chemical composition and mechanical properties of the cell wall are linked with plant cell growth and [[morphogenesis]].<ref name="bid">{{cite journal | vauthors = Bidhendi AJ, Geitmann A | title = Relating the mechanics of the primary plant cell wall to morphogenesis | journal = Journal of Experimental Botany | volume = 67 | issue = 2 | pages = 449–61 | date = January 2016 | pmid = 26689854 | doi = 10.1093/jxb/erv535 | url = https://academic.oup.com/jxb/article/67/2/449/2884961 | doi-access = free }}</ref> In multicellular organisms, they permit the organism to build and hold a definite shape. Cell walls also limit the entry of large molecules that may be toxic to the cell. They further permit the creation of stable [[osmotic]] environments by preventing [[Cytolysis|osmotic lysis]] and helping to retain water. Their composition, properties, and form may change during the [[cell cycle]] and depend on growth conditions.<ref name=bid/> |
||
===Rigidity of cell walls=== |
===Rigidity of cell walls=== |
||
In most cells, the cell wall is flexible, meaning that it will bend rather than holding a fixed shape, but has considerable [[tensile strength]]. The apparent rigidity of primary plant tissues is enabled by cell walls, but is not due to the walls' stiffness. Hydraulic [[turgor pressure]] creates this rigidity, along with the wall structure. The flexibility of the cell walls is seen when plants wilt, so that the stems and leaves begin to droop, or in [[seaweed]]s that bend in [[Ocean current|water current]]s. As John Howland explains |
In most cells, the cell wall is flexible, meaning that it will bend rather than holding a fixed shape, but has considerable [[tensile strength]]. The apparent rigidity of primary plant tissues is enabled by cell walls, but is not due to the walls' stiffness. Hydraulic [[turgor pressure]] creates this rigidity, along with the wall structure. The flexibility of the cell walls is seen when plants wilt, so that the stems and leaves begin to droop, or in [[seaweed]]s that bend in [[Ocean current|water current]]s. As John Howland explains |
||
{{ |
{{Blockquote|Think of the cell wall as a wicker basket in which a balloon has been inflated so that it exerts pressure from the inside. Such a basket is very rigid and resistant to mechanical damage. Thus does the prokaryote cell (and eukaryotic cell that possesses a cell wall) gain strength from a flexible plasma membrane pressing against a rigid cell wall.<ref name="Howland 2000">{{cite book| last = Howland | first = John L. | name-list-style = vanc | year = 2000 | title = The Surprising Archaea: Discovering Another Domain of Life | pages = 69–71 | publisher = Oxford University Press | location = Oxford | isbn = 978-0-19-511183-5}}</ref>}} |
||
The apparent rigidity of the cell wall thus results from inflation of the cell contained within. This [[turgor pressure|inflation]] is a result of the [[osmosis|passive uptake of water]]. |
The apparent rigidity of the cell wall thus results from inflation of the cell contained within. This [[turgor pressure|inflation]] is a result of the [[osmosis|passive uptake of water]]. |
||
In plants, a '''secondary cell wall''' is a thicker additional layer of cellulose which increases wall rigidity. Additional layers may be formed by [[lignin]] in [[xylem]] cell walls, or [[suberin]] in [[cork cambium|cork]] cell walls. These compounds are [[Structural rigidity|rigid]] and [[waterproof]], making the secondary wall stiff. Both [[wood]] and [[bark]] cells of [[tree]]s have secondary walls. Other parts of plants such as the [[petiole (botany)|leaf stalk]] may acquire similar reinforcement to resist the strain of physical forces. |
In plants, a '''secondary cell wall''' is a thicker additional layer of cellulose which increases wall rigidity. Additional layers may be formed by [[lignin]] in [[xylem]] cell walls, or [[suberin]] in [[cork cambium|cork]] cell walls. These compounds are [[Structural rigidity|rigid]] and [[waterproof]], making the secondary wall stiff. Both [[wood]] and [[Bark (botany)|bark]] cells of [[tree]]s have secondary walls. Other parts of plants such as the [[petiole (botany)|leaf stalk]] may acquire similar reinforcement to resist the strain of physical forces. |
||
===Permeability=== |
===Permeability=== |
||
The primary cell wall of most [[plant cell]]s is freely permeable to small molecules including small proteins, with size exclusion estimated to be 30-60 [[Atomic mass unit|kDa]].{{ |
The primary cell wall of most [[plant cell]]s is freely permeable to small molecules including small [[Protein|proteins]], with size exclusion estimated to be 30-60 [[Atomic mass unit|kDa]].<ref>{{cite book |author1=Harvey Lodish |author2=Arnold Berk |author3=Chris A. Kaiser |author4=Monty Krieger |author5=Matthew P. Scott |author6=Anthony Bretscher |author7=Hidde Ploegh |author8=Paul Matsudaira |title=Loose-leaf Version for Molecular Cell Biology |url=https://books.google.com/books?id=0bUVAAAAQBAJ |date=1 September 2012|publisher=W. H. Freeman|isbn=978-1-4641-2746-5}}</ref> The pH is an important factor governing the transport of molecules through cell walls.<ref>{{cite book | first = C. Michael | last = Hogan | date = 2010 | chapter-url = http://www.eoearth.org/article/Abiotic_factor?topic=49461 | chapter = Abiotic factor | title = Encyclopedia of Earth | veditors = Monosson E, Cleveland C | publisher = National Council for Science and the Environment | location = Washington DC | archive-url = https://web.archive.org/web/20130608071757/http://www.eoearth.org/article/Abiotic_factor?topic=49461 | archive-date = 2013-06-08 }}</ref> |
||
==Evolution== |
==Evolution== |
||
{{Expand section|date=October 2013}} |
{{Expand section|date=October 2013}} |
||
Cell walls evolved independently in many groups. |
|||
Cell walls evolved independently in many groups, even in the photosynthetic eukaryotes. In these lineages, the cell wall is closely related to the evolution of [[multicellularity]], terrestrialization and vascularization.<ref name=popper>{{cite journal |first1=Zoe A. |last=Popper |first2=Gurvan |last2=Michel |first3=Cecile |last3=Hervé |first4=David S. |last4=Domozych |first5=William G.T. |last5=Willats |first6=Maria G. |last6=Tuohy |first7=Bernard |last7=Kloareg |first8=Dagmar B. |last8=Stengel |title=Evolution and diversity of plant cell walls: from algae to flowering plants |journal=Annual Review of Plant Biology |date=2011 |volume=62 |pages=567–590 |url=http://public.wsu.edu/~lange-m/Documnets/Teaching2011/Popper2011.pdf }}</ref> |
|||
The [[photosynthesis|photosynthetic]] [[eukaryote]]s (so-called plant and algae) is one group with cellulose cell walls, where the cell wall is closely related to the evolution of [[multicellularity]], terrestrialization and vascularization. The [[Cellulose synthase (UDP-forming)|CesA cellulose synthase]] evolved in ''[[Cyanobacteria]]'' and was part of [[Archaeplastida]] since [[Symbiogenesis|endosymbiosis]]; [[secondary endosymbiosis]] events transferred it (with the [[arabinogalactan]] proteins) further into [[brown algae]] and [[oomycetes]]. Plants later evolved various genes from CesA, including the Csl (cellulose synthase-like) family of proteins and additional Ces proteins. Combined with the various glycosyltransferases (GT), they enable more complex chemical structures to be built.<ref name=popper>{{cite journal | vauthors = Popper ZA, Michel G, Hervé C, Domozych DS, Willats WG, Tuohy MG, Kloareg B, Stengel DB | s2cid = 11961888 | display-authors = 6 | title = Evolution and diversity of plant cell walls: from algae to flowering plants | journal = Annual Review of Plant Biology | volume = 62 | pages = 567–90 | date = 2011 | pmid = 21351878 | doi = 10.1146/annurev-arplant-042110-103809 | hdl = 10379/6762 | hdl-access = free }}</ref><!-- try 10.3389/fpls.2012.00152 --> |
|||
Fungi use a [[Chitin-glucan complex|chitin-glucan-protein]] cell wall.<ref name="Webster_2007" /> They share the 1,3-β-glucan synthesis pathway with plants, using homologous GT48 family [[1,3-Beta-glucan synthase]]s to perform the task, suggesting that such an enzyme is very ancient within the eukaryotes. Their glycoproteins are rich in [[mannose]]. The cell wall might have evolved to deter viral infections. Proteins embedded in cell walls are variable, contained in [[tandem repeat]]s subject to [[homologous recombination]].<ref>{{cite journal | vauthors = Xie X, Lipke PN | title = On the evolution of fungal and yeast cell walls | journal = Yeast | volume = 27 | issue = 8 | pages = 479–88 | date = August 2010 | pmid = 20641026 | pmc = 3074402 | doi = 10.1002/yea.1787 }}</ref> An alternative scenario is that fungi started with a [[chitin]]-based cell wall and later acquired the GT-48 enzymes for the 1,3-β-glucans via [[horizontal gene transfer]]. The pathway leading to 1,6-β-glucan synthesis is not sufficiently known in either case.<ref>{{cite journal | vauthors = Ruiz-Herrera J, Ortiz-Castellanos L | title = Analysis of the phylogenetic relationships and evolution of the cell walls from yeasts and fungi | journal = FEMS Yeast Research | volume = 10 | issue = 3 | pages = 225–43 | date = May 2010 | pmid = 19891730 | doi = 10.1111/j.1567-1364.2009.00589.x | doi-access = free }}</ref> |
|||
==Plant cell walls== |
==Plant cell walls== |
||
The walls of plant cells must have sufficient tensile strength to withstand internal [[osmotic pressure]]s of several times [[atmospheric pressure]] that result from the difference in solute concentration between the cell interior and external solutions.<ref |
The walls of plant cells must have sufficient tensile strength to withstand internal [[osmotic pressure]]s of several times [[atmospheric pressure]] that result from the difference in solute concentration between the cell interior and external solutions.<ref name=Romaniuk/> Plant cell walls vary from 0.1 to several μm in thickness.<ref>{{Cite book | title = Biology | url = https://archive.org/details/essentialbiology00camp_0 | url-access = registration | last1 = Campbell | last2 = Reece | last3 = Urry | last4 = Cain | last5 = Wasserman | last6 = Minorsky | last7 = Jackson | first1 = Neil A. | first2 = Jane B. | first3 = Lisa A. | first4 = Michael L. | first5 = Steven A. | first6 = Peter V. | first7 = Robert B. | name-list-style = vanc | edition = 8th | isbn = 978-0-8053-6844-4 | pages = [https://archive.org/details/essentialbiology00camp_0/page/118 118] | year = 2008 | publisher = Pearson Benjamin Cummings }}</ref> |
||
title=Biology| |
|||
last1=Campbell| |
|||
last2=Reece| |
|||
last3=Urry| |
|||
last4=Cain| |
|||
last5=Wasserman| |
|||
last6=Minorsky| |
|||
last7=Jackson| |
|||
first1=Neil A.| |
|||
first2=Jane B.| |
|||
first3=Lisa A.| |
|||
first4=Michael L.| |
|||
first5=Steven A.| |
|||
first6=Peter V.| |
|||
first7=Robert B.| |
|||
edition=8th| |
|||
isbn=978-0-8053-6844-4| |
|||
page=118| |
|||
year=2008}}</ref> |
|||
===Layers=== |
===Layers=== |
||
| ⚫ | |||
[[File:Plant Cell Wall.png|thumb|Cell wall in multicellular plants – its different layers and their placement with respect to protoplasm (highly diagrammatic)]] |
|||
| ⚫ | |||
| ⚫ | |||
| ⚫ | Up to three strata or layers may be found in plant cell walls:<ref>{{cite book | first1 = Bob B | last1 = Buchanan | first2 = Wilhelm | last2 = Gruissem | first3 = Russell L | last3 = Jones | name-list-style = vanc | title = Biochemistry & molecular biology of plants | edition = 1st | publisher = American society of plant physiology | year = 2000 | isbn = 978-0-943088-39-6 | url-access = registration | url = https://archive.org/details/biochemistrymole00buch }}</ref> |
||
*The '''primary cell wall''', generally a thin, flexible and extensible layer formed while the cell is growing. |
*The '''primary cell wall''', generally a thin, flexible and extensible layer formed while the cell is growing. |
||
| Line 65: | Line 57: | ||
===Composition=== |
===Composition=== |
||
In the primary (growing) plant cell wall, the major [[carbohydrate]]s are [[cellulose]], [[hemicellulose]] and [[pectin]]. The cellulose [[microfibril]]s are linked via hemicellulosic tethers to form the cellulose-hemicellulose network, which is embedded in the pectin matrix. The most common hemicellulose in the primary cell wall is [[xyloglucan]]. In grass cell walls, xyloglucan and pectin are reduced in abundance and partially replaced by |
In the primary (growing) plant cell wall, the major [[carbohydrate]]s are [[cellulose]], [[hemicellulose]] and [[pectin]]. The cellulose [[microfibril]]s are linked via hemicellulosic tethers to form the cellulose-hemicellulose network, which is embedded in the pectin matrix. The most common hemicellulose in the primary cell wall is [[xyloglucan]].<ref name="Fry1989">{{cite journal |last1=Fry |first1=Stephen C. | name-list-style = vanc |title=The Structure and Functions of Xyloglucan |journal=Journal of Experimental Botany |volume=40 |issue=1 |year=1989 |pages=1–11 |doi=10.1093/jxb/40.1.1}}</ref> In grass cell walls, xyloglucan and pectin are reduced in abundance and partially replaced by glucuronoarabinoxylan, another type of hemicellulose. Primary cell walls characteristically extend (grow) by a mechanism called [[acid growth]], mediated by [[expansin]]s, extracellular proteins activated by acidic conditions that modify the hydrogen bonds between [[pectin]] and cellulose.<ref>{{cite journal | vauthors = Braidwood L, Breuer C, Sugimoto K | title = My body is a cage: mechanisms and modulation of plant cell growth | journal = The New Phytologist | volume = 201 | issue = 2 | pages = 388–402 | date = January 2014 | pmid = 24033322 | doi = 10.1111/nph.12473 | doi-access = free }}</ref> This functions to increase cell wall extensibility. The outer part of the primary cell wall of the plant epidermis is usually impregnated with [[cutin]] and [[wax]], forming a permeability barrier known as the [[plant cuticle]]. |
||
Secondary cell walls contain a wide range of additional compounds that modify their mechanical properties and permeability. The major [[polymer]]s that make up [[wood]] (largely secondary cell walls) include: |
Secondary cell walls contain a wide range of additional compounds that modify their mechanical properties and permeability. The major [[polymer]]s that make up [[wood]] (largely secondary cell walls) include: |
||
| Line 72: | Line 64: | ||
* [[lignin]], 10-25%, a complex phenolic polymer that penetrates the spaces in the cell wall between cellulose, hemicellulose and pectin components, driving out water and strengthening the wall. |
* [[lignin]], 10-25%, a complex phenolic polymer that penetrates the spaces in the cell wall between cellulose, hemicellulose and pectin components, driving out water and strengthening the wall. |
||
| ⚫ | |||
Additionally, structural [[protein]]s (1-5%) are found in most plant cell walls; they are classified as hydroxyproline-rich glycoproteins (HRGP), [[arabinogalactan]] proteins (AGP), glycine-rich proteins (GRPs), and proline-rich proteins (PRPs). Each class of glycoprotein is defined by a characteristic, highly repetitive protein sequence. Most are [[glycosylation|glycosylated]], contain [[hydroxyproline]] (Hyp) and become cross-linked in the cell wall. These proteins are often concentrated in specialized cells and in cell corners. Cell walls of the [[Epidermis (botany)|epidermis]] may contain [[cutin]]. The [[Casparian strip]] in the [[endodermis]] roots and [[cork (material)|cork]] cells of plant bark contain [[suberin]]. Both cutin and suberin are polyesters that function as permeability barriers to the movement of water.<ref>{{ |
Additionally, structural [[protein]]s (1-5%) are found in most plant cell walls; they are classified as hydroxyproline-rich glycoproteins (HRGP), [[arabinogalactan]] proteins (AGP), glycine-rich proteins (GRPs), and proline-rich proteins (PRPs). Each class of glycoprotein is defined by a characteristic, highly repetitive protein sequence. Most are [[glycosylation|glycosylated]], contain [[hydroxyproline]] (Hyp) and become cross-linked in the cell wall. These proteins are often concentrated in specialized cells and in cell corners. Cell walls of the [[Epidermis (botany)|epidermis]] may contain [[cutin]]. The [[Casparian strip]] in the [[endodermis]] roots and [[cork (material)|cork]] cells of plant bark contain [[suberin]]. Both cutin and suberin are polyesters that function as permeability barriers to the movement of water.<ref>{{cite journal | vauthors = Moire L, Schmutz A, Buchala A, Yan B, Stark RE, Ryser U | title = Glycerol is a suberin monomer. New experimental evidence for an old hypothesis | journal = Plant Physiology | volume = 119 | issue = 3 | pages = 1137–46 | date = March 1999 | pmid = 10069853 | pmc = 32096 | doi = 10.1104/pp.119.3.1137 }}</ref> The relative composition of carbohydrates, secondary compounds and proteins varies between plants and between the cell type and age. Plant cells walls also contain numerous enzymes, such as hydrolases, esterases, peroxidases, and transglycosylases, that cut, trim and [[cross-link]] wall polymers. |
||
Secondary walls - especially in grasses - may also contain microscopic [[silica]] crystals, which may strengthen the wall and protect it from herbivores. |
Secondary walls - especially in grasses - may also contain microscopic [[silica]] crystals, which may strengthen the wall and protect it from herbivores. |
||
Cell walls in some plant tissues also function as storage deposits for carbohydrates that can be broken down and resorbed to supply the metabolic and growth needs of the plant. For example, endosperm cell walls in the seeds of cereal grasses, [[Tropaeolum majus|nasturtium]]<ref name=reid> Reid |
Cell walls in some plant tissues also function as storage deposits for carbohydrates that can be broken down and resorbed to supply the metabolic and growth needs of the plant. For example, endosperm cell walls in the seeds of cereal grasses, [[Tropaeolum majus|nasturtium]]<ref name=reid>{{cite book | vauthors = Reid J | chapter-url = https://books.google.com/books?id=NmKF0hxhpdMC&pg=PA228 | chapter = Carbohydrate metabolism:structural carbohydrates | veditors = Dey PM, Harborne JB | title = Plant Biochemistry | publisher = Academic Press | date = 1997 | pages = 205–236 | isbn = 978-0-12-214674-9 }}</ref>{{rp|228}} |
||
and other species, are rich in glucans and other polysaccharides that are readily digested by enzymes during seed germination to form simple sugars that nourish the growing embryo. |
and other species, are rich in glucans and other polysaccharides that are readily digested by enzymes during seed germination to form simple sugars that nourish the growing embryo. |
||
===Formation=== |
===Formation=== |
||
| ⚫ | The [[middle lamella]] is laid down first, formed from the [[cell plate]] during [[cytokinesis]], and the primary cell wall is then deposited inside the middle lamella.{{clarify|reason=how can this be, since the middle lamella is defined as the pectic material between the primary cell walls?|date=September 2016}} The actual structure of the cell wall is not clearly defined and several models exist - the covalently linked cross model, the tether model, the diffuse layer model and the stratified layer model. However, the primary cell wall, can be defined as composed of [[cellulose]] [[microfibrils]] aligned at all angles. Cellulose microfibrils are produced at the plasma membrane by the [[cellulose synthase (UDP-forming)|cellulose synthase complex]], which is proposed to be made of a hexameric rosette that contains three cellulose synthase catalytic subunits for each of the six units.<ref>{{cite journal | vauthors = Jarvis MC | title = Cellulose biosynthesis: counting the chains | journal = Plant Physiology | volume = 163 | issue = 4 | pages = 1485–6 | date = December 2013 | pmid = 24296786 | pmc = 3850196 | doi = 10.1104/pp.113.231092 }}</ref> Microfibrils are held together by hydrogen bonds to provide a high tensile strength. The cells are held together and share the gelatinous membrane (the middle lamella), which contains [[magnesium]] and [[calcium]] [[pectate]]s (salts of [[pectic acid]]). Cells interact though [[plasmodesma]]ta, which are inter-connecting channels of cytoplasm that connect to the protoplasts of adjacent cells across the cell wall. |
||
| ⚫ | |||
| ⚫ | The |
||
| ⚫ | In some plants and cell types, after a maximum size or point in development has been reached, a ''secondary wall'' is constructed between the plasma membrane and primary wall.<ref>{{Cite book| title=Biology| url=https://archive.org/details/essentialbiology00camp_0| url-access=registration|last1=Campbell |last2=Reece |last3=Urry|last4=Cain|last5=Wasserman|last6=Minorsky|last7=Jackson|first1=Neil A.|first2=Jane B.|first3=Lisa A. |first4=Michael L. |first5=Steven A.|first6=Peter V.|first7=Robert B. | name-list-style = vanc |edition=8th|isbn=978-0-8053-6844-4 |pages = [https://archive.org/details/essentialbiology00camp_0/page/119 119] |year=2008| publisher=Pearson Benjamin Cummings}}</ref> Unlike the primary wall, the cellulose microfibrils are aligned parallel in layers, the orientation changing slightly with each additional layer so that the structure becomes helicoidal.<ref name=Abeysekera>{{cite journal | vauthors = Abeysekera RM, Willison JH |title=A spiral helicoid in a plant cell wall |journal=Cell Biology International Reports |volume=11 |issue=2 |date=1987 |pages=75–79 |doi=10.1016/0309-1651(87)90106-8 }}</ref> Cells with secondary cell walls can be rigid, as in the gritty [[sclereid]] cells in [[pear]] and [[quince]] fruit. Cell to cell communication is possible through [[pit (botany)|pits]] in the secondary cell wall that allow plasmodesmata to connect cells through the secondary cell walls. |
||
In some plants and cell types, after a maximum size or point in development has been reached, a ''secondary wall'' is constructed between the plasma membrane and primary wall.<ref>{{Cite book| |
|||
title=Biology| |
|||
last1=Campbell| |
|||
last2=Reece| |
|||
last3=Urry| |
|||
last4=Cain| |
|||
last5=Wasserman| |
|||
last6=Minorsky| |
|||
last7=Jackson| |
|||
first1=Neil A.| |
|||
first2=Jane B.| |
|||
first3=Lisa A.| |
|||
first4=Michael L.| |
|||
first5=Steven A.| |
|||
first6=Peter V.| |
|||
first7=Robert B.| |
|||
edition=8th| |
|||
isbn=978-0-8053-6844-4| |
|||
page=119| |
|||
| ⚫ | year=2008}}</ref> Unlike the primary wall, the cellulose microfibrils are aligned parallel in layers, the orientation changing slightly with each additional layer so that the structure becomes helicoidal.<ref name=Abeysekera>{{cite journal | |
||
==Fungal cell walls== |
==Fungal cell walls== |
||
[[File:Chitin.svg|thumb |
[[File:Chitin.svg|thumb|Chemical structure of a unit from a [[chitin]] polymer chain]] |
||
There are several groups of organisms that have been called "fungi". Some of these groups ([[Oomycete]] and [[Myxogastria]]) have been transferred out of the Kingdom Fungi, in part because of fundamental biochemical differences in the composition of the cell wall. Most true fungi have a cell wall consisting largely of [[chitin]] and other [[polysaccharide]]s.<ref>Hudler |
There are several groups of organisms that have been called "fungi". Some of these groups ([[Oomycete]] and [[Myxogastria]]) have been transferred out of the Kingdom Fungi, in part because of fundamental biochemical differences in the composition of the cell wall. Most true fungi have a cell wall consisting largely of [[chitin]] and other [[polysaccharide]]s.<ref>{{cite book | last = Hudler | first = George W. | name-list-style = vanc | date = 1998 | title = Magical Mushrooms, Mischievous Molds | location = Princeton, NJ | publisher = Princeton University Press | page = [https://archive.org/details/magicalmushrooms00hudl/page/7 7] | isbn = 978-0-691-02873-6 | url-access = registration | url = https://archive.org/details/magicalmushrooms00hudl/page/7 }}</ref> True fungi do not have [[cellulose]] in their cell walls.<ref name="Webster_2007">{{cite book | vauthors = Webster J, Weber RW | date = 2007 | title = Introduction to Fungi | url = https://archive.org/details/introductiontofu00jweb | url-access = limited | location = New York, NY | publisher = Cambridge University Press | pages = [https://archive.org/details/introductiontofu00jweb/page/n24 5]–7 }}</ref> |
||
| ⚫ | |||
===True fungi=== |
|||
| ⚫ | * [[chitin]]: [[polymer]]s consisting mainly of unbranched chains of β-(1,4)-linked-[[N-Acetylglucosamine]] in the [[Ascomycota]] and [[Basidiomycota]], or poly-β-(1,4)-linked-[[N-Acetylglucosamine]] ([[chitosan]]) in the [[Zygomycota]]. Both [[chitin]] and [[chitosan]] are synthesized and extruded at the [[plasma membrane]].<ref name="Webster_2007"/> |
||
| ⚫ | |||
| ⚫ | * [[glucan]]s: glucose [[polymer]]s that function to cross-link [[chitin]] or [[chitosan]] polymers. β-glucans are glucose molecules linked via β-(1,3)- or β-(1,6)- bonds and provide rigidity to the cell wall while α-glucans are defined by α-(1,3)- and/or α-(1,4) bonds and function as part of the matrix.<ref name="Webster_2007"/> |
||
| ⚫ | * [[chitin]]: [[polymer]]s consisting mainly of unbranched chains of β-(1,4)-linked-[[N-Acetylglucosamine]] in the [[Ascomycota]] and [[Basidiomycota]], or poly-β-(1,4)-linked-[[N-Acetylglucosamine]] ([[chitosan]]) in the [[Zygomycota]]. Both [[chitin]] and [[chitosan]] are synthesized and extruded at the [[plasma membrane]].<ref name=" |
||
| ⚫ | * [[protein]]s: enzymes necessary for cell wall synthesis and lysis in addition to structural proteins are all present in the cell wall. Most of the structural proteins found in the cell wall are [[Glycosylation|glycosylated]] and contain [[mannose]], thus these proteins are called mannoproteins or [[Mannan (polysaccharide)|mannan]]s.<ref name="Webster_2007"/> |
||
| ⚫ | * [[glucan]]s: glucose [[polymer]]s that function to cross-link [[chitin]] or [[chitosan]] polymers. β-glucans are glucose molecules linked via β-(1,3)- or β-(1,6)- bonds and provide rigidity to the cell wall while α-glucans are defined by α-(1,3)- and/or α-(1,4) bonds and function as part of the matrix.<ref name=" |
||
| ⚫ | * [[protein]]s: enzymes necessary for cell wall synthesis and lysis in addition to structural proteins are all present in the cell wall. Most of the structural proteins found in the cell wall are [[glycosylated]] and contain [[mannose]], thus these proteins are called mannoproteins or [[mannan]]s.<ref name=" |
||
==Other eukaryotic cell walls== |
==Other eukaryotic cell walls== |
||
===Algae=== |
===Algae=== |
||
[[File:Diatoms.png|thumb |
[[File:Diatoms.png|thumb|right|[[Scanning electron microscope|Scanning electron]] [[micrograph]]s of [[diatom]]s showing the external appearance of the cell wall]] |
||
Like plants, algae have cell walls.<ref> |
Like plants, algae have cell walls.<ref>{{cite web | last = Sengbusch | first = Peter V. | name-list-style = vanc | date = 2003-07-31 | url = http://www.biologie.uni-hamburg.de/b-online/e26/26d.htm | title = Cell Walls of Algae | archive-url = https://web.archive.org/web/20051128095106/http://www.biologie.uni-hamburg.de/b-online/e26/26d.htm | archive-date = November 28, 2005 | work = Botany Online | publisher = biologie.uni-hamburg.de | access-date = 2007-10-29 }}</ref> Algal cell walls contain either [[polysaccharide]]s (such as cellulose (a [[glucan]])) or a variety of [[glycoprotein]]s ([[Volvocales]]) or both. The inclusion of additional [[polysaccharide]]s in algal cells walls is used as a feature for algal [[taxonomy (biology)|taxonomy]]. |
||
* [[Mannan]]s: They form microfibrils in the cell walls of a number of marine [[green algae]] including those from the genera, ''Codium'', ''Dasycladus'', and ''Acetabularia'' as well as in the walls of some [[red algae]], like ''Porphyra'' and ''Bangia''. |
* [[Mannan (polysaccharide)|Mannan]]s: They form microfibrils in the cell walls of a number of [[Marine (ocean)|marine]] [[green algae]] including those from the [[genera]], ''[[Codium]]'', ''[[Dasycladus]]'', and ''[[Acetabularia]]'' as well as in the walls of some [[red algae]], like ''[[Porphyra]]'' and ''[[Bangia]]''. |
||
* [[Xylan]]s: |
* [[Xylan]]s: |
||
* [[Alginic acid]]: It is a common polysaccharide in the cell walls of [[brown algae]]. |
* [[Alginic acid]]: It is a common polysaccharide in the cell walls of [[brown algae]]. |
||
* Sulfonated polysaccharides: They occur in the cell walls of most algae; those common in red algae include [[agar]]ose, [[carrageenan]], [[porphyra]]n, furcelleran and [[funoran]]. |
* [[Sulfonated]] polysaccharides: They occur in the cell walls of most algae; those common in red algae include [[agar]]ose, [[carrageenan]], [[porphyra]]n, [[furcelleran]] and [[funoran]]. |
||
Other compounds that may accumulate in algal cell walls include [[sporopollenin]] and [[calcium|calcium ions]]. |
Other compounds that may accumulate in algal cell walls include [[sporopollenin]] and [[calcium|calcium ions]]. |
||
The group of [[algae]] known as the [[diatom]]s synthesize their cell walls (also known as frustules or valves) from [[silicic acid]] |
The group of [[algae]] known as the [[diatom]]s [[Biosynthesis|synthesize]] their cell walls (also known as [[frustules]] or valves) from [[silicon dioxide|silicic acid]]. Significantly, relative to the organic cell walls produced by other groups, silica frustules require less energy to synthesize (approximately 8%), potentially a major saving on the overall cell energy budget<ref>{{Cite journal | doi = 10.1111/j.1469-185X.1983.tb00385.x | vauthors = Raven JA | year = 1983 | title = The transport and function of silicon in plants | journal = Biol. Rev. | volume = 58 | issue = 2| pages = 179–207 | s2cid = 86067386 }}</ref> and possibly an explanation for higher growth rates in diatoms.<ref>{{Cite journal | doi = 10.1093/plankt/12.6.1117 | vauthors = Furnas MJ | year = 1990 | title = ''In situ'' growth rates of marine phytoplankton : Approaches to measurement, community and species growth rates | journal = J. Plankton Res. | volume = 12 | issue = 6| pages = 1117–1151 }}</ref> |
||
In [[brown alga]]e, [[phlorotannin]]s may be a constituent of the cell walls.<ref>{{ |
In [[brown alga]]e, [[phlorotannin]]s may be a constituent of the cell walls.<ref>{{cite journal | vauthors = Koivikko R, Loponen J, Honkanen T, Jormalainen V | title = Contents of soluble, cell-wall-bound and exuded phlorotannins in the brown alga Fucus vesiculosus, with implications on their ecological functions | journal = Journal of Chemical Ecology | volume = 31 | issue = 1 | pages = 195–212 | date = January 2005 | pmid = 15839490 | doi = 10.1007/s10886-005-0984-2 | url = http://users.utu.fi/veijor/project/2005JCE.pdf | citeseerx = 10.1.1.320.5895 | s2cid = 1540749 }}</ref> |
||
===Water molds=== |
===Water molds=== |
||
The group [[Oomycete]]s, also known as water molds, are [[saprotroph]]ic [[Plant pathology|plant pathogens]] like fungi. Until recently they were widely believed to be fungi, but [[organelle|structural]] and [[molecular biology|molecular]] evidence<ref>Sengbusch |
The group [[Oomycete]]s, also known as water molds, are [[saprotroph]]ic [[Plant pathology|plant pathogens]] like fungi. Until recently they were widely believed to be fungi, but [[organelle|structural]] and [[molecular biology|molecular]] evidence<ref name="Sengbusch_2003">{{cite web | last = Sengbusch | first = Peter V. | name-list-style = vanc | date = 2003-07-31 | url = http://www.biologie.uni-hamburg.de/b-online/e33/33.htm | title = Interactions between Plants and Fungi: the Evolution of their Parasitic and Symbiotic Relations | archive-url = https://web.archive.org/web/20061208024328/http://www.biologie.uni-hamburg.de/b-online/e33/33.htm | archive-date = December 8, 2006 | work = Biology Online | access-date = 2007-10-29 }}</ref> has led to their reclassification as [[heterokont]]s, related to [[autotroph]]ic [[brown algae]] and [[diatom]]s. Unlike fungi, oomycetes typically possess cell walls of cellulose and [[glucan]]s rather than chitin, although some genera (such as ''[[Achlya]]'' and ''[[Saprolegnia]]'') do have chitin in their walls.<ref name="Alexopoulos 1996">{{cite book | vauthors = Alexopoulos CJ, Mims W, Blackwell M | date = 1996 | title = Introductory Mycology | chapter = 4 | location = New York | publisher = John Wiley & Sons | pages = 687–688 | isbn = 978-0-471-52229-4 }}</ref> The fraction of cellulose in the walls is no more than 4 to 20%, far less than the fraction of glucans.<ref name="Alexopoulos 1996" /> Oomycete cell walls also contain the [[amino acid]] [[hydroxyproline]], which is not found in fungal cell walls. |
||
===Slime molds=== |
===Slime molds=== |
||
The [[dictyostelid]]s are another group formerly classified among the fungi. They are [[slime mold]]s that feed as unicellular [[amoeba]]e, but aggregate into a reproductive stalk and [[sporangium]] under certain conditions. Cells of the reproductive stalk, as well as the [[spore]]s formed at the apex, possess a [[cellulose]] wall.<ref name="Raper 1984">Raper |
The [[dictyostelid]]s are another group formerly classified among the fungi. They are [[slime mold]]s that feed as unicellular [[amoeba]]e, but aggregate into a reproductive stalk and [[sporangium]] under certain conditions. Cells of the reproductive stalk, as well as the [[spore]]s formed at the apex, possess a [[cellulose]] wall.<ref name="Raper 1984">{{cite book | last1 = Raper | first1 = Kenneth B. | first2 = Ann Worley | last2 = Rahn | name-list-style = vanc | date = 1984 | title = The Dictyostelids | location = Princeton, NJ | publisher = Princeton University Press | pages = 99–100| isbn = 978-0-691-08345-2 }}</ref> The spore wall has three layers, the middle one composed primarily of cellulose, while the innermost is sensitive to [[cellulase]] and [[pronase]].<ref name="Raper 1984" /> |
||
==Prokaryotic cell walls== |
==Prokaryotic cell walls== |
||
| Line 141: | Line 113: | ||
===Bacterial cell walls=== |
===Bacterial cell walls=== |
||
<!-- This section is linked from [[Bacteriocin]] --> |
<!-- This section is linked from [[Bacteriocin]] --> |
||
[[File:Prokaryote cell.svg|thumb |
[[File:Prokaryote cell.svg|thumb|right|Illustration of a typical [[Gram-positive bacteria|gram-positive bacterium]]. The cell envelope comprises a [[cell membrane|plasma membrane]], seen here in light brown, and a thick [[peptidoglycan]]-containing cell wall (the purple layer). No [[bacterial outer membrane|outer lipid membrane]] is present, as would be the case in [[gram-negative bacteria]]. The red layer, known as the [[bacterial capsule|capsule]], is distinct from the cell envelope.]] |
||
{{Further|Cell envelope|Bacterial cell structure}} |
|||
| ⚫ | Around the outside of the cell membrane is the bacterial cell wall. Bacterial cell walls are made of [[peptidoglycan]] (also called murein), which is made from [[polysaccharide]] chains cross-linked by unusual [[peptide]]s containing D-[[amino acid]]s.<ref>{{cite journal | author = van Heijenoort J | title = Formation of the glycan chains in the synthesis of bacterial peptidoglycan | url=http://glycob.oxfordjournals.org/cgi/content/full/11/3/25R | journal = Glycobiology | volume = 11 | issue = 3 | pages = 25R – 36R | year = 2001 | pmid = 11320055 | doi = 10.1093/glycob/11.3.25R | doi-access = free }}</ref> Bacterial cell walls are different from the cell walls of [[plant]]s and [[fungi]] which are made of [[cellulose]] and [[chitin]], respectively.<ref name=Koch>{{cite journal | vauthors = Koch AL | title = Bacterial wall as target for attack: past, present, and future research | journal = Clinical Microbiology Reviews | volume = 16 | issue = 4 | pages = 673–87 | date = October 2003 | pmid = 14557293 | pmc = 207114 | doi = 10.1128/CMR.16.4.673-687.2003 }}</ref> The cell wall of bacteria is also distinct from that of Archaea, which do not contain peptidoglycan. The cell wall is essential to the survival of many bacteria, although [[L-form bacteria]] can be produced in the laboratory that lack a cell wall.<ref name=Joseleau>{{cite journal | vauthors = Joseleau-Petit D, Liébart JC, Ayala JA, D'Ari R | title = Unstable Escherichia coli L forms revisited: growth requires peptidoglycan synthesis | journal = Journal of Bacteriology | volume = 189 | issue = 18 | pages = 6512–20 | date = September 2007 | pmid = 17586646 | pmc = 2045188 | doi = 10.1128/JB.00273-07 }}</ref> The antibiotic [[penicillin]] is able to kill bacteria by preventing the cross-linking of peptidoglycan and this causes the cell wall to weaken and lyse.<ref name=Koch/> The [[lysozyme]] enzyme can also damage bacterial cell walls. |
||
| ⚫ | There are broadly speaking two different types of cell wall in bacteria, called [[gram-positive]] and [[gram-negative]]. The names originate from the reaction of cells to the [[Gram stain]], a test long-employed for the classification of bacterial species.<ref name=Gram>{{cite journal | last = Gram | first = HC | author-link = Hans Christian Gram | year = 1884 | title = Über die isolierte Färbung der Schizomyceten in Schnitt- und Trockenpräparaten | journal = Fortschr. Med. | volume = 2 | pages = 185–189 }}</ref> |
||
{{further|Cell envelope}} |
|||
Gram-positive bacteria possess a thick cell wall containing many layers of peptidoglycan and [[teichoic acid]]s. |
|||
| ⚫ | Around the outside of the cell membrane is the bacterial cell wall. Bacterial cell walls are made of [[peptidoglycan]] (also called murein), which is made from [[polysaccharide]] chains cross-linked by unusual [[peptide]]s containing D-[[amino acid]]s.<ref>{{cite |
||
Gram-negative bacteria have a relatively thin cell wall consisting of a few layers of peptidoglycan surrounded by a second lipid membrane containing [[lipopolysaccharide]]s and [[lipoprotein]]s. Most bacteria have the gram-negative cell wall and only the [[Bacillota]] and [[Actinomycetota]] (previously known as the low G+C and high G+C gram-positive bacteria, respectively) have the alternative gram-positive arrangement.<ref>{{cite journal | vauthors = Hugenholtz P | title = Exploring prokaryotic diversity in the genomic era | journal = Genome Biology | volume = 3 | issue = 2 | pages = REVIEWS0003 | year = 2002 | pmid = 11864374 | pmc = 139013 | doi = 10.1186/gb-2002-3-2-reviews0003 | doi-access = free }}</ref> |
|||
| ⚫ | There are broadly speaking two different types of cell wall in bacteria, called [[ |
||
These differences in structure produce differences in antibiotic susceptibility. The [[beta-lactam antibiotics]] (e.g. [[penicillin]], [[cephalosporin]]) only work against gram-negative pathogens, such as ''[[Haemophilus influenzae]]'' or ''[[Pseudomonas aeruginosa]]''. The [[glycopeptide antibiotic]]s (e.g. [[vancomycin]], [[teicoplanin]], [[telavancin]]) only work against gram-positive pathogens such as ''[[Staphylococcus aureus]]'' <ref>{{cite journal |vauthors=Walsh F, Amyes S | title = Microbiology and drug resistance mechanisms of fully resistant pathogens. | journal = Curr Opin Microbiol | volume = 7 | issue = 5 | pages = 439–44 | year = 2004 | pmid = 15451497 | doi = 10.1016/j.mib.2004.08.007 | url = http://mural.maynoothuniversity.ie/13551/1/FW-Microbiology-2004.pdf }}</ref> |
|||
===Archaeal cell walls=== |
===Archaeal cell walls=== |
||
Although not truly unique, the cell walls of [[Archaea]] are unusual. Whereas [[peptidoglycan]] is a standard component of all bacterial cell walls, all archaeal cell walls lack [[peptidoglycan]],<ref name="White 1995">White |
Although not truly unique, the cell walls of [[Archaea]] are unusual. Whereas [[peptidoglycan]] is a standard component of all bacterial cell walls, all archaeal cell walls lack [[peptidoglycan]],<ref name="White 1995">{{cite book | last = White | first = David | name-list-style = vanc | date = 1995 | title = The Physiology and Biochemistry of Prokaryotes | pages = 6, 12–21 | location = Oxford | publisher = Oxford University Press | isbn = 978-0-19-508439-9 }}</ref> though some [[methanogen]]s have a cell wall made of a similar polymer called [[pseudopeptidoglycan]].<ref name="Howland 2000" /> There are four types of cell wall currently known among the Archaea. |
||
One type of archaeal cell wall is that composed of [[pseudopeptidoglycan]] (also called pseudomurein). This type of wall is found in some [[methanogen]]s, such as ''[[Methanobacterium]]'' and ''[[Methanothermus]]''.<ref name="Brock 1994"> |
One type of archaeal cell wall is that composed of [[pseudopeptidoglycan]] (also called [[pseudomurein]]). This type of wall is found in some [[methanogen]]s, such as ''[[Methanobacterium]]'' and ''[[Methanothermus]]''.<ref name="Brock 1994">{{cite book | vauthors = Brock TD, Madigan MT, Martinko JM, Parker J | author-link1 = Thomas D. Brock | date = 1994 | title = Biology of Microorganisms | edition = 7th | pages = 818–819, 824 | location = Englewood Cliffs, NJ | publisher = Prentice Hall | isbn = 978-0-13-042169-2 }}</ref> While the overall structure of archaeal ''pseudo''peptidoglycan superficially resembles that of bacterial peptidoglycan, there are a number of significant chemical differences. Like the peptidoglycan found in bacterial cell walls, pseudopeptidoglycan consists of [[polymer]] chains of [[glycan]] cross-linked by short [[peptide]] connections. However, unlike peptidoglycan, the sugar [[N-Acetylmuramic acid|N-acetylmuramic acid]] is replaced by [[N-Acetyltalosaminuronic acid|N-acetyltalosaminuronic acid]],<ref name="White 1995" /> and the two sugars are bonded with a ''β'',1-3 glycosidic linkage instead of ''β'',1-4. Additionally, the cross-linking peptides are [[L-amino acid]]s rather than D-amino acids as they are in bacteria.<ref name="Brock 1994" /> |
||
A second type of archaeal cell wall is found in ''[[Methanosarcina]]'' and ''[[Halococcus]]''. This type of cell wall is composed entirely of a thick layer of [[polysaccharide]]s, which may be [[sulfate]]d in the case of ''Halococcus''.<ref name="Brock 1994" /> Structure in this type of wall is complex and not fully investigated. |
A second type of archaeal cell wall is found in ''[[Methanosarcina]]'' and ''[[Halococcus]]''. This type of cell wall is composed entirely of a thick layer of [[polysaccharide]]s, which may be [[sulfate]]d in the case of ''Halococcus''.<ref name="Brock 1994" /> Structure in this type of wall is complex and not fully investigated. |
||
A third type of wall among the [[Archaea]] consists of [[glycoprotein]], and occurs in the [[hyperthermophile]]s, ''[[Halobacterium]]'', and some [[methanogen]]s. In ''Halobacterium'', the [[protein]]s in the wall have a high content of [[acid]]ic [[amino acid]]s, giving the wall an overall negative charge. The result is an unstable structure that is stabilized by the presence of large quantities of positive [[sodium]] [[ion]]s that neutralize the charge.<ref name="Brock 1994" /> Consequently, ''Halobacterium'' thrives only under conditions with high [[salinity]]. |
A third type of wall among the [[Archaea]] consists of [[glycoprotein]], and occurs in the [[hyperthermophile]]s, ''[[Halobacterium]]'', and some [[methanogen]]s. In ''Halobacterium'', the [[protein]]s in the wall have a high content of [[acid]]ic [[amino acid]]s, giving the wall an overall negative charge. The result is an unstable structure that is stabilized by the presence of large quantities of positive [[sodium]] [[ion]]s that [[Neutralization (chemistry)|neutralize]] the charge.<ref name="Brock 1994" /> Consequently, ''Halobacterium'' thrives only under conditions with high [[salinity]]. |
||
In other Archaea, such as ''[[Methanomicrobium]]'' and ''[[Desulfurococcus]]'', the wall may be composed only of surface-layer [[protein]]s,<ref name="Howland 2000"/> known as an ''S-layer''. S-layers are common in |
In other Archaea, such as ''[[Methanomicrobium]]'' and ''[[Desulfurococcus]]'', the wall may be composed only of surface-layer [[protein]]s,<ref name="Howland 2000"/> known as an ''[[S-layer]]''. S-layers are common in bacteria, where they serve as either the sole cell-wall component or an outer layer in conjunction with [[polysaccharides]]. Most Archaea are Gram-negative, though at least one Gram-positive member is known.<ref name="Howland 2000" /> |
||
==Other cell coverings== |
==Other cell coverings== |
||
Many [[protist]]s and [[bacteria]] produce other cell surface structures apart from cell walls, external ([[extracellular matrix]]) or internal.<ref>Preisig |
Many [[protist]]s and [[bacteria]] produce other cell surface structures apart from cell walls, external ([[extracellular matrix]]) or internal.<ref>{{cite book | vauthors = Preisig HR | chapter = Terminology and nomenclature of protist cell surface structures | title = The Protistan Cell Surface | pages = 1–28 | edition = Protoplasma special | date = 1994 | doi = 10.1007/978-3-7091-9378-5_1 | isbn = 978-3-7091-9380-8 }}</ref><ref>{{cite book | vauthors = Becker B | chapter = The cell surface of flagellates. | chapter-url = http://www.uni-koeln.de/math-nat-fak/botanik/bot1/AGBecker/Publikationen/Cell%20Surfaces%20of%20Flagellates/CELLSURFACE.htm | title = The Flagellates. Unity, diversity and evolution | veditors = Leadbeater BS, Green JC | publisher = Taylor and Francis | location = London | year = 2000 | archive-url = https://web.archive.org/web/20130212024846/http://www.uni-koeln.de/math-nat-fak/botanik/bot1/AGBecker/Publikationen/Cell%20Surfaces%20of%20Flagellates/CELLSURFACE.htm | archive-date = 2013-02-12 }}</ref><ref>{{cite book | last1 = Barsanti | first1 = Laura | last2 = Gualtieri | first2 = Paolo | name-list-style = vanc | date = 2006 | title = Algae: anatomy, biochemistry, and biotechnology | location = Florida, USA | publisher = CRC Press }}</ref> Many [[algae]] have a sheath or envelope of [[mucilage]] outside the cell made of [[exopolysaccharides]]. [[Diatom]]s build a [[frustule]] from [[silica]] extracted from the surrounding water; [[radiolarian]]s, [[foraminiferan]]s, [[testate amoebae]] and [[silicoflagellate]]s also produce a skeleton from [[mineral]]s, called [[test (biology)|test]] in some groups. Many [[green algae]], such as ''[[Halimeda]]'' and the [[Dasycladales]], and some [[red algae]], the [[Corallinales]], encase their cells in a [[secreted]] skeleton of [[calcium carbonate]]. In each case, the wall is rigid and essentially [[inorganic]]. It is the non-living component of cell. Some [[golden algae]], [[ciliate]]s and [[choanoflagellate]]s produces a shell-like protective outer covering called [[lorica (biology)|lorica]]. Some [[dinoflagellate]]s have a [[theca]] of [[cellulose]] plates, and [[coccolithophorid]]s have [[coccolith]]s. |
||
An [[extracellular matrix]] is also present in [[metazoans]]. Its composition varies between cells, but [[collagen]]s are the most abundant protein in the ECM.<ref>{{ |
An [[extracellular matrix]] (ECM) is also present in [[metazoans]]. Its [[Chemical composition|composition]] varies between cells, but [[collagen]]s are the most [[abundance (chemistry)|abundant]] protein in the ECM.<ref>{{cite journal | vauthors = Frantz C, Stewart KM, Weaver VM | title = The extracellular matrix at a glance | journal = Journal of Cell Science | volume = 123 | issue = Pt 24 | pages = 4195–200 | date = December 2010 | pmid = 21123617 | pmc = 2995612 | doi = 10.1242/jcs.023820 }}</ref><ref>{{cite book|last1=Alberts |first1=Bruce | first2 = Alexander | last2 = Johnson | first3 = Julian | last3 = Lewis | first4 = Martin | last4 = Raff | first5 = Keith | last5 = Roberts | first6 = Peter | last6 = Walter | name-list-style = vanc |title=Molecular biology of the cell|date=2002|publisher=Garland|location=New York |isbn=978-0-8153-4072-0 |pages = 1065 |edition=4th}}</ref> |
||
==See also== |
== See also == |
||
* [[Extracellular matrix]] |
|||
* [[Bacterial cell structure]] |
* [[Bacterial cell structure]] |
||
* [[Plant cell]] |
* [[Plant cell]] |
||
==References== |
== References == |
||
{{ |
{{Reflist}} |
||
==External links== |
== External links == |
||
{{wiktionary}} |
{{wiktionary}} |
||
* [http://micro.magnet.fsu.edu/cells/plants/cellwall.html Cell wall ultrastructure] |
* [http://micro.magnet.fsu.edu/cells/plants/cellwall.html Cell wall ultrastructure] |
||
* [https://web.archive.org/web/20070325121027/http://www.palaeos.com |
* [https://web.archive.org/web/20070325121027/http://www.palaeos.com/Fungi/FPieces/CellWall.html The Cell Wall] |
||
{{Clear}} |
{{Clear}} |
||
| Line 186: | Line 162: | ||
{{Fungus structure}} |
{{Fungus structure}} |
||
{{Protist}} |
{{Protist}} |
||
{{Authority control}} |
|||
{{DEFAULTSORT:Cell Wall}} |
{{DEFAULTSORT:Cell Wall}} |
||
Latest revision as of 05:01, 2 November 2024
| Cell biology | |
|---|---|
| Plant cell diagram | |
 Components of a typical plant cell:
|
A cell wall is a structural layer that surrounds some cell types, found immediately outside the cell membrane. It can be tough, flexible, and sometimes rigid. Primarily, it provides the cell with structural support, shape, protection, and functions as a selective barrier.[1] Another vital role of the cell wall is to help the cell withstand osmotic pressure and mechanical stress. While absent in many eukaryotes, including animals, cell walls are prevalent in other organisms such as fungi, algae and plants, and are commonly found in most prokaryotes, with the exception of mollicute bacteria.
The composition of cell walls varies across taxonomic groups, species, cell type, and the cell cycle. In land plants, the primary cell wall comprises polysaccharides like cellulose, hemicelluloses, and pectin. Often, other polymers such as lignin, suberin or cutin are anchored to or embedded in plant cell walls. Algae exhibit cell walls composed of glycoproteins and polysaccharides, such as carrageenan and agar, distinct from those in land plants. Bacterial cell walls contain peptidoglycan, while archaeal cell walls vary in composition, potentially consisting of glycoprotein S-layers, pseudopeptidoglycan, or polysaccharides. Fungi possess cell walls constructed from the polymer chitin, specifically N-acetylglucosamine. diatoms have a unique cell wall composed of biogenic silica.[2]
History
A plant cell wall was first observed and named (simply as a "wall") by Robert Hooke in 1665.[3] However, "the dead excrusion product of the living protoplast" was forgotten, for almost three centuries, being the subject of scientific interest mainly as a resource for industrial processing or in relation to animal or human health.[4]
In 1804, Karl Rudolphi and J.H.F. Link proved that cells had independent cell walls.[5][6] Before, it had been thought that cells shared walls and that fluid passed between them this way.
The mode of formation of the cell wall was controversial in the 19th century. Hugo von Mohl (1853, 1858) advocated the idea that the cell wall grows by apposition. Carl Nägeli (1858, 1862, 1863) believed that the growth of the wall in thickness and in area was due to a process termed intussusception. Each theory was improved in the following decades: the apposition (or lamination) theory by Eduard Strasburger (1882, 1889), and the intussusception theory by Julius Wiesner (1886).[7]
In 1930, Ernst Münch coined the term apoplast in order to separate the "living" symplast from the "dead" plant region, the latter of which included the cell wall.[8]
By the 1980s, some authors suggested replacing the term "cell wall", particularly as it was used for plants, with the more precise term "extracellular matrix", as used for animal cells,[9][4]: 168 but others preferred the older term.[10]
Properties
This section needs additional citations for verification. (November 2017) |

Cell walls serve similar purposes in those organisms that possess them. They may give cells rigidity and strength, offering protection against mechanical stress. The chemical composition and mechanical properties of the cell wall are linked with plant cell growth and morphogenesis.[11] In multicellular organisms, they permit the organism to build and hold a definite shape. Cell walls also limit the entry of large molecules that may be toxic to the cell. They further permit the creation of stable osmotic environments by preventing osmotic lysis and helping to retain water. Their composition, properties, and form may change during the cell cycle and depend on growth conditions.[11]
Rigidity of cell walls
In most cells, the cell wall is flexible, meaning that it will bend rather than holding a fixed shape, but has considerable tensile strength. The apparent rigidity of primary plant tissues is enabled by cell walls, but is not due to the walls' stiffness. Hydraulic turgor pressure creates this rigidity, along with the wall structure. The flexibility of the cell walls is seen when plants wilt, so that the stems and leaves begin to droop, or in seaweeds that bend in water currents. As John Howland explains
Think of the cell wall as a wicker basket in which a balloon has been inflated so that it exerts pressure from the inside. Such a basket is very rigid and resistant to mechanical damage. Thus does the prokaryote cell (and eukaryotic cell that possesses a cell wall) gain strength from a flexible plasma membrane pressing against a rigid cell wall.[12]
The apparent rigidity of the cell wall thus results from inflation of the cell contained within. This inflation is a result of the passive uptake of water.
In plants, a secondary cell wall is a thicker additional layer of cellulose which increases wall rigidity. Additional layers may be formed by lignin in xylem cell walls, or suberin in cork cell walls. These compounds are rigid and waterproof, making the secondary wall stiff. Both wood and bark cells of trees have secondary walls. Other parts of plants such as the leaf stalk may acquire similar reinforcement to resist the strain of physical forces.
Permeability
The primary cell wall of most plant cells is freely permeable to small molecules including small proteins, with size exclusion estimated to be 30-60 kDa.[13] The pH is an important factor governing the transport of molecules through cell walls.[14]
Evolution
Cell walls evolved independently in many groups.
The photosynthetic eukaryotes (so-called plant and algae) is one group with cellulose cell walls, where the cell wall is closely related to the evolution of multicellularity, terrestrialization and vascularization. The CesA cellulose synthase evolved in Cyanobacteria and was part of Archaeplastida since endosymbiosis; secondary endosymbiosis events transferred it (with the arabinogalactan proteins) further into brown algae and oomycetes. Plants later evolved various genes from CesA, including the Csl (cellulose synthase-like) family of proteins and additional Ces proteins. Combined with the various glycosyltransferases (GT), they enable more complex chemical structures to be built.[15]
Fungi use a chitin-glucan-protein cell wall.[16] They share the 1,3-β-glucan synthesis pathway with plants, using homologous GT48 family 1,3-Beta-glucan synthases to perform the task, suggesting that such an enzyme is very ancient within the eukaryotes. Their glycoproteins are rich in mannose. The cell wall might have evolved to deter viral infections. Proteins embedded in cell walls are variable, contained in tandem repeats subject to homologous recombination.[17] An alternative scenario is that fungi started with a chitin-based cell wall and later acquired the GT-48 enzymes for the 1,3-β-glucans via horizontal gene transfer. The pathway leading to 1,6-β-glucan synthesis is not sufficiently known in either case.[18]
Plant cell walls
The walls of plant cells must have sufficient tensile strength to withstand internal osmotic pressures of several times atmospheric pressure that result from the difference in solute concentration between the cell interior and external solutions.[1] Plant cell walls vary from 0.1 to several μm in thickness.[19]
Layers


Up to three strata or layers may be found in plant cell walls:[20]
- The primary cell wall, generally a thin, flexible and extensible layer formed while the cell is growing.
- The secondary cell wall, a thick layer formed inside the primary cell wall after the cell is fully grown. It is not found in all cell types. Some cells, such as the conducting cells in xylem, possess a secondary wall containing lignin, which strengthens and waterproofs the wall.
- The middle lamella, a layer rich in pectins. This outermost layer forms the interface between adjacent plant cells and glues them together.
Composition
In the primary (growing) plant cell wall, the major carbohydrates are cellulose, hemicellulose and pectin. The cellulose microfibrils are linked via hemicellulosic tethers to form the cellulose-hemicellulose network, which is embedded in the pectin matrix. The most common hemicellulose in the primary cell wall is xyloglucan.[21] In grass cell walls, xyloglucan and pectin are reduced in abundance and partially replaced by glucuronoarabinoxylan, another type of hemicellulose. Primary cell walls characteristically extend (grow) by a mechanism called acid growth, mediated by expansins, extracellular proteins activated by acidic conditions that modify the hydrogen bonds between pectin and cellulose.[22] This functions to increase cell wall extensibility. The outer part of the primary cell wall of the plant epidermis is usually impregnated with cutin and wax, forming a permeability barrier known as the plant cuticle.
Secondary cell walls contain a wide range of additional compounds that modify their mechanical properties and permeability. The major polymers that make up wood (largely secondary cell walls) include:
- cellulose, 35-50%
- xylan, 20-35%, a type of hemicellulose
- lignin, 10-25%, a complex phenolic polymer that penetrates the spaces in the cell wall between cellulose, hemicellulose and pectin components, driving out water and strengthening the wall.

Additionally, structural proteins (1-5%) are found in most plant cell walls; they are classified as hydroxyproline-rich glycoproteins (HRGP), arabinogalactan proteins (AGP), glycine-rich proteins (GRPs), and proline-rich proteins (PRPs). Each class of glycoprotein is defined by a characteristic, highly repetitive protein sequence. Most are glycosylated, contain hydroxyproline (Hyp) and become cross-linked in the cell wall. These proteins are often concentrated in specialized cells and in cell corners. Cell walls of the epidermis may contain cutin. The Casparian strip in the endodermis roots and cork cells of plant bark contain suberin. Both cutin and suberin are polyesters that function as permeability barriers to the movement of water.[23] The relative composition of carbohydrates, secondary compounds and proteins varies between plants and between the cell type and age. Plant cells walls also contain numerous enzymes, such as hydrolases, esterases, peroxidases, and transglycosylases, that cut, trim and cross-link wall polymers.
Secondary walls - especially in grasses - may also contain microscopic silica crystals, which may strengthen the wall and protect it from herbivores.
Cell walls in some plant tissues also function as storage deposits for carbohydrates that can be broken down and resorbed to supply the metabolic and growth needs of the plant. For example, endosperm cell walls in the seeds of cereal grasses, nasturtium[24]: 228 and other species, are rich in glucans and other polysaccharides that are readily digested by enzymes during seed germination to form simple sugars that nourish the growing embryo.
Formation
The middle lamella is laid down first, formed from the cell plate during cytokinesis, and the primary cell wall is then deposited inside the middle lamella.[clarification needed] The actual structure of the cell wall is not clearly defined and several models exist - the covalently linked cross model, the tether model, the diffuse layer model and the stratified layer model. However, the primary cell wall, can be defined as composed of cellulose microfibrils aligned at all angles. Cellulose microfibrils are produced at the plasma membrane by the cellulose synthase complex, which is proposed to be made of a hexameric rosette that contains three cellulose synthase catalytic subunits for each of the six units.[25] Microfibrils are held together by hydrogen bonds to provide a high tensile strength. The cells are held together and share the gelatinous membrane (the middle lamella), which contains magnesium and calcium pectates (salts of pectic acid). Cells interact though plasmodesmata, which are inter-connecting channels of cytoplasm that connect to the protoplasts of adjacent cells across the cell wall.
In some plants and cell types, after a maximum size or point in development has been reached, a secondary wall is constructed between the plasma membrane and primary wall.[26] Unlike the primary wall, the cellulose microfibrils are aligned parallel in layers, the orientation changing slightly with each additional layer so that the structure becomes helicoidal.[27] Cells with secondary cell walls can be rigid, as in the gritty sclereid cells in pear and quince fruit. Cell to cell communication is possible through pits in the secondary cell wall that allow plasmodesmata to connect cells through the secondary cell walls.
Fungal cell walls

There are several groups of organisms that have been called "fungi". Some of these groups (Oomycete and Myxogastria) have been transferred out of the Kingdom Fungi, in part because of fundamental biochemical differences in the composition of the cell wall. Most true fungi have a cell wall consisting largely of chitin and other polysaccharides.[28] True fungi do not have cellulose in their cell walls.[16]
In fungi, the cell wall is the outer-most layer, external to the plasma membrane. The fungal cell wall is a matrix of three main components:[16]
- chitin: polymers consisting mainly of unbranched chains of β-(1,4)-linked-N-Acetylglucosamine in the Ascomycota and Basidiomycota, or poly-β-(1,4)-linked-N-Acetylglucosamine (chitosan) in the Zygomycota. Both chitin and chitosan are synthesized and extruded at the plasma membrane.[16]
- glucans: glucose polymers that function to cross-link chitin or chitosan polymers. β-glucans are glucose molecules linked via β-(1,3)- or β-(1,6)- bonds and provide rigidity to the cell wall while α-glucans are defined by α-(1,3)- and/or α-(1,4) bonds and function as part of the matrix.[16]
- proteins: enzymes necessary for cell wall synthesis and lysis in addition to structural proteins are all present in the cell wall. Most of the structural proteins found in the cell wall are glycosylated and contain mannose, thus these proteins are called mannoproteins or mannans.[16]
Other eukaryotic cell walls
Algae

Like plants, algae have cell walls.[29] Algal cell walls contain either polysaccharides (such as cellulose (a glucan)) or a variety of glycoproteins (Volvocales) or both. The inclusion of additional polysaccharides in algal cells walls is used as a feature for algal taxonomy.
- Mannans: They form microfibrils in the cell walls of a number of marine green algae including those from the genera, Codium, Dasycladus, and Acetabularia as well as in the walls of some red algae, like Porphyra and Bangia.
- Xylans:
- Alginic acid: It is a common polysaccharide in the cell walls of brown algae.
- Sulfonated polysaccharides: They occur in the cell walls of most algae; those common in red algae include agarose, carrageenan, porphyran, furcelleran and funoran.
Other compounds that may accumulate in algal cell walls include sporopollenin and calcium ions.
The group of algae known as the diatoms synthesize their cell walls (also known as frustules or valves) from silicic acid. Significantly, relative to the organic cell walls produced by other groups, silica frustules require less energy to synthesize (approximately 8%), potentially a major saving on the overall cell energy budget[30] and possibly an explanation for higher growth rates in diatoms.[31]
In brown algae, phlorotannins may be a constituent of the cell walls.[32]
Water molds
The group Oomycetes, also known as water molds, are saprotrophic plant pathogens like fungi. Until recently they were widely believed to be fungi, but structural and molecular evidence[33] has led to their reclassification as heterokonts, related to autotrophic brown algae and diatoms. Unlike fungi, oomycetes typically possess cell walls of cellulose and glucans rather than chitin, although some genera (such as Achlya and Saprolegnia) do have chitin in their walls.[34] The fraction of cellulose in the walls is no more than 4 to 20%, far less than the fraction of glucans.[34] Oomycete cell walls also contain the amino acid hydroxyproline, which is not found in fungal cell walls.
Slime molds
The dictyostelids are another group formerly classified among the fungi. They are slime molds that feed as unicellular amoebae, but aggregate into a reproductive stalk and sporangium under certain conditions. Cells of the reproductive stalk, as well as the spores formed at the apex, possess a cellulose wall.[35] The spore wall has three layers, the middle one composed primarily of cellulose, while the innermost is sensitive to cellulase and pronase.[35]
Prokaryotic cell walls
Bacterial cell walls
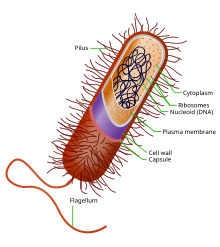
Around the outside of the cell membrane is the bacterial cell wall. Bacterial cell walls are made of peptidoglycan (also called murein), which is made from polysaccharide chains cross-linked by unusual peptides containing D-amino acids.[36] Bacterial cell walls are different from the cell walls of plants and fungi which are made of cellulose and chitin, respectively.[37] The cell wall of bacteria is also distinct from that of Archaea, which do not contain peptidoglycan. The cell wall is essential to the survival of many bacteria, although L-form bacteria can be produced in the laboratory that lack a cell wall.[38] The antibiotic penicillin is able to kill bacteria by preventing the cross-linking of peptidoglycan and this causes the cell wall to weaken and lyse.[37] The lysozyme enzyme can also damage bacterial cell walls.
There are broadly speaking two different types of cell wall in bacteria, called gram-positive and gram-negative. The names originate from the reaction of cells to the Gram stain, a test long-employed for the classification of bacterial species.[39]
Gram-positive bacteria possess a thick cell wall containing many layers of peptidoglycan and teichoic acids.
Gram-negative bacteria have a relatively thin cell wall consisting of a few layers of peptidoglycan surrounded by a second lipid membrane containing lipopolysaccharides and lipoproteins. Most bacteria have the gram-negative cell wall and only the Bacillota and Actinomycetota (previously known as the low G+C and high G+C gram-positive bacteria, respectively) have the alternative gram-positive arrangement.[40]
These differences in structure produce differences in antibiotic susceptibility. The beta-lactam antibiotics (e.g. penicillin, cephalosporin) only work against gram-negative pathogens, such as Haemophilus influenzae or Pseudomonas aeruginosa. The glycopeptide antibiotics (e.g. vancomycin, teicoplanin, telavancin) only work against gram-positive pathogens such as Staphylococcus aureus [41]
Archaeal cell walls
Although not truly unique, the cell walls of Archaea are unusual. Whereas peptidoglycan is a standard component of all bacterial cell walls, all archaeal cell walls lack peptidoglycan,[42] though some methanogens have a cell wall made of a similar polymer called pseudopeptidoglycan.[12] There are four types of cell wall currently known among the Archaea.
One type of archaeal cell wall is that composed of pseudopeptidoglycan (also called pseudomurein). This type of wall is found in some methanogens, such as Methanobacterium and Methanothermus.[43] While the overall structure of archaeal pseudopeptidoglycan superficially resembles that of bacterial peptidoglycan, there are a number of significant chemical differences. Like the peptidoglycan found in bacterial cell walls, pseudopeptidoglycan consists of polymer chains of glycan cross-linked by short peptide connections. However, unlike peptidoglycan, the sugar N-acetylmuramic acid is replaced by N-acetyltalosaminuronic acid,[42] and the two sugars are bonded with a β,1-3 glycosidic linkage instead of β,1-4. Additionally, the cross-linking peptides are L-amino acids rather than D-amino acids as they are in bacteria.[43]
A second type of archaeal cell wall is found in Methanosarcina and Halococcus. This type of cell wall is composed entirely of a thick layer of polysaccharides, which may be sulfated in the case of Halococcus.[43] Structure in this type of wall is complex and not fully investigated.
A third type of wall among the Archaea consists of glycoprotein, and occurs in the hyperthermophiles, Halobacterium, and some methanogens. In Halobacterium, the proteins in the wall have a high content of acidic amino acids, giving the wall an overall negative charge. The result is an unstable structure that is stabilized by the presence of large quantities of positive sodium ions that neutralize the charge.[43] Consequently, Halobacterium thrives only under conditions with high salinity.
In other Archaea, such as Methanomicrobium and Desulfurococcus, the wall may be composed only of surface-layer proteins,[12] known as an S-layer. S-layers are common in bacteria, where they serve as either the sole cell-wall component or an outer layer in conjunction with polysaccharides. Most Archaea are Gram-negative, though at least one Gram-positive member is known.[12]
Other cell coverings
Many protists and bacteria produce other cell surface structures apart from cell walls, external (extracellular matrix) or internal.[44][45][46] Many algae have a sheath or envelope of mucilage outside the cell made of exopolysaccharides. Diatoms build a frustule from silica extracted from the surrounding water; radiolarians, foraminiferans, testate amoebae and silicoflagellates also produce a skeleton from minerals, called test in some groups. Many green algae, such as Halimeda and the Dasycladales, and some red algae, the Corallinales, encase their cells in a secreted skeleton of calcium carbonate. In each case, the wall is rigid and essentially inorganic. It is the non-living component of cell. Some golden algae, ciliates and choanoflagellates produces a shell-like protective outer covering called lorica. Some dinoflagellates have a theca of cellulose plates, and coccolithophorids have coccoliths.
An extracellular matrix (ECM) is also present in metazoans. Its composition varies between cells, but collagens are the most abundant protein in the ECM.[47][48]
See also
References
- ^ a b Romaniuk JA, Cegelski L (October 2015). "Bacterial cell wall composition and the influence of antibiotics by cell-wall and whole-cell NMR". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 370 (1679): 20150024. doi:10.1098/rstb.2015.0024. PMC 4632600. PMID 26370936.
- ^ Rutledge RD, Wright DW (2013). "Biomineralization: Peptide-Mediated Synthesis of Materials". In Lukehart CM, Scott RA (eds.). Nanomaterials: Inorganic and Bioinorganic Perspectives. EIC Books. Wiley. ISBN 978-1-118-62522-4. Retrieved 2016-03-14.
- ^ Hooke R (1665). Martyn J, Allestry J (eds.). Micrographia: or, Some physiological descriptions of minute bodies made by magnifying glasses. London.
- ^ a b Sattelmacher B (2000). "The apoplast and its significance for plant mineral nutrition". New Phytologist. 149 (2): 167–192. doi:10.1046/j.1469-8137.2001.00034.x. PMID 33874640.
- ^ Link HF (1807). Grundlehren der anatomie und physiologie der pflanzen. Danckwerts.
- ^ Baker JR (June 1952). "The Cell-Theory: A Restatement, History, and Critique: Part III. The Cell as a Morphological Unit". Journal of Cell Science. 3 (22): 157–90. doi:10.1242/jcs.s3-93.22.157.
- ^ Sharp LW (1921). Introduction To Cytology. New York: McGraw Hill. p. 25.
- ^ Münch E (1930). Die Stoffbewegungen in der Pflanze. Jena: Verlag von Gustav Fischer.
- ^ Roberts K (October 1994). "The plant extracellular matrix: in a new expansive mood". Current Opinion in Cell Biology. 6 (5): 688–94. doi:10.1016/0955-0674(89)90074-4. PMID 7833049.
- ^ Evert RF (2006). Esau's Plant Anatomy: Meristems, Cells, and Tissues of the Plant Body: Their Structure, Function, and Development (3rd ed.). Hoboken, New Jersey: John Wiley & Sons, Inc. pp. 65–66. ISBN 978-0-470-04737-8.
- ^ a b Bidhendi AJ, Geitmann A (January 2016). "Relating the mechanics of the primary plant cell wall to morphogenesis". Journal of Experimental Botany. 67 (2): 449–61. doi:10.1093/jxb/erv535. PMID 26689854.
- ^ a b c d Howland JL (2000). The Surprising Archaea: Discovering Another Domain of Life. Oxford: Oxford University Press. pp. 69–71. ISBN 978-0-19-511183-5.
- ^ Harvey Lodish; Arnold Berk; Chris A. Kaiser; Monty Krieger; Matthew P. Scott; Anthony Bretscher; Hidde Ploegh; Paul Matsudaira (1 September 2012). Loose-leaf Version for Molecular Cell Biology. W. H. Freeman. ISBN 978-1-4641-2746-5.
- ^ Hogan CM (2010). "Abiotic factor". In Monosson E, Cleveland C (eds.). Encyclopedia of Earth. Washington DC: National Council for Science and the Environment. Archived from the original on 2013-06-08.
- ^ Popper ZA, Michel G, Hervé C, Domozych DS, Willats WG, Tuohy MG, et al. (2011). "Evolution and diversity of plant cell walls: from algae to flowering plants". Annual Review of Plant Biology. 62: 567–90. doi:10.1146/annurev-arplant-042110-103809. hdl:10379/6762. PMID 21351878. S2CID 11961888.
- ^ a b c d e f Webster J, Weber RW (2007). Introduction to Fungi. New York, NY: Cambridge University Press. pp. 5–7.
- ^ Xie X, Lipke PN (August 2010). "On the evolution of fungal and yeast cell walls". Yeast. 27 (8): 479–88. doi:10.1002/yea.1787. PMC 3074402. PMID 20641026.
- ^ Ruiz-Herrera J, Ortiz-Castellanos L (May 2010). "Analysis of the phylogenetic relationships and evolution of the cell walls from yeasts and fungi". FEMS Yeast Research. 10 (3): 225–43. doi:10.1111/j.1567-1364.2009.00589.x. PMID 19891730.
- ^ Campbell NA, Reece JB, Urry LA, Cain ML, Wasserman SA, Minorsky PV, Jackson RB (2008). Biology (8th ed.). Pearson Benjamin Cummings. pp. 118. ISBN 978-0-8053-6844-4.
- ^ Buchanan BB, Gruissem W, Jones RL (2000). Biochemistry & molecular biology of plants (1st ed.). American society of plant physiology. ISBN 978-0-943088-39-6.
- ^ Fry SC (1989). "The Structure and Functions of Xyloglucan". Journal of Experimental Botany. 40 (1): 1–11. doi:10.1093/jxb/40.1.1.
- ^ Braidwood L, Breuer C, Sugimoto K (January 2014). "My body is a cage: mechanisms and modulation of plant cell growth". The New Phytologist. 201 (2): 388–402. doi:10.1111/nph.12473. PMID 24033322.
- ^ Moire L, Schmutz A, Buchala A, Yan B, Stark RE, Ryser U (March 1999). "Glycerol is a suberin monomer. New experimental evidence for an old hypothesis". Plant Physiology. 119 (3): 1137–46. doi:10.1104/pp.119.3.1137. PMC 32096. PMID 10069853.
- ^ Reid J (1997). "Carbohydrate metabolism:structural carbohydrates". In Dey PM, Harborne JB (eds.). Plant Biochemistry. Academic Press. pp. 205–236. ISBN 978-0-12-214674-9.
- ^ Jarvis MC (December 2013). "Cellulose biosynthesis: counting the chains". Plant Physiology. 163 (4): 1485–6. doi:10.1104/pp.113.231092. PMC 3850196. PMID 24296786.
- ^ Campbell NA, Reece JB, Urry LA, Cain ML, Wasserman SA, Minorsky PV, Jackson RB (2008). Biology (8th ed.). Pearson Benjamin Cummings. pp. 119. ISBN 978-0-8053-6844-4.
- ^ Abeysekera RM, Willison JH (1987). "A spiral helicoid in a plant cell wall". Cell Biology International Reports. 11 (2): 75–79. doi:10.1016/0309-1651(87)90106-8.
- ^ Hudler GW (1998). Magical Mushrooms, Mischievous Molds. Princeton, NJ: Princeton University Press. p. 7. ISBN 978-0-691-02873-6.
- ^ Sengbusch PV (2003-07-31). "Cell Walls of Algae". Botany Online. biologie.uni-hamburg.de. Archived from the original on November 28, 2005. Retrieved 2007-10-29.
- ^ Raven JA (1983). "The transport and function of silicon in plants". Biol. Rev. 58 (2): 179–207. doi:10.1111/j.1469-185X.1983.tb00385.x. S2CID 86067386.
- ^ Furnas MJ (1990). "In situ growth rates of marine phytoplankton : Approaches to measurement, community and species growth rates". J. Plankton Res. 12 (6): 1117–1151. doi:10.1093/plankt/12.6.1117.
- ^ Koivikko R, Loponen J, Honkanen T, Jormalainen V (January 2005). "Contents of soluble, cell-wall-bound and exuded phlorotannins in the brown alga Fucus vesiculosus, with implications on their ecological functions" (PDF). Journal of Chemical Ecology. 31 (1): 195–212. CiteSeerX 10.1.1.320.5895. doi:10.1007/s10886-005-0984-2. PMID 15839490. S2CID 1540749.
- ^ Sengbusch PV (2003-07-31). "Interactions between Plants and Fungi: the Evolution of their Parasitic and Symbiotic Relations". Biology Online. Archived from the original on December 8, 2006. Retrieved 2007-10-29.
- ^ a b Alexopoulos CJ, Mims W, Blackwell M (1996). "4". Introductory Mycology. New York: John Wiley & Sons. pp. 687–688. ISBN 978-0-471-52229-4.
- ^ a b Raper KB, Rahn AW (1984). The Dictyostelids. Princeton, NJ: Princeton University Press. pp. 99–100. ISBN 978-0-691-08345-2.
- ^ van Heijenoort J (2001). "Formation of the glycan chains in the synthesis of bacterial peptidoglycan". Glycobiology. 11 (3): 25R–36R. doi:10.1093/glycob/11.3.25R. PMID 11320055.
- ^ a b Koch AL (October 2003). "Bacterial wall as target for attack: past, present, and future research". Clinical Microbiology Reviews. 16 (4): 673–87. doi:10.1128/CMR.16.4.673-687.2003. PMC 207114. PMID 14557293.
- ^ Joseleau-Petit D, Liébart JC, Ayala JA, D'Ari R (September 2007). "Unstable Escherichia coli L forms revisited: growth requires peptidoglycan synthesis". Journal of Bacteriology. 189 (18): 6512–20. doi:10.1128/JB.00273-07. PMC 2045188. PMID 17586646.
- ^ Gram, HC (1884). "Über die isolierte Färbung der Schizomyceten in Schnitt- und Trockenpräparaten". Fortschr. Med. 2: 185–189.
- ^ Hugenholtz P (2002). "Exploring prokaryotic diversity in the genomic era". Genome Biology. 3 (2): REVIEWS0003. doi:10.1186/gb-2002-3-2-reviews0003. PMC 139013. PMID 11864374.
- ^ Walsh F, Amyes S (2004). "Microbiology and drug resistance mechanisms of fully resistant pathogens" (PDF). Curr Opin Microbiol. 7 (5): 439–44. doi:10.1016/j.mib.2004.08.007. PMID 15451497.
- ^ a b White D (1995). The Physiology and Biochemistry of Prokaryotes. Oxford: Oxford University Press. pp. 6, 12–21. ISBN 978-0-19-508439-9.
- ^ a b c d Brock TD, Madigan MT, Martinko JM, Parker J (1994). Biology of Microorganisms (7th ed.). Englewood Cliffs, NJ: Prentice Hall. pp. 818–819, 824. ISBN 978-0-13-042169-2.
- ^ Preisig HR (1994). "Terminology and nomenclature of protist cell surface structures". The Protistan Cell Surface (Protoplasma special ed.). pp. 1–28. doi:10.1007/978-3-7091-9378-5_1. ISBN 978-3-7091-9380-8.
- ^ Becker B (2000). "The cell surface of flagellates.". In Leadbeater BS, Green JC (eds.). The Flagellates. Unity, diversity and evolution. London: Taylor and Francis. Archived from the original on 2013-02-12.
- ^ Barsanti L, Gualtieri P (2006). Algae: anatomy, biochemistry, and biotechnology. Florida, USA: CRC Press.
- ^ Frantz C, Stewart KM, Weaver VM (December 2010). "The extracellular matrix at a glance". Journal of Cell Science. 123 (Pt 24): 4195–200. doi:10.1242/jcs.023820. PMC 2995612. PMID 21123617.
- ^ Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P (2002). Molecular biology of the cell (4th ed.). New York: Garland. p. 1065. ISBN 978-0-8153-4072-0.
