Acid–base titration: Difference between revisions
No edit summary |
m minor copy edits |
||
| (146 intermediate revisions by 47 users not shown) | |||
| Line 1: | Line 1: | ||
{{short description|Method of chemical quantitative analysis}} |
|||
[[Image:Titration.gif|frame|100px|frame|Titration setup. The burette would normally be held by a clamp, not shown here. The pink is most likely caused by use of the [[phenolphthalein]] indicator.]] |
|||
[[File:Titration NaOH HCl PP.ogv|thumb|The endpoint of an acid–base titration is reached as the indicator suddenly changes colour.|261x261px]] |
|||
{{Acids and bases}} |
{{Acids and bases}} |
||
An '''acid–base titration''' is a method of [[Quantitative analysis (chemistry)|quantitative analysis]] for determining the [[concentration]] of [[Brønsted–Lowry acid–base theory|Brønsted-Lowry acid or base]] (titrate) by [[neutralization reaction|neutralizing]] it using a solution of known concentration (titrant).<ref name="PennState">{{Cite web |last= |first= |title=Acid-Base Titrations 14.7 |url=https://psu.pb.unizin.org/chem112spring2020luz4/chapter/acid-base-titrations/ |access-date=2023-12-07 |website=PennState}}</ref> A [[pH indicator]] is used to monitor the progress of the [[acid–base reaction]] and a [[titration curve]] can be constructed.<ref name="PennState" /> |
|||
This differs from other modern modes of titrations, such as [[Redox titration|oxidation-reduction titrations]], precipitation titrations, & [[complexometric titration]]s.<ref name="www.britannica.com">{{Cite web |title=Titration {{!}} Definition, Types, & Facts {{!}} Britannica |url=https://www.britannica.com/science/titration |access-date=2023-12-06 |website=www.britannica.com |language=en}}</ref> Although these types of titrations are also used to determine unknown amounts of substances, these substances vary from ions to metals.<ref name="www.britannica.com" /> |
|||
An '''acid–base [[titration]]''' is the determination of the [[concentration]] of an acid or base by exactly neutralizing the acid or base with an acid or base of known concentration. This allows for [[Quantitative analysis (chemistry)|quantitative analysis]] of the concentration of an unknown [[acid]] or [[Base (chemistry)|base]] [[solution]]. It makes use of the [[neutralization reaction]] that occurs between acids and bases. pKa and Ka (acid constants) can also be determined from a pH titration graph. |
|||
Acid–base titration finds extensive applications in various scientific fields, such as pharmaceuticals, environmental monitoring, and quality control in industries.<ref>{{Cite journal |last1=Rajendraprasad |first1=Nagaraju |last2=Basavaiah |first2=Kanakapura |last3=Vinay |first3=Basavaiah Kanakapura |date=2010 |title=Acid-base titrimetric assay of hydroxyzine dihydrochloride in pharmaceutical samples |url=https://doiserbia.nb.rs/Article.aspx?id=1451-93721000014R |journal=Chemical Industry and Chemical Engineering Quarterly |volume=16 |issue=2 |pages=127–132}}</ref> This method's precision and simplicity makes it an important tool in quantitative chemical analysis, contributing significantly to the general understanding of solution chemistry.<ref>{{Cite book |last1=Li |first1=Na |url=https://books.google.com/books?id=Stw7DQAAQBAJ&dq=quantitative+chemical+analysis+acid-base+titration&pg=PR5 |title=Quantitative Chemical Analysis |last2=Hefferren |first2=John J. |last3=Li |first3=Ke'an |date=2013-04-26 |publisher=World Scientific Publishing Company |isbn=978-981-4452-31-1 |language=en}}</ref> |
|||
Acid–base titrations can also be used to find percent purity of chemicals. |
|||
== History == |
|||
[[File:Svante_Arrhenius_01.jpg|left|thumb|255x255px|Svante Arrhenius]] |
|||
The history of acid-base titration dates back to the late 19th century when advancements in analytical chemistry fostered the development of systematic techniques for quantitative analysis.<ref name="Szabadváry-1979">{{Cite journal |last1=Szabadváry |first1=Ferenc |last2=Chalmers® |first2=Robert A. |date=1979-08-01 |title=Carl Friedrich Mohr and analytical chemistry in Germany |url=https://dx.doi.org/10.1016/0039-9140%2879%2980165-4 |journal=Talanta |volume=26 |issue=8 |pages=609–617 |doi=10.1016/0039-9140(79)80165-4 |issn=0039-9140}}</ref> The origins of titration methods can be linked to the work of chemists such as [[Karl Friedrich Mohr]] in the mid-1800s.<ref name="Szabadváry-1979" /> His contributions laid the groundwork for understanding titrations involving acids and bases. |
|||
Theoretical progress came with the research of Swedish chemist [[Svante Arrhenius]], who in the late 19th century, introduced the Arrhenius theory, providing a theoretical framework for acid-base reactions.<ref name="Kousathana-2005">{{Cite journal |last1=Kousathana |first1=Margarita |last2=Demerouti |first2=Margarita |last3=Tsaparlis |first3=Georgios |date=2005-02-01 |title=Instructional Misconceptions in Acid-Base Equilibria: An Analysis from a History and Philosophy of Science Perspective |url=https://doi.org/10.1007/s11191-005-5719-9 |journal=Science & Education |language=en |volume=14 |issue=2 |pages=173–193 |doi=10.1007/s11191-005-5719-9 |issn=1573-1901}}</ref> This theoretical foundation, along with ongoing experimental refinements, contributed to the evolution of acid-base titration as a precise and widely applicable analytical method.<ref name="Kousathana-2005" /> |
|||
Over time, the method has undergone further refinements and adaptations, establishing itself as an essential tool in laboratories across various scientific disciplines. |
|||
==Alkalimetry and acidimetry== |
==Alkalimetry and acidimetry== |
||
Alkalimetry and acidimetry are |
Alkalimetry and acidimetry are types of volumetric analyses in which the fundamental reaction is a [[Neutralization (chemistry)|neutralization]] reaction. They involve the controlled addition of either an acid or a base (titrant) of known concentration to the solution of the unknown concentration (titrate) until the reaction reaches its stoichiometric equivalence point. At this point, the moles of acid and base are equal, resulting in a neutral solution:<ref>{{Cite web |title=Lesson 6.9: Neutralizing Acids and Bases |url=https://www.acs.org/middleschoolchemistry/lessonplans/chapter6/lesson9.html |access-date=2023-12-07 |website=American Chemical Society |language=en}}</ref> |
||
[[File:Titration_of_HCl_with_methyl_orange_indicator.png|thumb|444x444px|Titration of a standard solution using methyl orange indicator. Titrate is in Erlenmeyer flask, titrant is in burette.]] |
|||
:acid + base → salt + water |
|||
For example: |
|||
:HCl + NaOH → NaCl + H<sub>2</sub>O |
|||
Acidimetry is the specialized analytical use of acid-base titration to determine the concentration of a basic (alkaline) substance using standard acid. This can be used for weak bases and strong bases.<ref name="Hesperides2">{{cite book |url=https://books.google.com/books?id=Ae138bkVCqoC&pg=PA14 |title=The Chemical Age – Chemical Dictionary – Chemical Terms |date=2007-03-15 |publisher=Hesperides |isbn=978-1-4067-5758-3 |page=14}}</ref> An example of an acidimetric titration involving a strong base is as follows: |
|||
:Ba(OH)<sub>2</sub> + 2 H<sup>+</sup> → Ba<sup>2+</sup> + 2 H<sub>2</sub>O |
|||
In this case, the strong base (Ba(OH)<sub>2</sub>) is neutralized by the acid until all of the base has reacted. This allows the viewer to calculate the concentration of the base from the volume of the standard acid that is used. |
|||
Alkalimetry follows uses same concept of specialized analytic acid-base titration, but to determine the concentration of an acidic substance using standard base.<ref name="Hesperides2" /> An example of an alkalimetric titration involving a strong acid is as follows: |
|||
:H<sub>2</sub>SO<sub>4</sub> + 2 OH<sup>−</sup> → SO<sub>4</sub><sup>2-</sup> + 2 H<sub>2</sub>O |
|||
In this case, the strong acid (H<sub>2</sub>SO<sub>4</sub>) is neutralized by the base until all of the acid has reacted. This allows the viewer to calculate the concentration of the acid from the volume of the standard base that is used. |
|||
==Equipment== |
|||
The key equipment used in a titration are: |
|||
* [[Burette]] |
|||
* White tile – used to see a colour change in the solution |
|||
* [[Pipette]] |
|||
* [[pH indicator]] (the one used varies depending on the reactants) |
|||
* [[Erlenmeyer flask]] / Conical flask |
|||
* Titrant or titrator (a [[standard solution]] of known concentration, a common one is aqueous [[sodium carbonate]]) |
|||
* [[Analyte]] or titrand (solution of unknown concentration) |
|||
The standard solution (titrant) is stored in the [[burette]], while the solution of unknown concentration (analyte/titrate) is placed in the [[Erlenmeyer flask]] below it with an indicator.<ref name="groups.chem.ubc.ca">{{Cite web |title=Titration Curves |url=https://groups.chem.ubc.ca/courseware/pH/section14/index.html |access-date=2023-12-07 |website=groups.chem.ubc.ca}}</ref> |
|||
==Method== |
|||
==Indicator choice== |
|||
Before starting the titration a suitable [[pH indicator]] must be chosen. The [[equivalence point]] of the reaction, the point at which equivalent amounts of the reactants have reacted, will have a pH dependent on the relative strengths of the acid and base used. The pH of the equivalence point can be [[Approximation|estimated]] using the following rules: |
|||
A suitable pH indicator must be chosen in order to detect the end point of the titration.<ref name="groups.chem.ubc.ca-2">{{Cite web |title=Acid-Base Indicators |url=https://groups.chem.ubc.ca/courseware/pH/section15/index.html |access-date=2023-12-07 |website=groups.chem.ubc.ca}}</ref> The colour change or other effect should occur close to the [[equivalence point]] of the reaction so that the experimenter can accurately determine when that point is reached. The pH of the equivalence point can be [[Approximation|estimated]] using the following rules: |
|||
* A strong acid will react with a strong base to form a neutral (pH = 7) solution. |
* A strong acid will react with a strong base to form a neutral (pH = 7) solution. |
||
* A strong acid will react with a weak base to form an acidic (pH < 7) solution. |
* A strong acid will react with a weak base to form an acidic (pH < 7) solution. |
||
* A weak acid will react with a strong base to form a basic (pH > 7) solution. |
* A weak acid will react with a strong base to form a basic (pH > 7) solution. |
||
These indicators are essential tools in chemistry and biology, aiding in the determination of a solution's acidity or alkalinity through the observation of colour transitions.<ref name="groups.chem.ubc.ca-2" /> The table below serves as a reference guide for these indicator choices, offering insights into the pH ranges and colour transformations associated with specific indicators: |
|||
When a weak acid reacts with a weak base, the equivalence point solution will be basic if the base is stronger and acidic if the acid is stronger. If both are of equal strength, then the equivalence pH will be neutral. However, weak acids are not often titrated against weak bases because the colour change shown with the indicator is often quick, and therefore very difficult for the observer to see the change of colour. |
|||
{| class="wikitable" |
|||
The point at which the indicator changes colour is called the end point. A suitable indicator should be chosen, preferably one that will experience a change in colour (an end point) close to the equivalence point of the reaction. |
|||
|+Titration indicator table<ref>{{Cite journal |last1=Kahlert |first1=Heike |last2=Meyer |first2=Gabriele |last3=Albrecht |first3=Anja |date=2016-04-29 |title=Colour maps of acid–base titrations with colour indicators: how to choose the appropriate indicator and how to estimate the systematic titration errors |url=https://doi.org/10.1007/s40828-016-0026-4 |journal=ChemTexts |language=en |volume=2 |issue=2 |pages=7 |doi=10.1007/s40828-016-0026-4 |issn=2199-3793|doi-access=free }}</ref> |
|||
!Indicator name |
|||
!Indicator colour |
|||
!Transition interval (pH range) |
|||
!Color after high pH conditions |
|||
|- |
|||
|Methyl Orange |
|||
|Orange/red |
|||
|3.1 - 4.4 |
|||
|Yellow |
|||
|- |
|||
|Methyl Red |
|||
|Red |
|||
|4.4 - 6.3 |
|||
|Yellow |
|||
|- |
|||
|Congo Red |
|||
|Blue |
|||
|3.0 - 5.2 |
|||
|Red |
|||
|- |
|||
|Phenolphthalein |
|||
|Colourless |
|||
|8.3 - 10.0 |
|||
|Pink |
|||
|- |
|||
|Thymolphthalein |
|||
|Colourless |
|||
|9.3 - 10.5 |
|||
|Blue |
|||
|- |
|||
|Bromophenol Blue |
|||
|Yellow |
|||
|3.0 - 4.6 |
|||
|Blue |
|||
|- |
|||
|Bromocresol Green |
|||
|Yellow |
|||
|3.8 - 5.6 |
|||
|Blue |
|||
|- |
|||
|Thymol Blue |
|||
|Red |
|||
|1.2 - 2.8; 8.0 - 9.6 |
|||
|Blue |
|||
|- |
|||
|Cresol Red |
|||
|Yellow |
|||
|7.2 - 8.8 |
|||
|Violet |
|||
|- |
|||
|Neutral Red |
|||
|Red |
|||
|6.8 - 8.0 |
|||
|Yellow |
|||
|} |
|||
[[File:Výsledek_alkalimetrické_titrace.jpg|left|thumb|397x397px|Three different points in an acid-base titration using phenolphthalein as the indicator]] |
|||
[[Phenolphthalein]] is widely recognized as one of the most commonly used acid-base indicators in chemistry.<ref name="www.britannica.com-2023">{{Cite web |date=2023-09-15 |title=Phenolphthalein {{!}} pH indicator, acid-base titration, indicator dye {{!}} Britannica |url=https://www.britannica.com/science/phenolphthalein |access-date=2023-11-05 |website=www.britannica.com |language=en}}</ref> Its popularity is because of its effectiveness in a broad pH range and its distinct colour transitions.<ref name="www.britannica.com-2023" /> Its sharp and easily detectable colour changes makes phenolphthalein a valuable tool for determining the endpoint of acid-base titrations, as a precise pH change signifies the completion of the reaction. |
|||
First, the burette should be rinsed with the standard solution, the pipette with the unknown solution, and the conical flask with distilled water. |
|||
When a weak acid reacts with a weak base, the equivalence point solution will be basic if the base is stronger and acidic if the acid is stronger. If both are of equal strength, then the equivalence pH will be neutral.<ref name="Chemistry LibreTexts-2016">{{Cite web |date=2016-02-13 |title=13.5: Acid/Base Titration |url=https://chem.libretexts.org/Bookshelves/General_Chemistry/Chem1_(Lower)/13%3A_Acid-Base_Equilibria/13.05%3A_Acid_Base_Titration |access-date=2023-10-08 |website=Chemistry LibreTexts |language=en}}</ref> However, weak acids are not often titrated against weak bases because the colour change shown with the indicator is often quick, and therefore very difficult for the observer to see the change of colour. |
|||
Secondly, a known volume of the unknown concentration solution should be taken with the pipette and placed into the conical flask, along with a small amount of the indicator chosen. |
|||
The point at which the indicator changes colour is called the ''endpoint''.<ref name="groups.chem.ubc.ca-2" /> A suitable indicator should be chosen, preferably one that will experience a change in colour (an endpoint) close to the equivalence point of the reaction. |
|||
The known solution should then be allowed out of the burette, into the conical flask. At this stage we want a rough estimate of the amount of this solution it took to neutralize the unknown solution. The solution should be let out of the burette until the indicator changes colour and the value on the burette should be recorded. This is the first (or rough) titration volume and should be excluded from any calculation. |
|||
In addition to the wide variety of indicator solutions, pH papers, crafted from paper or plastic infused with combinations of these indicators, serve as a practical alternative.<ref name="Chemistry LibreTexts-2016" /> The pH of a solution can be estimated by immersing a strip of pH paper into it and matching the observed colour to the reference standards provided on the container.<ref name="Chemistry LibreTexts-2016" /> |
|||
At least three more titrations should be performed, this time more accurately, taking into account roughly where the end point will occur. The initial and final readings on the burette (prior to starting the titration and at the end point, respectively) should be recorded. Subtracting the initial volume from the final volume will yield the amount of titrant used to reach the end point. The end point is reached when the indicator just changes colour permanently. |
|||
== Overshot titration == |
|||
Acid–base titration is performed with a [[bromothymol blue]] indicator, when it is a strong acid – strong base titration, a [[phenolphthalein]] indicator in weak acid – strong base reactions, and a [[methyl orange]] indicator for strong acid – weak base reactions. If the base is off the scale, i.e. a pH of >13.5, and the acid has a pH >5.5, then an [[Alizarine Yellow R|Alizarine yellow]] indicator may be used. On the other hand, if the acid is off the scale, i.e. a pH of <0.5, and the base has a pH <8.5, then a [[Thymol blue|Thymol Blue]] indicator may be used. |
|||
[[File:Pink_colour_in_a_titration_conical_flask.jpg|thumb|261x261px|An overshot titration using phenolphthalein indicator]] |
|||
Overshot titrations are a common phenomenon, and refer to a situation where the volume of titrant added during a chemical titration exceeds the amount required to reach the equivalence point.<ref name="Kim-2009">{{Cite web |last=Kim |first=Myung-Hoon |date=October 2009 |title=How to Save Overshot Titrations |url=https://www.researchgate.net/publication/267355930 }}</ref> This excess titrant leads to an outcome where the solution becomes slightly more alkaline or over-acidified.<ref name="Kim-2009" /> |
|||
==Titration of weak acid== |
|||
[[File:Titration of weak acid with strong base.PNG|Titration of weak acid with strong base|400px|right]] |
|||
Overshooting the equivalence point can occur due to various factors, such as errors in burette readings, imperfect reaction stoichiometry, or issues with endpoint detection.<ref name="Kim-2009" /> The consequences of overshot titrations can affect the accuracy of the analytical results, particularly in quantitative analysis.<ref name="Kim-2009" /> |
|||
The pH of a [[weak acid]] solution being titrated with a strong base solution can be found at different points along the way. These points fall into one of four categories:<ref name=Harris>''Quantitative Chemical Analysis, 7Ed.'' by Daniel C. Harris. Freeman and Company 2007.</ref> |
|||
Researchers and analysts often employ corrective measures, such as back-titration<ref>{{Cite web |title=What is Back Titration? |url=https://www.thoughtco.com/back-titration-definition-608731 |access-date=2023-11-05 |website=ThoughtCo |language=en}}</ref> and using more precise titration techniques, to mitigate the impact of overshooting and obtain reliable and precise measurements. Understanding the causes, consequences, and solutions related to overshot titrations is crucial in achieving accurate and reproducible results in the field of chemistry. |
|||
== Mathematical analysis: titration of weak acid == |
|||
[[File:Titration_Curve.png|thumb|258x258px|Titration of a weak acid with a strong base showing pH level, volume of titrant, and different points throughout the titration process]] |
|||
For calculating concentrations, an [[ICE table]] can be used.<ref>{{Cite web |last=Gabi |date=2021-08-05 |title=Using an ICE Table |url=https://chemistrytalk.org/ice-table-chemistry/ |access-date=2023-12-06 |website=ChemTalk |language=en-US}}</ref><ref name="PennState" /> ICE stands for ''initial'', ''change'', and ''equilibrium''. |
|||
The pH of a [[weak acid]] solution being titrated with a strong base solution can be found at different points along the way. These points fall into one of four categories:<ref name="Harris">''Quantitative Chemical Analysis, 7Ed.'' by Daniel C. Harris. Freeman and Company 2007.</ref> |
|||
# initial pH |
# initial pH |
||
# pH before the equivalence point |
# pH before the equivalence point |
||
# pH at the equivalence point |
# pH at the equivalence point |
||
# pH after the equivalence point |
# pH after the equivalence point |
||
1. '''The initial pH''' is approximated for a weak acid solution in water using the equation:<ref name="PennState" /> |
|||
The equations and methods below assume that the concentration of the acid and base are at least 1000 times greater than the <math>K_a</math>of the acid. If not, a more rigorous calculation using an [[RICE chart]] is required. In fact the equations below are a simplification of the RICE chart. |
|||
<math chem="">\ce{pH} =-\log[\ce{H3O+}]_0</math> where <chem>[H3O+]0</chem> is the initial concentration of the [[Hydronium|hydronium ion]]. |
|||
'''1. The initial pH''' is approximated for a [[weak acid]] solution in water using the equation |
|||
2. '''The pH before the equivalence point''' depends on the amount of weak acid remaining and the amount of conjugate base formed. The pH can be calculated approximately by the [[Henderson–Hasselbalch equation]]:<ref name="PennState" /><math chem=""> \ce{pH} = -\log K_a +\log \frac\text{[Conjugate Base]}\text{[Weak Acid]} </math> where K<sub>a</sub> is the [[acid dissociation constant]]. |
|||
:<math>\mathrm{pH} = -\log \sqrt { K_a F }</math> |
|||
3. '''The pH at the equivalence point''' depends on how much the weak acid is consumed to be converted into its conjugate base. Note that when an acid neutralizes a base, the pH may or may not be neutral (pH = 7). The pH depends on the strengths of the acid and base. In the case of a weak acid and strong base titration, the pH is greater than 7 at the equivalence point. Thus pH can be calculated using the following formula:<ref name="PennState" /> |
|||
where <math>K_a</math> is the dissociation constant and <math>F</math> is the concentration of the acid. |
|||
<math chem=""> \ce{pH}_{eq}=-\log[\ce{H3O+}]_{eq}=14+\log[\ce{OH-}]_{eq} </math> Where <chem>{[OH^{-}]}</chem> is the concentration of the hydroxide ion. The concentration of the hydroxide ion is calculated from the concentration of the hydronium ion and using the following relationship: |
|||
'''2. The pH before the equivalence point''' depends on the amount of weak acid remaining and the amount of conjugate base formed. The pH can be calculated by the following formula (which is a variation of the [[Henderson-Hasselbalch equation]]): |
|||
<math chem=""> K_a K_b=K_w=10^{-14} </math> Where K<sub>b</sub> is the [[Acid dissociation constant|base dissociation constant]], K<sub>w</sub> is the water dissociation constant. |
|||
:<math chem> \ce{pH} = \ce{p}K_a + \log( \frac{n_\ce{OH^-\ added}}{n_\ce{HA\ initial}-n_\ce{OH^-\ added}} )</math> |
|||
4. '''The pH after the equivalence point''' depends on the concentration of the conjugate base of the weak acid and the strong base of the titrant. However, the base of the titrant is stronger than the conjugate base of the acid. Therefore, the pH in this region is controlled by the strong base. As such the pH can be found using the following:<ref name="PennState" /> |
|||
where: |
|||
* <math>\mathrm{p}K_a</math> is the negative log of the [[acid dissociation constant]] of the weak acid. |
|||
* <math>n_{OH^-}</math> is the number of moles of added strong base in the solution. |
|||
* <math>n_{HA}</math> is the number of moles the weak acid initially present. |
|||
<math chem=""> \ce{pH} = 14+\log[\ce{OH^-}]= 14 + \log \frac {(C_bV_b)-(C_aV_a)} { V_a + V_b } </math> where <math chem=""> C_{b} </math> is the concentration of the strong base that is added, <math chem=""> V_{b} </math> is the volume of base added until the equilibrium, <math chem=""> C_{a} </math> is the concentration of the strong acid that is added, and <math chem=""> V_{a} </math> is the initial volume of the acid. |
|||
When the numerator of the log term equals the denominator (<math chem> {n_\ce{OH^-\ added}}={n_\ce{HA\ initial}-{n_\ce{OH^-\ added}}}</math>), then the ratio goes to 1 and the log term goes to zero. Thus the pH will equal the pK<sub>a</sub> which occurs half-way to the equivalence point. The half-titration can be used to determine the K<sub>a</sub> value of an acid or base that is unknown. |
|||
=== Single formula === |
|||
[[File:Titolazione.gif|thumb|250px|Animation of titration with base titrant]] |
|||
More accurately, a single formula<ref name="de Levie">{{cite journal|title=Explicit expressions of the general form of the titration curve in terms of concentration: Writing a single closed-form expression for the titration curve for a variety of titrations without using approximations or segmentation|journal=Journal of Chemical Education|volume=70|issue=3|pages=209|doi=10.1021/ed070p209|bibcode = 1993JChEd..70..209D |year=1993|last1=De Levie|first1=Robert|author-link1=Robert de Levie}}</ref> that describes the titration of a weak acid with a strong base from start to finish is given below: |
|||
'''3. At the equivalence point''', the weak acid is consumed and converted to its conjugate base. The pH will be greater than 7 and can be calculated from an equation derived from the following relationships: |
|||
# <math>\mathrm{pH + pOH = 14}</math> |
|||
# <math>K_a K_b=10^{-14}</math> |
|||
# at equivalence <math>C_a V_a = C_b V_b</math> |
|||
: <math chem="">\phi = \frac{C_b V_b }{C_a V_a}</math> |
|||
The previous 3 relationships are used to generate the equivalence point pH formula below: |
|||
where |
|||
" φ = fraction of completion of the titration (φ < 1 is before the equivalence point, φ = 1 is the equivalence point, and φ > 1 is after the equivalence point) |
|||
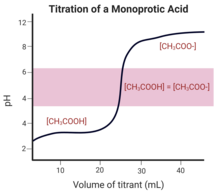
: [[File:Monoprotic_acid_titration.png|thumb|223x223px|Monoprotic acid titration curve. Highlighted pink region depicts equivalence point.]] |
|||
<math>C_a, C_b</math> = the concentrations of the acid and base respectively |
|||
:<math> |
|||
: <math>V_a, V_b</math> = the volumes of the acid and base respectively |
|||
\mathrm{pH} = 14 + \log \sqrt { \frac {C_a C_b K_w} {(C_a + C_b) K_a} } |
|||
</math> |
|||
* <math>C_a</math> = concentration of acid and <math>C_b</math> = concentration of base |
|||
* <math>K_w</math> = dissociation constant for water and <math>K_a></math> = dissociation constant for the acid |
|||
==Graphical methods== |
|||
Note that when an acid neutralizes a base, the pH may or may not be neutral (pH = 7). The pH depends on the strengths of the acid and base. |
|||
Identifying the pH associated with any stage in the titration process is relatively simple for monoprotic acids and bases. A monoprotic acid is an acid that donates one proton. A monoprotic base is a base that accepts one proton. A monoprotic acid or base only has one equivalence point on a titration curve.<ref name="Chemistry LibreTexts-2016" /><ref name="groups.chem.ubc.ca" /> |
|||
[[File:Diprotic_Acid_Titration.png|thumb|223x223px|Diprotic acid titration curve. Highlighted pink regions depict equivalence points.]] |
|||
'''4. After the equivalence point''', the solution will contain two bases: the conjugate base of the acid and the strong base of the titrant. However, the base of the titrant is stronger than the conjugate base of the acid. Therefore, the pH in this region is controlled by the strong base. As such the pH can be found using the following: |
|||
A diprotic acid donates two protons and a diprotic base accepts two protons. The titration curve for a diprotic solution has two equivalence points.<ref name="Chemistry LibreTexts-2016" /><ref name="groups.chem.ubc.ca" /> |
|||
:<math> |
|||
\mathrm{pH} = 14 + \log \frac {C_b V_b - C_a V_a} {(V_a + V_b )} |
|||
</math> |
|||
A polyprotic substance has multiple equivalence points.<ref name="groups.chem.ubc.ca" /> |
|||
'''Single formula'''. More accurately, a single formula<ref name="de Levie">{{cite journal|title=Explicit expressions of the general form of the [[titration curve]] in terms of concentration: Writing a single closed-form expression for the titration curve for a variety of titrations without using approximations or segmentation|doi=10.1021/ed070p209|bibcode = 1993JChEd..70..209D }}</ref> that describes the titration of a weak acid with a strong base from start to finish is given below: |
|||
All titration reactions contain small buffer regions that appear horizontal on the graph. These regions contain comparable concentrations of acid and base, preventing sudden changes in pH when additional acid or base is added.<ref>{{Cite web |title=Titration pH Curves – HSC Chemistry |url=https://scienceready.com.au/pages/titration-curves |access-date=2023-12-06 |website=Science Ready |language=en}}</ref><ref name="groups.chem.ubc.ca" /> |
|||
:<math chem>\phi = \frac{ C_b V_b }{C_a V_a} = \frac{\alpha_\ce{A^-} - \frac\ce{[H^+] - [OH^-]}{C_a}}{1 + \frac\ce{[H^+] - [OH^-]}{C_b}}</math> |
|||
== Pharmaceutical applications == |
|||
:<math chem>\alpha_{A^-} = \frac {K_a}{[\ce H^+] + K_a}</math> |
|||
[[File:Chemist_woman.jpg|left|thumb|267x267px|A chemist performing an acid-base titration in lab]] |
|||
* <math chem>\phi</math> = fraction of completion of the titration (φ < 1 is before the equivalence point, φ = 1 is the equivalence point, and φ > 1 is after the equivalence point) |
|||
* <math chem>C_a, C_b</math> = the concentrations of the acid and base respectively |
|||
* <math chem>V_a, V_b</math> = the volumes of the acid and base respectively |
|||
* <math chem>\alpha_\ce{A^-}</math> = the fraction of the weak acid that is ionized |
|||
* <math chem>K_a</math> = the dissociation constant for the acid |
|||
* <math chem>\ce{[H^+], [OH^-]}</math> = concentrations of the H<sup>+</sup> and OH<sup>–</sup> ions respectively |
|||
In the pharmaceutical industry, acid-base titration serves as a fundamental analytical technique with diverse applications. One primary use involves the determination of the concentration of [[Active ingredient|Active Pharmaceutical Ingredients]] (APIs) in drug formulations, ensuring product quality and compliance with regulatory standards.<ref>{{Cite web |last1=Alhamdany |first1=Hayder |last2=Alfahad |first2=Mohanad |date=Jul–Sep 2021 |title=Stability evaluation of Acetylsalicylic acid in commercial Aspirin tablets available in the Iraqi market |url=https://japer.in/storage/files/article/07ee90b8-d943-46f7-b4ab-3e7733ae0894-6j0xFKQqQmKFumVE/japer-vol-11-iss-3-20-24-8001.pdf }}</ref> |
|||
This formula is somewhat cumbersome, but does describe the titration curve as a single equation. |
|||
Acid–base titration is particularly valuable in quantifying acidic or basic functional groups with pharmaceutical compounds. Additionally, the method is employed for the analysis of additives or ingredients, making it easier to adjust and control how a product is made.<ref name="Chapman-1949">{{Cite journal |last=Chapman |first=O. W. |date=1949 |title=Statistical Quality Control in College Analytical Laboratories |url=https://www.jstor.org/stable/3626169 |journal=Transactions of the Kansas Academy of Science |volume=52 |issue=2 |pages=160–167 |doi=10.2307/3626169 |jstor=3626169 |issn=0022-8443}}</ref> Quality control laboratories utilize acid-base titration to assess the purity of raw materials and to monitor various stages of drug manufacturing processes.<ref name="Chapman-1949" /> |
|||
== Gallery == |
|||
<gallery> |
|||
Acidobazna titracija 001.jpg| |
|||
Acidobazna titracija 002.jpg| |
|||
</gallery> |
|||
The technique's reliability and simplicity make it an integral tool in pharmaceutical research and development, contributing to the production of safe and effective medications. |
|||
==Graphical Methods== |
|||
The titration process creates solutions with compositions ranging from pure acid to pure base. Identifying the pH associated with any stage in the titration process is relatively simple for monoprotic acids and bases. The presence of more than one acid or base group complicates these computations. Graphical methods,<ref> |
|||
== Environmental monitoring applications == |
|||
{{Cite web |
|||
[[File:Soil_fertility_analysis_9_Titration_of_extractable_acidity.jpg|thumb|Analysis of soil fertility using acid-base titration]] |
|||
| title = The Equligraph: Revisiting an old tool |
|||
| url = http://www.tahosa.us/Equiligraph/Equiligraph/Equiligraph_Basics.html |
|||
Acid–base titration plays a crucial role in environmental monitoring by providing a quantitative analytical method for assessing the acidity or alkalinity of water samples.<ref name="Marle-2005">{{Cite journal |last1=Marle |first1=Leanne |last2=Greenway |first2=Gillian M. |date=2005-10-01 |title=Microfluidic devices for environmental monitoring |url=https://www.sciencedirect.com/science/article/pii/S0165993605001883 |journal=TrAC Trends in Analytical Chemistry |volume=24 |issue=9 |pages=795–802 |doi=10.1016/j.trac.2005.08.003 |issn=0165-9936}}</ref> The measurement of parameters such as pH, total alkalinity, and acidity is essential in evaluating the environmental impact of industrial discharges, [[Agricultural pollution|agricultural runoff]], and other sources of [[Water pollution|water contamination]].<ref name="Marle-2005" /> |
|||
| accessdate = 4 October 2015 |
|||
}}</ref> such as the equiligraph,<ref>{{Cite book |
|||
Acid–base titration allows for the determination of the [[Buffer solution|buffering capacity]] of natural water systems, aiding in the assessment of their ability to resist changes in pH.<ref name="journals.biologists.com">{{Cite web |title=Urea production, acid–base regulation and their interactions in the lake magadi tilapia, a unique teleost adapted to a highly alkaline environment |url=https://journals.biologists.com/jeb/article/189/1/13/6778/Urea-production-acid-base-regulation-and-their |access-date=2023-12-06 |website=journals.biologists.com}}</ref> Monitoring pH levels is important for preserving aquatic ecosystems and ensuring compliance with environmental regulations.<ref name="journals.biologists.com" /> |
|||
| last = Freiser |
|||
| first = H. |
|||
Acid–base titration is also utilized in the analysis of acid rain effects on soil and water bodies, contributing to the overall understanding and management of environmental quality.<ref name="Karmanovskaya-2021">{{Cite web |last1=Karmanovskaya |first1=Natalia V |last2=Nosova |first2=Olga V |last3=Galishevskaya |first3=Victoria V |date=February 2, 2021 |title=Public Environmental Monitoring of the Quality of Water Bodies in Norilsk and Taimyr |url=https://www.academia.edu/49206163/Public_Environmental_Monitoring_of_the_Quality_of_Water_Bodies_in_Norilsk_and_Taimyr |via=[[Academia.edu]] }}</ref> The method's prevision and reliability make it a valuable tool in safeguarding ecosystems and assessing the impact of human activities on natural water resources.<ref name="Karmanovskaya-2021" /> |
|||
| title = Ionic Equilibria in Analytical Chemistry |
|||
| publisher = Kreiger |
|||
| year = 1963 |
|||
| isbn = 0-88275-955-8 |
|||
}}</ref> have long been used to account for the interaction of coupled equilibria. These graphical solution methods are simple to implement, however they are infrequently used. |
|||
== See also == |
== See also == |
||
* [[Titration]] |
|||
* [[Titration curve]] |
|||
* [[Equivalence point]] |
|||
* [[Acid dissociation constant]] |
|||
* [[Acid–base reaction]] |
|||
* [[Henderson–Hasselbalch equation]] |
* [[Henderson–Hasselbalch equation]] |
||
* [[pH indicator]] |
|||
==References== |
==References== |
||
| Line 135: | Line 198: | ||
==External links== |
==External links== |
||
{{Commons category| |
{{Commons category|Acid-base titration}} |
||
* [http://www.tahosa.us/Equiligraph/Equiligraph/Equiligraph_Basics.html Graphical method to solve acid-base problems, including titrations] |
* [http://www.tahosa.us/Equiligraph/Equiligraph/Equiligraph_Basics.html Graphical method to solve acid-base problems, including titrations] |
||
* [http://www.tahosa.us/Equiligraph/Equiligraph/Titration_App.html Graphic and numerical solver for general acid-base problems - Software Program for phone and tablets] |
* [http://www.tahosa.us/Equiligraph/Equiligraph/Titration_App.html Graphic and numerical solver for general acid-base problems - Software Program for phone and tablets] |
||
* {{cite journal |url= http://www.thenucleuspak.org.pk/index.php/Nucleus/article/view/675 |title=Simple analytical formulas for the titration of polyprotic acids |last=Khan |first=A.S.A. |journal=The Nucleus |volume=51 |year=2014 |issue=4 |pages=448–454 |issn=2306-6539 }} |
|||
{{Analytical chemistry}} |
{{Analytical chemistry}} |
||
{{Authority control}} |
|||
{{DEFAULTSORT:Acid-base titration}} |
{{DEFAULTSORT:Acid-base titration}} |
||
Latest revision as of 17:24, 5 November 2024
An acid–base titration is a method of quantitative analysis for determining the concentration of Brønsted-Lowry acid or base (titrate) by neutralizing it using a solution of known concentration (titrant).[1] A pH indicator is used to monitor the progress of the acid–base reaction and a titration curve can be constructed.[1]
This differs from other modern modes of titrations, such as oxidation-reduction titrations, precipitation titrations, & complexometric titrations.[2] Although these types of titrations are also used to determine unknown amounts of substances, these substances vary from ions to metals.[2]
Acid–base titration finds extensive applications in various scientific fields, such as pharmaceuticals, environmental monitoring, and quality control in industries.[3] This method's precision and simplicity makes it an important tool in quantitative chemical analysis, contributing significantly to the general understanding of solution chemistry.[4]
History
[edit]
The history of acid-base titration dates back to the late 19th century when advancements in analytical chemistry fostered the development of systematic techniques for quantitative analysis.[5] The origins of titration methods can be linked to the work of chemists such as Karl Friedrich Mohr in the mid-1800s.[5] His contributions laid the groundwork for understanding titrations involving acids and bases.
Theoretical progress came with the research of Swedish chemist Svante Arrhenius, who in the late 19th century, introduced the Arrhenius theory, providing a theoretical framework for acid-base reactions.[6] This theoretical foundation, along with ongoing experimental refinements, contributed to the evolution of acid-base titration as a precise and widely applicable analytical method.[6]
Over time, the method has undergone further refinements and adaptations, establishing itself as an essential tool in laboratories across various scientific disciplines.
Alkalimetry and acidimetry
[edit]Alkalimetry and acidimetry are types of volumetric analyses in which the fundamental reaction is a neutralization reaction. They involve the controlled addition of either an acid or a base (titrant) of known concentration to the solution of the unknown concentration (titrate) until the reaction reaches its stoichiometric equivalence point. At this point, the moles of acid and base are equal, resulting in a neutral solution:[7]

- acid + base → salt + water
For example:
- HCl + NaOH → NaCl + H2O
Acidimetry is the specialized analytical use of acid-base titration to determine the concentration of a basic (alkaline) substance using standard acid. This can be used for weak bases and strong bases.[8] An example of an acidimetric titration involving a strong base is as follows:
- Ba(OH)2 + 2 H+ → Ba2+ + 2 H2O
In this case, the strong base (Ba(OH)2) is neutralized by the acid until all of the base has reacted. This allows the viewer to calculate the concentration of the base from the volume of the standard acid that is used.
Alkalimetry follows uses same concept of specialized analytic acid-base titration, but to determine the concentration of an acidic substance using standard base.[8] An example of an alkalimetric titration involving a strong acid is as follows:
- H2SO4 + 2 OH− → SO42- + 2 H2O
In this case, the strong acid (H2SO4) is neutralized by the base until all of the acid has reacted. This allows the viewer to calculate the concentration of the acid from the volume of the standard base that is used.
The standard solution (titrant) is stored in the burette, while the solution of unknown concentration (analyte/titrate) is placed in the Erlenmeyer flask below it with an indicator.[9]
Indicator choice
[edit]A suitable pH indicator must be chosen in order to detect the end point of the titration.[10] The colour change or other effect should occur close to the equivalence point of the reaction so that the experimenter can accurately determine when that point is reached. The pH of the equivalence point can be estimated using the following rules:
- A strong acid will react with a strong base to form a neutral (pH = 7) solution.
- A strong acid will react with a weak base to form an acidic (pH < 7) solution.
- A weak acid will react with a strong base to form a basic (pH > 7) solution.
These indicators are essential tools in chemistry and biology, aiding in the determination of a solution's acidity or alkalinity through the observation of colour transitions.[10] The table below serves as a reference guide for these indicator choices, offering insights into the pH ranges and colour transformations associated with specific indicators:
| Indicator name | Indicator colour | Transition interval (pH range) | Color after high pH conditions |
|---|---|---|---|
| Methyl Orange | Orange/red | 3.1 - 4.4 | Yellow |
| Methyl Red | Red | 4.4 - 6.3 | Yellow |
| Congo Red | Blue | 3.0 - 5.2 | Red |
| Phenolphthalein | Colourless | 8.3 - 10.0 | Pink |
| Thymolphthalein | Colourless | 9.3 - 10.5 | Blue |
| Bromophenol Blue | Yellow | 3.0 - 4.6 | Blue |
| Bromocresol Green | Yellow | 3.8 - 5.6 | Blue |
| Thymol Blue | Red | 1.2 - 2.8; 8.0 - 9.6 | Blue |
| Cresol Red | Yellow | 7.2 - 8.8 | Violet |
| Neutral Red | Red | 6.8 - 8.0 | Yellow |

Phenolphthalein is widely recognized as one of the most commonly used acid-base indicators in chemistry.[12] Its popularity is because of its effectiveness in a broad pH range and its distinct colour transitions.[12] Its sharp and easily detectable colour changes makes phenolphthalein a valuable tool for determining the endpoint of acid-base titrations, as a precise pH change signifies the completion of the reaction.
When a weak acid reacts with a weak base, the equivalence point solution will be basic if the base is stronger and acidic if the acid is stronger. If both are of equal strength, then the equivalence pH will be neutral.[13] However, weak acids are not often titrated against weak bases because the colour change shown with the indicator is often quick, and therefore very difficult for the observer to see the change of colour.
The point at which the indicator changes colour is called the endpoint.[10] A suitable indicator should be chosen, preferably one that will experience a change in colour (an endpoint) close to the equivalence point of the reaction.
In addition to the wide variety of indicator solutions, pH papers, crafted from paper or plastic infused with combinations of these indicators, serve as a practical alternative.[13] The pH of a solution can be estimated by immersing a strip of pH paper into it and matching the observed colour to the reference standards provided on the container.[13]
Overshot titration
[edit]
Overshot titrations are a common phenomenon, and refer to a situation where the volume of titrant added during a chemical titration exceeds the amount required to reach the equivalence point.[14] This excess titrant leads to an outcome where the solution becomes slightly more alkaline or over-acidified.[14]
Overshooting the equivalence point can occur due to various factors, such as errors in burette readings, imperfect reaction stoichiometry, or issues with endpoint detection.[14] The consequences of overshot titrations can affect the accuracy of the analytical results, particularly in quantitative analysis.[14]
Researchers and analysts often employ corrective measures, such as back-titration[15] and using more precise titration techniques, to mitigate the impact of overshooting and obtain reliable and precise measurements. Understanding the causes, consequences, and solutions related to overshot titrations is crucial in achieving accurate and reproducible results in the field of chemistry.
Mathematical analysis: titration of weak acid
[edit]
For calculating concentrations, an ICE table can be used.[16][1] ICE stands for initial, change, and equilibrium.
The pH of a weak acid solution being titrated with a strong base solution can be found at different points along the way. These points fall into one of four categories:[17]
- initial pH
- pH before the equivalence point
- pH at the equivalence point
- pH after the equivalence point
1. The initial pH is approximated for a weak acid solution in water using the equation:[1]
where is the initial concentration of the hydronium ion.
2. The pH before the equivalence point depends on the amount of weak acid remaining and the amount of conjugate base formed. The pH can be calculated approximately by the Henderson–Hasselbalch equation:[1] where Ka is the acid dissociation constant.
3. The pH at the equivalence point depends on how much the weak acid is consumed to be converted into its conjugate base. Note that when an acid neutralizes a base, the pH may or may not be neutral (pH = 7). The pH depends on the strengths of the acid and base. In the case of a weak acid and strong base titration, the pH is greater than 7 at the equivalence point. Thus pH can be calculated using the following formula:[1]
Where is the concentration of the hydroxide ion. The concentration of the hydroxide ion is calculated from the concentration of the hydronium ion and using the following relationship:
Where Kb is the base dissociation constant, Kw is the water dissociation constant.
4. The pH after the equivalence point depends on the concentration of the conjugate base of the weak acid and the strong base of the titrant. However, the base of the titrant is stronger than the conjugate base of the acid. Therefore, the pH in this region is controlled by the strong base. As such the pH can be found using the following:[1]
where is the concentration of the strong base that is added, is the volume of base added until the equilibrium, is the concentration of the strong acid that is added, and is the initial volume of the acid.
Single formula
[edit]More accurately, a single formula[18] that describes the titration of a weak acid with a strong base from start to finish is given below:
where " φ = fraction of completion of the titration (φ < 1 is before the equivalence point, φ = 1 is the equivalence point, and φ > 1 is after the equivalence point)
= the concentrations of the acid and base respectively
- = the volumes of the acid and base respectively
Graphical methods
[edit]Identifying the pH associated with any stage in the titration process is relatively simple for monoprotic acids and bases. A monoprotic acid is an acid that donates one proton. A monoprotic base is a base that accepts one proton. A monoprotic acid or base only has one equivalence point on a titration curve.[13][9]

A diprotic acid donates two protons and a diprotic base accepts two protons. The titration curve for a diprotic solution has two equivalence points.[13][9]
A polyprotic substance has multiple equivalence points.[9]
All titration reactions contain small buffer regions that appear horizontal on the graph. These regions contain comparable concentrations of acid and base, preventing sudden changes in pH when additional acid or base is added.[19][9]
Pharmaceutical applications
[edit]
In the pharmaceutical industry, acid-base titration serves as a fundamental analytical technique with diverse applications. One primary use involves the determination of the concentration of Active Pharmaceutical Ingredients (APIs) in drug formulations, ensuring product quality and compliance with regulatory standards.[20]
Acid–base titration is particularly valuable in quantifying acidic or basic functional groups with pharmaceutical compounds. Additionally, the method is employed for the analysis of additives or ingredients, making it easier to adjust and control how a product is made.[21] Quality control laboratories utilize acid-base titration to assess the purity of raw materials and to monitor various stages of drug manufacturing processes.[21]
The technique's reliability and simplicity make it an integral tool in pharmaceutical research and development, contributing to the production of safe and effective medications.
Environmental monitoring applications
[edit]
Acid–base titration plays a crucial role in environmental monitoring by providing a quantitative analytical method for assessing the acidity or alkalinity of water samples.[22] The measurement of parameters such as pH, total alkalinity, and acidity is essential in evaluating the environmental impact of industrial discharges, agricultural runoff, and other sources of water contamination.[22]
Acid–base titration allows for the determination of the buffering capacity of natural water systems, aiding in the assessment of their ability to resist changes in pH.[23] Monitoring pH levels is important for preserving aquatic ecosystems and ensuring compliance with environmental regulations.[23]
Acid–base titration is also utilized in the analysis of acid rain effects on soil and water bodies, contributing to the overall understanding and management of environmental quality.[24] The method's prevision and reliability make it a valuable tool in safeguarding ecosystems and assessing the impact of human activities on natural water resources.[24]
See also
[edit]References
[edit]- ^ a b c d e f g "Acid-Base Titrations 14.7". PennState. Retrieved 2023-12-07.
- ^ a b "Titration | Definition, Types, & Facts | Britannica". www.britannica.com. Retrieved 2023-12-06.
- ^ Rajendraprasad, Nagaraju; Basavaiah, Kanakapura; Vinay, Basavaiah Kanakapura (2010). "Acid-base titrimetric assay of hydroxyzine dihydrochloride in pharmaceutical samples". Chemical Industry and Chemical Engineering Quarterly. 16 (2): 127–132.
- ^ Li, Na; Hefferren, John J.; Li, Ke'an (2013-04-26). Quantitative Chemical Analysis. World Scientific Publishing Company. ISBN 978-981-4452-31-1.
- ^ a b Szabadváry, Ferenc; Chalmers®, Robert A. (1979-08-01). "Carl Friedrich Mohr and analytical chemistry in Germany". Talanta. 26 (8): 609–617. doi:10.1016/0039-9140(79)80165-4. ISSN 0039-9140.
- ^ a b Kousathana, Margarita; Demerouti, Margarita; Tsaparlis, Georgios (2005-02-01). "Instructional Misconceptions in Acid-Base Equilibria: An Analysis from a History and Philosophy of Science Perspective". Science & Education. 14 (2): 173–193. doi:10.1007/s11191-005-5719-9. ISSN 1573-1901.
- ^ "Lesson 6.9: Neutralizing Acids and Bases". American Chemical Society. Retrieved 2023-12-07.
- ^ a b The Chemical Age – Chemical Dictionary – Chemical Terms. Hesperides. 2007-03-15. p. 14. ISBN 978-1-4067-5758-3.
- ^ a b c d e "Titration Curves". groups.chem.ubc.ca. Retrieved 2023-12-07.
- ^ a b c "Acid-Base Indicators". groups.chem.ubc.ca. Retrieved 2023-12-07.
- ^ Kahlert, Heike; Meyer, Gabriele; Albrecht, Anja (2016-04-29). "Colour maps of acid–base titrations with colour indicators: how to choose the appropriate indicator and how to estimate the systematic titration errors". ChemTexts. 2 (2): 7. doi:10.1007/s40828-016-0026-4. ISSN 2199-3793.
- ^ a b "Phenolphthalein | pH indicator, acid-base titration, indicator dye | Britannica". www.britannica.com. 2023-09-15. Retrieved 2023-11-05.
- ^ a b c d e "13.5: Acid/Base Titration". Chemistry LibreTexts. 2016-02-13. Retrieved 2023-10-08.
- ^ a b c d Kim, Myung-Hoon (October 2009). "How to Save Overshot Titrations".
- ^ "What is Back Titration?". ThoughtCo. Retrieved 2023-11-05.
- ^ Gabi (2021-08-05). "Using an ICE Table". ChemTalk. Retrieved 2023-12-06.
- ^ Quantitative Chemical Analysis, 7Ed. by Daniel C. Harris. Freeman and Company 2007.
- ^ De Levie, Robert (1993). "Explicit expressions of the general form of the titration curve in terms of concentration: Writing a single closed-form expression for the titration curve for a variety of titrations without using approximations or segmentation". Journal of Chemical Education. 70 (3): 209. Bibcode:1993JChEd..70..209D. doi:10.1021/ed070p209.
- ^ "Titration pH Curves – HSC Chemistry". Science Ready. Retrieved 2023-12-06.
- ^ Alhamdany, Hayder; Alfahad, Mohanad (Jul–Sep 2021). "Stability evaluation of Acetylsalicylic acid in commercial Aspirin tablets available in the Iraqi market" (PDF).
- ^ a b Chapman, O. W. (1949). "Statistical Quality Control in College Analytical Laboratories". Transactions of the Kansas Academy of Science. 52 (2): 160–167. doi:10.2307/3626169. ISSN 0022-8443. JSTOR 3626169.
- ^ a b Marle, Leanne; Greenway, Gillian M. (2005-10-01). "Microfluidic devices for environmental monitoring". TrAC Trends in Analytical Chemistry. 24 (9): 795–802. doi:10.1016/j.trac.2005.08.003. ISSN 0165-9936.
- ^ a b "Urea production, acid–base regulation and their interactions in the lake magadi tilapia, a unique teleost adapted to a highly alkaline environment". journals.biologists.com. Retrieved 2023-12-06.
- ^ a b Karmanovskaya, Natalia V; Nosova, Olga V; Galishevskaya, Victoria V (February 2, 2021). "Public Environmental Monitoring of the Quality of Water Bodies in Norilsk and Taimyr" – via Academia.edu.
External links
[edit]- Graphical method to solve acid-base problems, including titrations
- Graphic and numerical solver for general acid-base problems - Software Program for phone and tablets
- Khan, A.S.A. (2014). "Simple analytical formulas for the titration of polyprotic acids". The Nucleus. 51 (4): 448–454. ISSN 2306-6539.


![{\displaystyle {\ce {pH}}=-\log[{\ce {H3O+}}]_{0}}](https://wikimedia.org/enwiki/api/rest_v1/media/math/render/svg/c4f9c03cfac5741d4d94fdcb4fd5192674871672)
![{\displaystyle {\ce {[H3O+]0}}}](https://wikimedia.org/enwiki/api/rest_v1/media/math/render/svg/cc694abd3878b10501071f967a62631f72990aaf)
![{\displaystyle {\ce {pH}}=-\log K_{a}+\log {\frac {\text{[Conjugate Base]}}{\text{[Weak Acid]}}}}](https://wikimedia.org/enwiki/api/rest_v1/media/math/render/svg/a639c98d02c1da328344ecfc0c793a39e9747212)
![{\displaystyle {\ce {pH}}_{eq}=-\log[{\ce {H3O+}}]_{eq}=14+\log[{\ce {OH-}}]_{eq}}](https://wikimedia.org/enwiki/api/rest_v1/media/math/render/svg/4670f51daf3aa3216775e5267108f091eaaeab1b)
![{\displaystyle {\ce {[OH^{-}]}}}](https://wikimedia.org/enwiki/api/rest_v1/media/math/render/svg/125fb7165b6c82998e782ceb2fc48fe797c4fe87)

![{\displaystyle {\ce {pH}}=14+\log[{\ce {OH^-}}]=14+\log {\frac {(C_{b}V_{b})-(C_{a}V_{a})}{V_{a}+V_{b}}}}](https://wikimedia.org/enwiki/api/rest_v1/media/math/render/svg/9ee3f7f37dbf56900008c1b226dbe6e47201ac49)