Allopregnanolone: Difference between revisions
Tags: Mobile edit Mobile web edit |
|||
| (273 intermediate revisions by 76 users not shown) | |||
| Line 1: | Line 1: | ||
{{Short description|Endogenous inhibitory neurosteroid}} |
|||
{{chembox |
|||
{{cs1 config|name-list-style=vanc}} |
|||
| Verifiedfields = changed |
|||
{{Use dmy dates|date=November 2019}} |
|||
| Watchedfields = changed |
|||
{{Infobox drug |
|||
| verifiedrevid = 477318018 |
|||
| Verifiedfields = |
|||
| ImageFile=Allopregnanolone.svg |
|||
| Watchedfields = |
|||
| ImageSize=250 |
|||
| verifiedrevid = |
|||
| ImageAlt = Skeletal formula of allopregnanolone |
|||
| |
| image = Allopregnanolone.svg |
||
| |
| width = 225 |
||
| |
| alt = Skeletal formula of allopregnanolone |
||
| image2 = Allopregnanolone-3D-balls.png |
|||
| IUPACName=1-(3-hydroxy-10,13-dimethyl-<br/>2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-<br/>1''H''-cyclopenta[a]<br/>phenanthren-17-yl)ethanone |
|||
| width2 = 225 |
|||
| OtherNames=ALLO; Allo; ALLOP; AlloP; 5α-Pregnan-3α-ol-20-one; 3α-Hydroxy-5α-pregnan-20-one; 3α,5α-Tetrahydroprogesterone; 3α,5α-THP |
|||
| alt2 = Ball-and-stick model of the allopregnanolone molecule |
|||
|Section1={{Chembox Identifiers |
|||
| USAN = brexanolone |
|||
| ChemSpiderID_Ref = {{chemspidercite|changed|chemspider}} |
|||
<!-- Clinical data --> |
|||
| pronounce = |
|||
| tradename = Zulresso |
|||
| Drugs.com = {{drugs.com|monograph|brexanolone}} |
|||
| MedlinePlus = a619037 |
|||
| licence_CA = <!-- Health Canada may use generic or brand name (generic name preferred) --> |
|||
| licence_EU = <!-- EMA uses INN (or special INN_EMA) --> |
|||
| DailyMedID = Brexanolone |
|||
| licence_US = Zulresso |
|||
| pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> |
|||
| pregnancy_AU_comment = |
|||
| pregnancy_category = |
|||
| routes_of_administration = [[Intravenous therapy|Intravenous]]<ref name="Zulresso label" /> |
|||
| class = [[Neurosteroid]]s; [[Antidepressant]]s |
|||
| ATCvet = |
|||
| ATC_prefix = N06 |
|||
| ATC_suffix = AX29 |
|||
| ATC_supplemental = |
|||
<!-- Legal status --> |
|||
| legal_AU = <!-- S2, S3, S4, S5, S6, S7, S8, S9 or Unscheduled --> |
|||
| legal_AU_comment = |
|||
| legal_BR = <!-- OTC, A1, A2, A3, B1, B2, C1, C2, C3, C4, C5, D1, D2, E, F --> |
|||
| legal_BR_comment = |
|||
| legal_CA = <!-- OTC, Rx-only, Schedule I, II, III, IV, V, VI, VII, VIII --> |
|||
| legal_CA_comment = |
|||
| legal_DE = <!-- Anlage I, II, III or Unscheduled --> |
|||
| legal_DE_comment = |
|||
| legal_NZ = <!-- Class A, B, C --> |
|||
| legal_NZ_comment = |
|||
| legal_UK = <!-- GSL, P, POM, CD, CD Lic, CD POM, CD No Reg POM, CD (Benz) POM, CD (Anab) POM or CD Inv POM / Class A, B, C --> |
|||
| legal_UK_comment = |
|||
| legal_US = Schedule IV |
|||
| legal_US_comment = <ref name="MPR">{{cite web | title=DEA Schedules Postpartum Depression Treatment Zulresso | website=Monthly Prescribing Reference | date=17 June 2019 | url=https://www.empr.com/home/news/dea-schedules-postpartum-depression-treatment-zulresso/ | archive-url=https://web.archive.org/web/20190903150157/https://www.empr.com/home/news/dea-schedules-postpartum-depression-treatment-zulresso/ | archive-date=3 September 2019 | url-status=live | access-date=24 November 2019}}</ref><ref name="Zulresso label" /> |
|||
| legal_UN = <!-- N I, II, III, IV / P I, II, III, IV --> |
|||
| legal_UN_comment = |
|||
| legal_status = <!-- For countries not listed above --> |
|||
<!-- Pharmacokinetic data --> |
|||
| bioavailability = [[Oral administration|Oral]]: <5%<ref name="Scott2019" /> |
|||
| protein_bound = >99%<ref name="Zulresso label" /><ref name="Scott2019" /> |
|||
| metabolism = Non-[[CYP450]] ([[ketone|keto]]-[[redox|reduction]] via [[aldo-keto reductase]]s (AKR), [[glucuronidation]] via [[glucuronosyltransferase]]s (UGT), [[sulfation]] via [[sulfotransferase]]s (SULT))<ref name="Zulresso label" /><ref name="Scott2019" /> |
|||
| metabolites = |
|||
| elimination_half-life = 9 hours<ref name="Zulresso label" /><ref name="Scott2019" /> |
|||
| duration_of_action = |
|||
| excretion = [[Feces]]: 47%<ref name="Zulresso label" /><ref name="Scott2019" /><br />[[Urine]]: 42%<ref name="Zulresso label" /><ref name="Scott2019" /> |
|||
<!-- Identifiers --> |
|||
| CAS_number_Ref = |
|||
| CAS_number = 516-54-1 |
|||
| CAS_supplemental = |
|||
| PubChem = 92786 |
|||
| IUPHAR_ligand = |
|||
| DrugBank_Ref = |
|||
| DrugBank = DB11859 |
|||
| ChemSpiderID_Ref = |
|||
| ChemSpiderID = 83760 |
| ChemSpiderID = 83760 |
||
| UNII_Ref = |
|||
| UNII = S39XZ5QV8Y |
|||
| KEGG_Ref = |
|||
| KEGG = D11149 |
|||
| ChEBI_Ref = |
|||
| ChEBI = 50169 |
|||
| ChEMBL_Ref = |
|||
| ChEMBL = 207538 |
|||
| NIAID_ChemDB = |
|||
| PDB_ligand = |
|||
| synonyms = ALLO; ALLOP; SAGE-547; SGE-102; 5α-Pregnan-3α-ol-20-one; 5α-Pregnane-3α-ol-20-one;<ref name="doi1010970000054219900900100702">{{cite journal |date=September 1990 |title=5α-Pregnane-3α-ol-20-one Identified as an Active Molecular Species of Steroid Anesthetic in Brain |doi=10.1097/00000542-199009001-00702 | vauthors=Krieger NR, Mok WM, Herschkowitz S |journal=Anesthesiology |volume=73|doi-access=free | title-link = doi }}</ref><ref name="pmid697360">{{cite journal | vauthors = Yagen B, Gallili GE, Mateles RI | title = Progesterone biotransformation by plant cell suspension cultures | journal = Applied and Environmental Microbiology | volume = 36 | issue = 2 | pages = 213–216 | date = August 1978 | pmid = 697360 | pmc = 291203 | doi = 10.1128/AEM.36.2.213-216.1978 | bibcode = 1978ApEnM..36..213Y }}</ref><ref name="pmid9038248">{{cite journal | vauthors = Meyer HH, Jewgenow K, Hodges JK | title = Binding activity of 5alpha-reduced gestagens to the progestin receptor from African elephant (Loxodonta africana) | journal = General and Comparative Endocrinology | volume = 105 | issue = 2 | pages = 164–167 | date = February 1997 | pmid = 9038248 | doi = 10.1006/gcen.1996.6813 }}</ref><ref name="pmid12175598">{{cite journal | vauthors = Frye C, Seliga A | title = Olanzapine and progesterone have dose-dependent and additive effects to enhance lordosis and progestin concentrations of rats | journal = Physiology & Behavior | volume = 76 | issue = 1 | pages = 151–158 | date = May 2002 | pmid = 12175598 | doi = 10.1016/s0031-9384(02)00689-3 | s2cid = 38249308 }}</ref><ref name="pmid15249131">{{cite journal | vauthors = Mahendroo M, Wilson JD, Richardson JA, Auchus RJ | title = Steroid 5alpha-reductase 1 promotes 5alpha-androstane-3alpha,17beta-diol synthesis in immature mouse testes by two pathways | journal = Molecular and Cellular Endocrinology | volume = 222 | issue = 1–2 | pages = 113–120 | date = July 2004 | pmid = 15249131 | doi = 10.1016/j.mce.2004.04.009 | s2cid = 54297812 }}</ref> 3α-Hydroxy-5α-pregnan-20-one; 3α,5α-Tetrahydroprogesterone; 3α,5α-THP |
|||
<!-- Chemical and physical data --> |
|||
| IUPAC_name = 1-[(3''R'',5''S'',8''R'',9''S'',10''S'',13''S'',14''S'',17''S'')-3-hydroxy-10,13-dimethyl-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1''H''-cyclopenta[''a'']phenanthren-17-yl]ethanone |
|||
| C=21 | H=34 | O=2 |
|||
| SMILES = CC(=O)[C@H]1CC[C@@H]2[C@@]1(CC[C@H]3[C@H]2CC[C@@H]4[C@@]3(CC[C@H](C4)O)C)C |
| SMILES = CC(=O)[C@H]1CC[C@@H]2[C@@]1(CC[C@H]3[C@H]2CC[C@@H]4[C@@]3(CC[C@H](C4)O)C)C |
||
| StdInChI_Ref = |
|||
| InChI = 1/C21H34O2/c1-13(22)17-6-7-18-16-5-4-14-12-15(23)8-10-20(14,2)19(16)9-11-21(17,18)3/h14-19,23H,4-12H2,1-3H3/t14-,15+,16-,17+,18-,19-,20-,21+/m0/s1 |
|||
| InChIKey = AURFZBICLPNKBZ-SYBPFIFIBJ |
|||
| StdInChI_Ref = {{stdinchicite|changed|chemspider}} |
|||
| StdInChI = 1S/C21H34O2/c1-13(22)17-6-7-18-16-5-4-14-12-15(23)8-10-20(14,2)19(16)9-11-21(17,18)3/h14-19,23H,4-12H2,1-3H3/t14-,15+,16-,17+,18-,19-,20-,21+/m0/s1 |
| StdInChI = 1S/C21H34O2/c1-13(22)17-6-7-18-16-5-4-14-12-15(23)8-10-20(14,2)19(16)9-11-21(17,18)3/h14-19,23H,4-12H2,1-3H3/t14-,15+,16-,17+,18-,19-,20-,21+/m0/s1 |
||
| StdInChI_comment = |
|||
| StdInChIKey_Ref = {{stdinchicite|changed|chemspider}} |
|||
| StdInChIKey_Ref = |
|||
| StdInChIKey = AURFZBICLPNKBZ-SYBPFIFISA-N |
| StdInChIKey = AURFZBICLPNKBZ-SYBPFIFISA-N |
||
| density = |
|||
| CASNo_Ref = {{cascite|changed|??}} |
|||
| density_notes = |
|||
| CASNo=516-54-1 |
|||
| melting_point = |
|||
| UNII_Ref = {{fdacite|changed|FDA}} |
|||
| melting_high = |
|||
| UNII = S39XZ5QV8Y |
|||
| melting_notes = |
|||
| PubChem=262961 |
|||
| boiling_point = |
|||
| ChEMBL_Ref = {{ebicite|changed|EBI}} |
|||
| boiling_notes = |
|||
| ChEMBL = 38856 |
|||
| solubility = |
|||
}} |
|||
| sol_units = |
|||
|Section2={{Chembox Properties |
|||
| specific_rotation = |
|||
| Formula=C<sub>21</sub>H<sub>34</sub>O<sub>2</sub> |
|||
| MolarMass=318.49 g/mol |
|||
| Appearance= |
|||
| Density= |
|||
| MeltingPt= |
|||
| BoilingPt= |
|||
| Solubility= |
|||
}} |
|||
|Section3={{Chembox Hazards |
|||
| MainHazards= |
|||
| FlashPt= |
|||
| AutoignitionPt = |
|||
}} |
|||
}} |
}} |
||
<!-- Definition and medical uses --> |
|||
'''Allopregnanolone''', also known as '''5α-pregnan-3α-ol-20-one''' or '''3α,5α-tetrahydroprogesterone''' ('''3α,5α-THP'''), as well as '''brexanolone''' ([[United States Adopted Name|USAN]]),<ref name="SageTherapeutics">http://investor.sagerx.com/releasedetail.cfm?releaseid=1014136</ref> is an [[endogenous]] [[inhibitory postsynaptic potential|inhibitory]] [[pregnane]] [[neurosteroid]].<ref name="pmid21094889">{{cite journal | author = Reddy DS | title = Neurosteroids: endogenous role in the human brain and therapeutic potentials | journal = Prog. Brain Res. | volume = 186 | issue = | pages = 113–37 | year = 2010 | pmid = 21094889 | pmc = 3139029 | doi = 10.1016/B978-0-444-53630-3.00008-7 | url = }}</ref> It is [[biosynthesis|synthesized]] from [[progesterone]], and is a potent [[positive allosteric modulator]] of the action of gamma-amininobutyric acid (GABA) at [[GABAA receptor|GABA<sub>A</sub> receptor]].<ref name="pmid21094889" /> Allopregnanolone has effects similar to those of other positive allosteric modulators of the GABA action at GABA<sub>A</sub> receptor such as the [[benzodiazepine]]s, including [[anxiolytic]], [[sedative]], and [[anticonvulsant]] activity.<ref name="pmid21094889" /> |
|||
'''Allopregnanolone''' is a [[natural product|naturally occurring]] [[neurosteroid]] which is made in the body from the [[hormone]] [[progesterone]].<ref name="Reddy_2010">{{cite book | vauthors = Reddy DS | title = Sex Differences in the Human Brain, their Underpinnings and Implications | chapter = Neurosteroids | series = Progress in Brain Research | volume = 186 | pages = 113–137 | year = 2010 | publisher = Elsevier | pmid = 21094889 | pmc = 3139029 | doi = 10.1016/B978-0-444-53630-3.00008-7 | isbn = 9780444536303 }}</ref><ref name=FDA2019/> As a medication, allopregnanolone is referred to as '''brexanolone''', sold under the brand name '''Zulresso''',<ref name="Zulresso label" /><ref>{{cite web|title=ChemIDplus - 516-54-1 - AURFZBICLPNKBZ-SYBPFIFISA-N - Brexanolone [USAN] - Similar structures search, synonyms, formulas, resource links, and other chemical information.|url=https://chem.nlm.nih.gov/chemidplus/rn/516-54-1|publisher=NIH Toxnet|access-date=26 December 2017}}</ref> and used to treat [[postpartum depression]].<ref name=FDA2019>{{cite press release | title=FDA approves first treatment for post-partum depression | website=U.S. [[Food and Drug Administration]] (FDA) | date=19 March 2019 | url=https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-post-partum-depression | archive-url=https://web.archive.org/web/20191011120005/https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-post-partum-depression | archive-date=11 October 2019 | url-status=live | access-date=21 March 2019 }} {{PD-notice}}</ref><ref name="pmid30790145">{{cite journal | vauthors = Frieder A, Fersh M, Hainline R, Deligiannidis KM | title = Pharmacotherapy of Postpartum Depression: Current Approaches and Novel Drug Development | journal = CNS Drugs | volume = 33 | issue = 3 | pages = 265–282 | date = March 2019 | pmid = 30790145 | pmc = 6424603 | doi = 10.1007/s40263-019-00605-7 }}</ref><ref name="pmid30447328">{{cite journal | vauthors = Wilkinson ST, Sanacora G | title = A new generation of antidepressants: an update on the pharmaceutical pipeline for novel and rapid-acting therapeutics in mood disorders based on glutamate/GABA neurotransmitter systems | journal = Drug Discovery Today | volume = 24 | issue = 2 | pages = 606–615 | date = February 2019 | pmid = 30447328 | pmc = 6397075 | doi = 10.1016/j.drudis.2018.11.007 }}</ref> It is given by [[intravenous|injection into a vein]].<ref name=FDA2019/><ref name="Zulresso label" /> |
|||
<!-- Side effects and mechanism of action --> |
|||
Endogenously produced allopregnanolone exerts a pivotal neurophysiological role by fine-tuning of [[GABAA receptor|GABA<sub>A</sub> receptor]] and modulating the action of several positive allosteric modulators and agonists at [[GABAA receptor|GABA<sub>A</sub> receptor]].<ref>{{Cite journal|title = Brain allopregnanolone regulates the potency of the GABAA receptor agonist muscimol|url = http://www.sciencedirect.com/science/article/pii/S0028390899001495|journal = Neuropharmacology|date = 2000-03-01|pages = 440–448|volume = 39|issue = 3|doi = 10.1016/S0028-3908(99)00149-5|first = G|last = Pinna|first2 = V|last2 = Uzunova|first3 = K|last3 = Matsumoto|first4 = G|last4 = Puia|first5 = J. -M|last5 = Mienville|first6 = E|last6 = Costa|first7 = A|last7 = Guidotti|pmid=10698010}}</ref> The 21-hydroxylated derivative of this compound, [[tetrahydrodeoxycorticosterone]] (THDOC), is an endogenous inhibitory neurosteroid with similar properties to those of allopregnanolone, and the 3β-methyl [[structural analog|analogue]] of allopregnanolone, [[ganaxolone]], is under development to treat [[epilepsy]] and other conditions, including [[post-traumatic stress disorder]] (PTSD).<ref name="pmid21094889" /> |
|||
[[Side effect]]s of brexanolone may include [[sedation]], [[sleepiness]], [[dry mouth]], [[hot flash]]es, and [[loss of consciousness]].<ref name="Zulresso label" /><ref name="FDA2019" /> It is a [[neurosteroid]] and acts as a [[positive allosteric modulator]] of the [[GABAA receptor|GABA<sub>A</sub> receptor]], the major [[biological target]] of the [[inhibitory postsynaptic potential|inhibitory]] [[neurotransmitter]] [[γ-aminobutyric acid]] (GABA).<ref name="Zulresso label">{{cite web | title=Zulresso- brexanolone injection, solution | website=[[DailyMed]] | date=18 November 2019 | url=https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b40f3b2a-1859-4ed6-8551-444300806d13 | access-date=23 November 2019}}</ref> |
|||
<!-- History, society, and culture --> |
|||
==Biochemistry== |
|||
Brexanolone was approved for medical use in the United States in 2019.<ref name=FDA2019 /><ref name="FDA approval">{{cite web | title=Drug Approval Package: Zulresso | website=U.S. [[Food and Drug Administration]] (FDA) | date=7 February 2019 | url=https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/211371Orig1s000TOC.cfm | access-date=6 August 2020}}</ref> The U.S. [[Food and Drug Administration]] (FDA) considers it to be a [[first-in-class medication]].<ref>{{cite web | title=New Drug Therapy Approvals 2019 | website=U.S. Food and Drug Administration | date=31 December 2019 | url=https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/new-drug-therapy-approvals-2019 | access-date=15 September 2020}}</ref> The long administration time, as well as the cost for a one-time treatment, have raised concerns about accessibility for many women.<ref name="NPR2019">{{cite news | vauthors = Chatterjee R |title=New Postpartum Depression Drug Could Be Hard To Access For Moms Most In Need |url=https://www.npr.org/sections/health-shots/2019/03/21/705545014/new-postpartum-depression-drug-could-be-hard-to-access-for-moms-most-in-need |work=[[NPR]] |date=21 March 2019 | access-date=22 March 2019 }}</ref> |
|||
{{TOC limit}} |
|||
===Biosynthesis=== |
|||
The [[biosynthesis]] of allopregnanolone in the brain starts with the conversion of progesterone into [[5α-dihydroprogesterone]] by [[5α-reductase]] [[SRD5A1|type I]]. After that, [[3α-hydroxysteroid dehydrogenase]] converts this [[metabolic intermediate|intermediate]] into allopregnanolone.<ref name="pmid21094889" /> Allopregnanolone in the brain is produced by cortical and hippocampus pyramidal neurons and pyramidal-like neurons of the basolateral amygdala.<ref>{{Cite journal|title = Characterization of brain neurons that express enzymes mediating neurosteroid biosynthesis|url = http://www.pnas.org/content/103/39/14602|journal = Proceedings of the National Academy of Sciences|date = 2006-09-26|issn = 0027-8424|pmc = 1600006|pmid = 16984997|pages = 14602–14607|volume = 103|issue = 39|doi = 10.1073/pnas.0606544103|language = en|first = Roberto C.|last = Agís-Balboa|first2 = Graziano|last2 = Pinna|first3 = Adrian|last3 = Zhubi|first4 = Ekrem|last4 = Maloku|first5 = Marin|last5 = Veldic|first6 = Erminio|last6 = Costa|first7 = Alessandro|last7 = Guidotti}}</ref> |
|||
==Medical uses== |
|||
[[Depression (mood)|Depression]],<ref>{{Cite journal|title = Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar major depression who are receiving fluoxetine or fluvoxamine|url = http://www.pnas.org/content/95/6/3239|journal = Proceedings of the National Academy of Sciences|date = 1998-03-17|issn = 0027-8424|pmc = 19726|pmid = 9501247|pages = 3239–3244|volume = 95|issue = 6|language = en|first = V.|last = Uzunova|first2 = Y.|last2 = Sheline|first3 = J. M.|last3 = Davis|first4 = A.|last4 = Rasmusson|first5 = D. P.|last5 = Uzunov|first6 = E.|last6 = Costa|first7 = A.|last7 = Guidotti|doi=10.1073/pnas.95.6.3239}}</ref> [[anxiety]], and post-traumatic stress disorder (PTSD)<ref>{{Cite web|title = Decreased Cerebrospinal Fluid Allopregnanolone Levels in Women with Posttraumatic Stress Disorder - Biological Psychiatry|url = http://www.biologicalpsychiatryjournal.com/article/S0006-3223(06)00402-1/fulltext|website = www.biologicalpsychiatryjournal.com|access-date = 2016-01-26}}</ref> have been associated with a decrease of cerebral spinal fluid (CSF) levels of allopregnanolone, likewise mood disorders and [[sexual dysfunction]] are frequently-seen [[side effect]]s of [[5α-reductase inhibitor]]s such as [[finasteride]], and are thought to be caused, in part, by interfering with the normal production of allopregnanolone.<ref name="pmid21122055">{{cite journal |vauthors=Römer B, Gass P | title = Finasteride-induced depression: new insights into possible pathomechanisms | journal = J Cosmet Dermatol | volume = 9 | issue = 4 | pages = 331–2 |date=December 2010 | pmid = 21122055 | doi = 10.1111/j.1473-2165.2010.00533.x | url = }}</ref> |
|||
Brexanolone is used to treat postpartum depression in adult women, administered as a continuous [[intravenous infusion]] over a period of 60 hours and essential tremor.<ref name=FDA2019/><ref>{{cite web |title=Zulresso (brexanolone) dosing, indications, interactions, adverse effects, and more |url=https://reference.medscape.com/drug/zulresso-brexanolone-1000299 |access-date=2023-09-30 |website=reference.medscape.com}}</ref> |
|||
== Clinical efficacy == |
|||
===Metabolism=== |
|||
Allopregnanolone [[metabolism|metabolized]] into [[allopregnanediol]]s, [[conjugation (biochemistry)|conjugated]], and then [[excretion|excreted]].{{Citation needed|date=December 2016}} |
|||
Women experiencing moderate to severe postpartum depression when treated with a single dose of intravenous brexanolone display a significant reduction in [[Hamilton Depression Rating Scale|HAM-D scores]] which persisted 30 days post-treatment.<ref name="pmid34594247">{{cite journal | vauthors = Edinoff AN, Odisho AS, Lewis K, Kaskas A, Hunt G, Cornett EM, Kaye AD, Kaye A, Morgan J, Barrilleaux PS, Lewis D, Viswanath O, Urits I | display-authors = 6 | title = Brexanolone, a GABA<sub>A</sub> Modulator, in the Treatment of Postpartum Depression in Adults: A Comprehensive Review | journal = Frontiers in Psychiatry | volume = 12 | issue = | pages = 699740 | date = 2021 | pmid = 34594247 | pmc = 8477036 | doi = 10.3389/fpsyt.2021.699740 | doi-access = free }}</ref> |
|||
==Biological activity== |
|||
Allopregnanolone acts as a highly potent [[positive allosteric modulator]] of the [[GABAA receptor|GABA<sub>A</sub> receptor]].<ref name="pmid21094889" /> While allopregnanolone, like other inhibitory neurosteroids such as THDOC, positively modulates all GABA<sub>A</sub> receptor isoforms, those isoforms containing [[GABRD|δ subunits]] exhibit the greatest potentiation.<ref>{{cite journal |vauthors=Mousavi Nik A, Pressly B, Singh V, Antrobus S, Hulsizer S, Rogawski MA, Wulff H, Pessah IN |title=Rapid Throughput Analysis of GABAA Receptor Subtype Modulators and Blockers Using DiSBAC1(3) Membrane Potential Red Dye |journal=Mol. Pharmacol. |volume= |issue= |pages= |year=2017 |pmid=28428226 |doi=10.1124/mol.117.108563 |url=}}</ref> Allopregnanolone has also been found to act as a positive allosteric modulator of the [[GABAA-rho receptor|GABA<sub>A</sub>-ρ receptor]], though the implications of this action are unclear.<ref name="pmid10496958">{{cite journal |vauthors=Morris KD, Moorefield CN, Amin J | title = Differential modulation of the gamma-aminobutyric acid type C receptor by neuroactive steroids | journal = Mol. Pharmacol. | volume = 56 | issue = 4 | pages = 752–9 |date=October 1999 | pmid = 10496958 | doi = | url = }}</ref><ref name="pmid17636008">{{cite journal |vauthors=Li W, Jin X, Covey DF, Steinbach JH | title = Neuroactive steroids and human recombinant rho1 GABAC receptors | journal = J. Pharmacol. Exp. Ther. | volume = 323 | issue = 1 | pages = 236–47 |date=October 2007 | pmid = 17636008 | doi = 10.1124/jpet.107.127365 | url = }}</ref> In addition to its actions on GABA receptors, allopregnanolone, like progesterone, is known to be a [[negative allosteric modulator]] of [[nicotinic acetylcholine receptor|nACh receptor]]s,<ref name="pmid9166735">{{cite journal |vauthors=Bullock AE, Clark AL, Grady SR | title = Neurosteroids modulate nicotinic receptor function in mouse striatal and thalamic synaptosomes | journal = J. Neurochem. | volume = 68 | issue = 6 | pages = 2412–23 |date=June 1997 | pmid = 9166735 | doi = 10.1046/j.1471-4159.1997.68062412.x |display-authors=etal}}</ref> and also appears to act as a negative allosteric modulator of the [[5-HT3 receptor|5-HT<sub>3</sub> receptor]].<ref name="pmid9731711">{{cite journal |vauthors=Wetzel CH, Hermann B, Behl C | title = Functional antagonism of gonadal steroids at the 5-hydroxytryptamine type 3 receptor | journal = Mol. Endocrinol. | volume = 12 | issue = 9 | pages = 1441–51 |date=September 1998 | pmid = 9731711 | doi = 10.1210/mend.12.9.0163 | url = |display-authors=etal}}</ref> Along with the other inhibitory neurosteroids, allopregnanolone appears to have little or no action at other [[ligand-gated ion channel]]s, including the [[NMDA receptor|NMDA]], [[AMPA receptor|AMPA]], [[kainate receptor|kainate]], and [[glycine receptor]]s.<ref name="pmid17651807">{{cite journal | author = Mellon SH | title = Neurosteroid regulation of central nervous system development | journal = Pharmacol. Ther. | volume = 116 | issue = 1 | pages = 107–24 |date=October 2007 | pmid = 17651807 | pmc = 2386997 | doi = 10.1016/j.pharmthera.2007.04.011 | url = http://linkinghub.elsevier.com/retrieve/pii/S0163-7258(07)00109-X}}</ref> |
|||
==Side effects== |
|||
Unlike progesterone, allopregnanolone is inactive at the [[progesterone receptor|nuclear progesterone receptor]] (nPR).<ref name="pmid17651807" /> However, allopregnanolone can be intracellularly oxidized into [[5α-dihydroprogesterone]], which ''is'' an [[agonist]] of the nPR, and thus/in accordance, allopregnanolone does appear to have indirect nPR-mediated [[progestogen]]ic effects.<ref name="pmid8398145">{{cite journal |vauthors=Rupprecht R, Reul JM, Trapp T | title = Progesterone receptor-mediated effects of neuroactive steroids | journal = Neuron | volume = 11 | issue = 3 | pages = 523–30 |date=September 1993 | pmid = 8398145 | doi = 10.1016/0896-6273(93)90156-l| url = |display-authors=etal}}</ref> In addition, allopregnanolone has recently been found to be an agonist of the newly discovered [[membrane progesterone receptor]]s (mPR), including [[PAQR6|mPRδ]], [[PAQR7|mPRα]], and [[PAQR8|mPRβ]], with its activity at these receptors about a magnitude more potent than at the GABA<sub>A</sub> receptor.<ref name="pmid22687885">{{cite journal |vauthors=Thomas P, Pang Y | title = Membrane progesterone receptors: evidence for neuroprotective, neurosteroid signaling and neuroendocrine functions in neuronal cells | journal = Neuroendocrinology | volume = 96 | issue = 2 | pages = 162–71 | year = 2012 | pmid = 22687885 | pmc = 3489003 | doi = 10.1159/000339822 | url = }}</ref><ref name="pmid23161870">{{cite journal |vauthors=Pang Y, Dong J, Thomas P | title = Characterization, neurosteroid binding and brain distribution of human membrane progesterone receptors δ and {epsilon} (mPRδ and mPR{epsilon}) and mPRδ involvement in neurosteroid inhibition of apoptosis | journal = Endocrinology | volume = 154 | issue = 1 | pages = 283–95 | date = January 2013 | pmid = 23161870 | pmc = 3529379 | doi = 10.1210/en.2012-1772 | url = }}</ref> The action of allopregnanolone at these receptors may be related, in part, to its [[neuroprotective]] and [[antigonadotropic]] properties.<ref name="pmid22687885" /><ref name="pmid19423765">{{cite journal |vauthors=Sleiter N, Pang Y, Park C | title = Progesterone receptor A (PRA) and PRB-independent effects of progesterone on gonadotropin-releasing hormone release | journal = Endocrinology | volume = 150 | issue = 8 | pages = 3833–44 |date=August 2009 | pmid = 19423765 | pmc = 2717864 | doi = 10.1210/en.2008-0774 | url = |display-authors=etal}}</ref> Also like progesterone, recent evidence has shown that allopregnanolone is an activator of the [[pregnane X receptor]].<ref name="pmid17651807" /><ref name="pmid15364541">{{cite journal |vauthors=Lamba V, Yasuda K, Lamba JK | title = PXR (NR1I2): splice variants in human tissues, including brain, and identification of neurosteroids and nicotine as PXR activators | journal = Toxicol. Appl. Pharmacol. | volume = 199 | issue = 3 | pages = 251–65 |date=September 2004 | pmid = 15364541 | doi = 10.1016/j.taap.2003.12.027 | url = |display-authors=etal}}</ref> |
|||
Side effects of brexanolone include dizziness (10–20%), [[sedation]] (13–21%), headache (18%), nausea (10%), dry mouth (3–11%), loss of consciousness (3–5%), and [[flushing (physiology)|flushing]] (2–5%).<ref name="Zulresso label" /><ref name="FDA2019" /><ref name="Scott2019" /><ref name="Review of Allopregnanolone Agonist">{{cite journal | vauthors = Walkery A, Leader LD, Cooke E, VandenBerg A | title = Review of Allopregnanolone Agonist Therapy for the Treatment of Depressive Disorders | language = English | journal = Drug Design, Development and Therapy | volume = 15 | pages = 3017–3026 | date = 2021-07-09 | pmid = 34267503 | pmc = 8276990 | doi = 10.2147/DDDT.S240856 | doi-access = free }}</ref> It can produce [[euphoria]] to a degree similar to that of [[alprazolam]] (3–13% at infusion doses of 90–270 μg over a one-hour period).<ref name="Zulresso label" /> Serious or severe adverse effects are rare but may include [[altered state of consciousness]], [[Syncope (medicine)|syncope]], presyncope, fatigue, and [[insomnia]].<ref name="Review of Allopregnanolone Agonist"/> |
|||
Similarly to many other GABA<sub>A</sub> receptor positive allosteric modulators, allopregnanolone has been found to act as an [[channel blocker|inhibitor]] of [[L-type voltage-gated calcium channel]]s (L-VGCCs),<ref name="pmid17151597">{{cite journal |vauthors=Hu AQ, Wang ZM, Lan DM | title = Inhibition of evoked glutamate release by neurosteroid allopregnanolone via inhibition of L-type calcium channels in rat medial prefrontal cortex | journal = Neuropsychopharmacology | volume = 32 | issue = 7 | pages = 1477–89 |date=July 2007 | pmid = 17151597 | doi = 10.1038/sj.npp.1301261 | url = |display-authors=etal}}</ref> including [[voltage-dependent calcium channel#α1 Subunit|α<sub>1</sub> subtype]]s [[Cav1.2|Ca<sub>v</sub>1.2]] and [[Cav1.3|Ca<sub>v</sub>1.3]].<ref name="pmid21262851">{{cite journal |vauthors=Earl DE, Tietz EI | title = Inhibition of recombinant L-type voltage-gated calcium channels by positive allosteric modulators of GABAA receptors | journal = J. Pharmacol. Exp. Ther. | volume = 337 | issue = 1 | pages = 301–11 |date=April 2011 | pmid = 21262851 | pmc = 3063747 | doi = 10.1124/jpet.110.178244 | url = }}</ref> However, the threshold concentration of allopregnanolone to inhibit L-VGCCs was determined to be 3 μM (3,000 nM), which is far greater than the concentration of 5 nM that has been estimated to be naturally produced in the human brain.<ref name="pmid21262851" /> Thus, inhibition of L-VGCCs is unlikely of any actual significance in the effects of endogenous allopregnanolone.<ref name="pmid21262851" /> Also, allopregnanolone, along with several other neurosteroids, has been found to activate the [[G protein-coupled bile acid receptor]] (GPBAR1, or TGR5).<ref name="pmid20665558">{{cite journal |vauthors=Keitel V, Görg B, Bidmon HJ | title = The bile acid receptor TGR5 (Gpbar-1) acts as a neurosteroid receptor in brain | journal = Glia | volume = 58 | issue = 15 | pages = 1794–805 |date=November 2010 | pmid = 20665558 | doi = 10.1002/glia.21049 | url = |display-authors=etal}}</ref> However, it is only able to do so at micromolar concentrations, which, similarly to the case of the L-VGCCs, are far greater than the low nanomolar concentrations of allopregnanolone estimated to be present in the brain.<ref name="pmid20665558" /> |
|||
==Biological function== |
==Biological function== |
||
Allopregnanolone possesses a wide variety of effects, including, in no particular order, [[antidepressant]], [[anxiolytic]], [[stress (psychological)|stress-reducing]], [[pleasure|rewarding]],<ref name="pmid12153544">{{cite journal |vauthors=Rougé-Pont F, Mayo W, Marinelli M, Gingras M, Le Moal M, Piazza PV | title = The neurosteroid allopregnanolone increases dopamine release and dopaminergic response to morphine in the rat nucleus accumbens | journal = |
Allopregnanolone possesses a wide variety of effects, including, in no particular order, [[antidepressant]], [[anxiolytic]], [[stress (psychological)|stress-reducing]], [[pleasure|rewarding]],<ref name="pmid12153544">{{cite journal | vauthors = Rougé-Pont F, Mayo W, Marinelli M, Gingras M, Le Moal M, Piazza PV | title = The neurosteroid allopregnanolone increases dopamine release and dopaminergic response to morphine in the rat nucleus accumbens | journal = The European Journal of Neuroscience | volume = 16 | issue = 1 | pages = 169–173 | date = July 2002 | pmid = 12153544 | doi = 10.1046/j.1460-9568.2002.02084.x | s2cid = 9953445 }}</ref> [[prosocial behavior|prosocial]],<ref name="pmid19656632">{{cite journal | vauthors = Frye CA | title = Neurosteroids' effects and mechanisms for social, cognitive, emotional, and physical functions | journal = Psychoneuroendocrinology | volume = 34 | issue = Suppl 1 | pages = S143–S161 | date = December 2009 | pmid = 19656632 | pmc = 2898141 | doi = 10.1016/j.psyneuen.2009.07.005 }}</ref> [[serenic|antiaggressive]],<ref name="pmid15677716">{{cite journal | vauthors = Pinna G, Costa E, Guidotti A | title = Changes in brain testosterone and allopregnanolone biosynthesis elicit aggressive behavior | journal = Proceedings of the National Academy of Sciences of the United States of America | volume = 102 | issue = 6 | pages = 2135–2140 | date = February 2005 | pmid = 15677716 | pmc = 548579 | doi = 10.1073/pnas.0409643102 | doi-access = free | bibcode = 2005PNAS..102.2135P | title-link = doi }}</ref> [[aphrodisiac|prosexual]],<ref name="pmid19656632" /> sedative, [[hypnotic|pro-sleep]],<ref name="pmid23092405">{{cite journal | vauthors = Terán-Pérez G, Arana-Lechuga Y, Esqueda-León E, Santana-Miranda R, Rojas-Zamorano JÁ, Velázquez Moctezuma J | title = Steroid hormones and sleep regulation | journal = Mini Reviews in Medicinal Chemistry | volume = 12 | issue = 11 | pages = 1040–1048 | date = October 2012 | pmid = 23092405 | doi = 10.2174/138955712802762167 }}</ref> [[cognitive deficit|cognitive]], [[cognitive deficit|memory-impairment]], [[analgesic]],<ref name="pmid23948490">{{cite journal | vauthors = Patte-Mensah C, Meyer L, Taleb O, Mensah-Nyagan AG | title = Potential role of allopregnanolone for a safe and effective therapy of neuropathic pain | journal = Progress in Neurobiology | volume = 113 | pages = 70–78 | date = February 2014 | pmid = 23948490 | doi = 10.1016/j.pneurobio.2013.07.004 | s2cid = 207407077 }}</ref> [[anesthetic]], [[anticonvulsant]], [[neuroprotective]], and [[neurogenic]] effects.<ref name="Reddy_2010" /> Fluctuations in the levels of allopregnanolone and the other neurosteroids seem to play an important role in the pathophysiology of [[depression (mood)|mood]], [[anxiety]], [[premenstrual syndrome]], [[catamenial epilepsy]], and various other neuropsychiatric conditions.<ref name="pmid14993039">{{cite journal | vauthors = Bäckström T, Andersson A, Andreé L, Birzniece V, Bixo M, Björn I, Haage D, Isaksson M, Johansson IM, Lindblad C, Lundgren P, Nyberg S, Odmark IS, Strömberg J, Sundström-Poromaa I, Turkmen S, Wahlström G, Wang M, Wihlbäck AC, Zhu D, Zingmark E | display-authors = 6 | title = Pathogenesis in menstrual cycle-linked CNS disorders | journal = Annals of the New York Academy of Sciences | volume = 1007 | issue = 1 | pages = 42–53 | date = December 2003 | pmid = 14993039 | doi = 10.1196/annals.1286.005 | s2cid = 20995334 | bibcode = 2003NYASA1007...42B | doi-access = free }}</ref><ref name="pmid18346939">{{cite journal | vauthors = Guille C, Spencer S, Cavus I, Epperson CN | title = The role of sex steroids in catamenial epilepsy and premenstrual dysphoric disorder: implications for diagnosis and treatment | journal = Epilepsy & Behavior | volume = 13 | issue = 1 | pages = 12–24 | date = July 2008 | pmid = 18346939 | pmc = 4112568 | doi = 10.1016/j.yebeh.2008.02.004 }}</ref><ref name="pmid21533709">{{cite journal | vauthors = Finocchi C, Ferrari M | title = Female reproductive steroids and neuronal excitability | journal = Neurological Sciences | volume = 32 | issue = Suppl 1 | pages = S31–S35 | date = May 2011 | pmid = 21533709 | doi = 10.1007/s10072-011-0532-5 | s2cid = 8885335 }}</ref> |
||
During [[pregnancy]], allopregnanolone and [[pregnanolone]] are involved in [[sedation]] and [[anesthesia]] of the [[fetus]].<ref name="pmid16269314">{{cite journal | vauthors = Mellor DJ, Diesch TJ, Gunn AJ, Bennet L | title = The importance of 'awareness' for understanding fetal pain | journal = Brain Research. Brain Research Reviews | volume = 49 | issue = 3 | pages = 455–471 | date = November 2005 | pmid = 16269314 | doi = 10.1016/j.brainresrev.2005.01.006 | s2cid = 9833426 }}</ref><ref name="pmid19092726">{{cite journal | vauthors = Lagercrantz H, Changeux JP | title = The emergence of human consciousness: from fetal to neonatal life | journal = Pediatric Research | volume = 65 | issue = 3 | pages = 255–260 | date = March 2009 | pmid = 19092726 | doi = 10.1203/PDR.0b013e3181973b0d | quote = [...] the fetus is sedated by the low oxygen tension of the fetal blood and the neurosteroid anesthetics pregnanolone and the sleep-inducing prostaglandin D2 provided by the placenta (36). | s2cid = 39391626 | doi-access = free | title-link = doi }}</ref> |
|||
Increased levels of allopregnanolone can produce paradoxical effects, including [[depression (mood)|negative mood]], [[anxiety]], [[irritability]], and [[aggression]].<ref name="pmid21600269">{{cite journal |vauthors=Bäckström T, Haage D, Löfgren M | title = Paradoxical effects of GABA-A modulators may explain sex steroid induced negative mood symptoms in some persons | journal = Neuroscience | volume = 191 | issue = | pages = 46–54 |date=September 2011 | pmid = 21600269 | doi = 10.1016/j.neuroscience.2011.03.061 | url = |display-authors=etal}}</ref><ref name="pmid19272715">{{cite journal |vauthors=Andréen L, Nyberg S, Turkmen S, van Wingen G, Fernández G, Bäckström T | title = Sex steroid induced negative mood may be explained by the paradoxical effect mediated by GABAA modulators | journal = Psychoneuroendocrinology | volume = 34 | issue = 8 | pages = 1121–32 |date=September 2009 | pmid = 19272715 | doi = 10.1016/j.psyneuen.2009.02.003 | url = }}</ref><ref name="pmid23978486">{{cite journal |vauthors=Bäckström T, Bixo M, Johansson M | title = Allopregnanolone and mood disorders | journal = Prog. Neurobiol. | volume = 113 | issue = | pages = 88–94 |date=February 2014 | pmid = 23978486 | doi = 10.1016/j.pneurobio.2013.07.005 | url = |display-authors=etal}}</ref> This appears to be because allopregnanolone possesses biphasic, U-shaped actions at the GABA<sub>A</sub> receptor – moderate level increases (in the range of 1.5–2 nM/L total allopregnanolone, which are approximately equivalent to [[luteal phase]] levels) inhibit the activity of the receptor, while lower and higher concentration increases stimulate it.<ref name="pmid21600269" /><ref name="pmid19272715" /> This seems to be a common effect of many GABA<sub>A</sub> receptor positive allosteric modulators.<ref name="pmid14993039" /><ref name="pmid23978486" /> In accordance, acute administration of low doses of [[micronized progesterone]] (which reliably elevates allopregnanolone levels), have been found to have negative effects on mood, while higher doses have a neutral effect.<ref name="pmid16724185">{{cite journal |vauthors=Andréen L, Sundström-Poromaa I, Bixo M, Nyberg S, Bäckström T | title = Allopregnanolone concentration and mood--a bimodal association in postmenopausal women treated with oral progesterone | journal = Psychopharmacology (Berl.) | volume = 187 | issue = 2 | pages = 209–21 |date=August 2006 | pmid = 16724185 | doi = 10.1007/s00213-006-0417-0 | url = }}</ref> |
|||
Allopregnanolone is a [[metabolic intermediate]] in an [[androgen backdoor pathway]] from [[progesterone]] to [[dihydrotestosterone]], which occurs during normal male [[fetus]] development; placental progesterone in the male fetus is the feedstock of this pathway; deficiencies in this pathway lead to insufficient [[virilization]] of the male fetus.<ref name=wj>{{cite journal|doi=10.15347/WJM/2023.003 |doi-access=free |title=Alternative androgen pathways |year=2023 | vauthors = Masiutin M, Yadav M |journal=WikiJournal of Medicine |volume=10 |pages=X |s2cid=257943362}}</ref> |
|||
==Mechanism of action== |
|||
===Molecular interactions=== |
|||
Allopregnanolone is an [[endogenous]] [[inhibitory postsynaptic potential|inhibitory]] [[pregnane]] [[neurosteroid]].<ref name="Reddy_2010"/> It is [[biosynthesis|made]] from [[pregnenolone]], and is a [[positive allosteric modulator]] of the action of γ-aminobutyric acid (GABA) at [[GABAA receptor|GABA<sub>A</sub> receptor]].<ref name="Reddy_2010" /> Allopregnanolone has effects similar to those of other positive allosteric modulators of the GABA action at GABA<sub>A</sub> receptor such as the [[benzodiazepine]]s, including anxiolytic, sedative, and anticonvulsant activity.<ref name="Reddy_2010" /><ref>{{cite journal | vauthors = Reddy DS, Rogawski MA, Rogawski MA, Olsen RW, Delgado-Escueta AV, Reddy DS, Rogawski MA | title = Neurosteroids — Endogenous Regulators of Seizure Susceptibility and Role in the Treatment of Epilepsy | journal = Jasper's Basic Mechanisms of the Epilepsies, 4th Edition | pages = 984–1002 | year = 2012 | pmid = 22787590 | doi = 10.1093/med/9780199746545.003.0077 | isbn = 9780199746545 }}</ref><ref>{{cite journal | vauthors = Kokate TG, Svensson BE, Rogawski MA | title = Anticonvulsant activity of neurosteroids: correlation with gamma-aminobutyric acid-evoked chloride current potentiation | journal = The Journal of Pharmacology and Experimental Therapeutics | volume = 270 | issue = 3 | pages = 1223–1229 | date = September 1994 | pmid = 7932175 }}</ref> Endogenously produced allopregnanolone exerts a neurophysiological role by fine-tuning of GABA<sub>A</sub> receptor and modulating the action of several positive allosteric modulators and agonists at GABA<sub>A</sub> receptor.<ref>{{cite journal | vauthors = Pinna G, Uzunova V, Matsumoto K, Puia G, Mienville JM, Costa E, Guidotti A | title = Brain allopregnanolone regulates the potency of the GABA(A) receptor agonist muscimol | journal = Neuropharmacology | volume = 39 | issue = 3 | pages = 440–448 | date = January 2000 | pmid = 10698010 | doi = 10.1016/S0028-3908(99)00149-5 | s2cid = 42753647 }}</ref> |
|||
Allopregnanolone acts as a highly potent positive allosteric modulator of the GABA<sub>A</sub> receptor.<ref name="Reddy_2010" /> While allopregnanolone, like other inhibitory neurosteroids such as THDOC, positively modulates all GABA<sub>A</sub> receptor isoforms, those isoforms containing [[GABRD|δ subunits]] exhibit the greatest potentiation.<ref>{{cite journal | vauthors = Nik AM, Pressly B, Singh V, Antrobus S, Hulsizer S, Rogawski MA, Wulff H, Pessah IN | display-authors = 6 | title = Rapid Throughput Analysis of GABA<sub>A</sub> Receptor Subtype Modulators and Blockers Using DiSBAC<sub>1</sub>(3) Membrane Potential Red Dye | journal = Molecular Pharmacology | volume = 92 | issue = 1 | pages = 88–99 | date = July 2017 | pmid = 28428226 | pmc = 5452057 | doi = 10.1124/mol.117.108563 }}</ref> Allopregnanolone has also been found to act as a positive allosteric modulator of the [[GABAA-rho receptor|GABA<sub>A</sub>-ρ receptor]], though the implications of this action are unclear.<ref name="pmid10496958">{{cite journal | vauthors = Morris KD, Moorefield CN, Amin J | title = Differential modulation of the gamma-aminobutyric acid type C receptor by neuroactive steroids | journal = Molecular Pharmacology | volume = 56 | issue = 4 | pages = 752–759 | date = October 1999 | pmid = 10496958 }}</ref><ref name="pmid17636008">{{cite journal | vauthors = Li W, Jin X, Covey DF, Steinbach JH | title = Neuroactive steroids and human recombinant rho1 GABAC receptors | journal = The Journal of Pharmacology and Experimental Therapeutics | volume = 323 | issue = 1 | pages = 236–247 | date = October 2007 | pmid = 17636008 | pmc = 3905684 | doi = 10.1124/jpet.107.127365 | s2cid = 12294587 }}</ref> In addition to its actions on GABA receptors, allopregnanolone, like progesterone, is known to be a [[negative allosteric modulator]] of [[nicotinic acetylcholine receptor|nACh receptor]]s,<ref name="pmid9166735">{{cite journal | vauthors = Bullock AE, Clark AL, Grady SR, Robinson SF, Slobe BS, Marks MJ, Collins AC | title = Neurosteroids modulate nicotinic receptor function in mouse striatal and thalamic synaptosomes | journal = Journal of Neurochemistry | volume = 68 | issue = 6 | pages = 2412–2423 | date = June 1997 | pmid = 9166735 | doi = 10.1046/j.1471-4159.1997.68062412.x | s2cid = 26195479 }}</ref> and also appears to act as a negative allosteric modulator of the [[5-HT3 receptor|5-HT<sub>3</sub> receptor]].<ref name="pmid9731711">{{cite journal | vauthors = Wetzel CH, Hermann B, Behl C, Pestel E, Rammes G, Zieglgänsberger W, Holsboer F, Rupprecht R | display-authors = 6 | title = Functional antagonism of gonadal steroids at the 5-hydroxytryptamine type 3 receptor | journal = Molecular Endocrinology | volume = 12 | issue = 9 | pages = 1441–1451 | date = September 1998 | pmid = 9731711 | doi = 10.1210/mend.12.9.0163 | doi-access = free | title-link = doi }}</ref> Along with the other inhibitory neurosteroids, allopregnanolone appears to have little or no action at other [[ligand-gated ion channel]]s, including the [[NMDA receptor|NMDA]], [[AMPA receptor|AMPA]], [[kainate receptor|kainate]], and [[glycine receptor]]s.<ref name="pmid17651807">{{cite journal | vauthors = Mellon SH | title = Neurosteroid regulation of central nervous system development | journal = Pharmacology & Therapeutics | volume = 116 | issue = 1 | pages = 107–124 | date = October 2007 | pmid = 17651807 | pmc = 2386997 | doi = 10.1016/j.pharmthera.2007.04.011 }}</ref> |
|||
Unlike progesterone, allopregnanolone is inactive at the classical [[nuclear receptor|nuclear]] [[progesterone receptor]] (PR).<ref name="pmid17651807" /> However, allopregnanolone can be intracellularly oxidized into [[5α-dihydroprogesterone]], which does act as an [[agonist]] of the PR, and for this reason, allopregnanolone can produce PR-mediated [[progestogen]]ic effects.<ref name="pmid8398145">{{cite journal | vauthors = Rupprecht R, Reul JM, Trapp T, van Steensel B, Wetzel C, Damm K, Zieglgänsberger W, Holsboer F | display-authors = 6 | title = Progesterone receptor-mediated effects of neuroactive steroids | journal = Neuron | volume = 11 | issue = 3 | pages = 523–530 | date = September 1993 | pmid = 8398145 | doi = 10.1016/0896-6273(93)90156-l | s2cid = 11205767 }}</ref><ref name="pmid27156439">{{cite journal | vauthors = Reddy DS, Estes WA | title = Clinical Potential of Neurosteroids for CNS Disorders | journal = Trends in Pharmacological Sciences | volume = 37 | issue = 7 | pages = 543–561 | date = July 2016 | pmid = 27156439 | pmc = 5310676 | doi = 10.1016/j.tips.2016.04.003 }}</ref> ([[5α-dihydroprogesterone]] is reduced to produce allopregnanolone, and progesterone is reduced to produce [[5α-dihydroprogesterone]]). In addition, allopregnanolone was reported in 2012 to be an agonist of the [[membrane progesterone receptor]]s (mPRs) discovered shortly before, including [[PAQR6|mPRδ]], [[PAQR7|mPRα]], and [[PAQR8|mPRβ]], with its activity at these receptors about a magnitude more potent than at the GABA<sub>A</sub> receptor.<ref name="pmid22687885">{{cite journal | vauthors = Thomas P, Pang Y | title = Membrane progesterone receptors: evidence for neuroprotective, neurosteroid signaling and neuroendocrine functions in neuronal cells | journal = Neuroendocrinology | volume = 96 | issue = 2 | pages = 162–171 | year = 2012 | pmid = 22687885 | pmc = 3489003 | doi = 10.1159/000339822 }}</ref><ref name="pmid23161870">{{cite journal | vauthors = Pang Y, Dong J, Thomas P | title = Characterization, neurosteroid binding and brain distribution of human membrane progesterone receptors δ and {epsilon} (mPRδ and mPR{epsilon}) and mPRδ involvement in neurosteroid inhibition of apoptosis | journal = Endocrinology | volume = 154 | issue = 1 | pages = 283–295 | date = January 2013 | pmid = 23161870 | pmc = 3529379 | doi = 10.1210/en.2012-1772 }}</ref> The action of allopregnanolone at these receptors may be related, in part, to its neuroprotective and [[antigonadotropic]] properties.<ref name="pmid22687885" /><ref name="pmid19423765">{{cite journal | vauthors = Sleiter N, Pang Y, Park C, Horton TH, Dong J, Thomas P, Levine JE | title = Progesterone receptor A (PRA) and PRB-independent effects of progesterone on gonadotropin-releasing hormone release | journal = Endocrinology | volume = 150 | issue = 8 | pages = 3833–3844 | date = August 2009 | pmid = 19423765 | pmc = 2717864 | doi = 10.1210/en.2008-0774 }}</ref> Also like progesterone, recent evidence has shown that allopregnanolone is an activator of the [[pregnane X receptor]].<ref name="pmid17651807" /><ref name="pmid15364541">{{cite journal | vauthors = Lamba V, Yasuda K, Lamba JK, Assem M, Davila J, Strom S, Schuetz EG | title = PXR (NR1I2): splice variants in human tissues, including brain, and identification of neurosteroids and nicotine as PXR activators | journal = Toxicology and Applied Pharmacology | volume = 199 | issue = 3 | pages = 251–265 | date = September 2004 | pmid = 15364541 | doi = 10.1016/j.taap.2003.12.027 | bibcode = 2004ToxAP.199..251L }}</ref> |
|||
Similarly to many other GABA<sub>A</sub> receptor positive allosteric modulators, allopregnanolone has been found to act as an [[channel blocker|inhibitor]] of [[L-type voltage-gated calcium channel]]s (L-VGCCs),<ref name="pmid17151597">{{cite journal | vauthors = Hu AQ, Wang ZM, Lan DM, Fu YM, Zhu YH, Dong Y, Zheng P | title = Inhibition of evoked glutamate release by neurosteroid allopregnanolone via inhibition of L-type calcium channels in rat medial prefrontal cortex | journal = Neuropsychopharmacology | volume = 32 | issue = 7 | pages = 1477–1489 | date = July 2007 | pmid = 17151597 | doi = 10.1038/sj.npp.1301261 | doi-access = free | title-link = doi }}</ref> including [[voltage-dependent calcium channel#α1 Subunit|α<sub>1</sub> subtype]]s [[Cav1.2|Ca<sub>v</sub>1.2]] and [[Cav1.3|Ca<sub>v</sub>1.3]].<ref name="pmid21262851">{{cite journal | vauthors = Earl DE, Tietz EI | title = Inhibition of recombinant L-type voltage-gated calcium channels by positive allosteric modulators of GABAA receptors | journal = The Journal of Pharmacology and Experimental Therapeutics | volume = 337 | issue = 1 | pages = 301–311 | date = April 2011 | pmid = 21262851 | pmc = 3063747 | doi = 10.1124/jpet.110.178244 }}</ref> However, the threshold concentration of allopregnanolone to inhibit L-VGCCs was determined to be 3 μM (3,000 nM), which is far greater than the concentration of 5 nM that has been estimated to be naturally produced in the human brain.<ref name="pmid21262851" /> Thus, inhibition of L-VGCCs is unlikely of any actual significance in the effects of endogenous allopregnanolone.<ref name="pmid21262851" /> Also, allopregnanolone, along with several other neurosteroids, has been found to activate the [[G protein-coupled bile acid receptor]] (GPBAR1, or TGR5).<ref name="pmid20665558">{{cite journal | vauthors = Keitel V, Görg B, Bidmon HJ, Zemtsova I, Spomer L, Zilles K, Häussinger D | title = The bile acid receptor TGR5 (Gpbar-1) acts as a neurosteroid receptor in brain | journal = Glia | volume = 58 | issue = 15 | pages = 1794–1805 | date = November 2010 | pmid = 20665558 | doi = 10.1002/glia.21049 | s2cid = 37368754 }}</ref> However, it is only able to do so at micromolar concentrations, which, similarly to the case of the L-VGCCs, are far greater than the low nanomolar concentrations of allopregnanolone estimated to be present in the brain.<ref name="pmid20665558" /> |
|||
====Biphasic actions at the GABA<sub>A</sub> receptor==== |
|||
Increased levels of allopregnanolone can produce paradoxical effects, including negative mood, anxiety, [[irritability]], and [[aggression]].<ref name="pmid21600269">{{cite journal | vauthors = Bäckström T, Haage D, Löfgren M, Johansson IM, Strömberg J, Nyberg S, Andréen L, Ossewaarde L, van Wingen GA, Turkmen S, Bengtsson SK | display-authors = 6 | title = Paradoxical effects of GABA-A modulators may explain sex steroid induced negative mood symptoms in some persons | journal = Neuroscience | volume = 191 | pages = 46–54 | date = September 2011 | pmid = 21600269 | doi = 10.1016/j.neuroscience.2011.03.061 | s2cid = 38928854 }}</ref><ref name="pmid19272715">{{cite journal | vauthors = Andréen L, Nyberg S, Turkmen S, van Wingen G, Fernández G, Bäckström T | title = Sex steroid induced negative mood may be explained by the paradoxical effect mediated by GABAA modulators | journal = Psychoneuroendocrinology | volume = 34 | issue = 8 | pages = 1121–1132 | date = September 2009 | pmid = 19272715 | doi = 10.1016/j.psyneuen.2009.02.003 | s2cid = 22259026 }}</ref><ref name="pmid23978486">{{cite journal | vauthors = Bäckström T, Bixo M, Johansson M, Nyberg S, Ossewaarde L, Ragagnin G, Savic I, Strömberg J, Timby E, van Broekhoven F, van Wingen G | display-authors = 6 | title = Allopregnanolone and mood disorders | journal = Progress in Neurobiology | volume = 113 | pages = 88–94 | date = February 2014 | pmid = 23978486 | doi = 10.1016/j.pneurobio.2013.07.005 | s2cid = 207407084 }}</ref> This appears to be because allopregnanolone possesses biphasic, U-shaped actions at the GABA<sub>A</sub> receptor – moderate level increases (in the range of 1.5–2 nmol/L total allopregnanolone, which are approximately equivalent to [[luteal phase]] levels) inhibit the activity of the receptor, while lower and higher concentration increases stimulate it.<ref name="pmid21600269" /><ref name="pmid19272715" /> This seems to be a common effect of many GABA<sub>A</sub> receptor positive allosteric modulators.<ref name="pmid14993039" /><ref name="pmid23978486" /> In accordance, acute administration of low doses of [[micronized progesterone]] (which reliably elevates allopregnanolone levels) has been found to have negative effects on mood, while higher doses have a neutral effect.<ref name="pmid16724185">{{cite journal | vauthors = Andréen L, Sundström-Poromaa I, Bixo M, Nyberg S, Bäckström T | title = Allopregnanolone concentration and mood--a bimodal association in postmenopausal women treated with oral progesterone | journal = Psychopharmacology | volume = 187 | issue = 2 | pages = 209–221 | date = August 2006 | pmid = 16724185 | doi = 10.1007/s00213-006-0417-0 | s2cid = 1933116 }}</ref> |
|||
===Antidepressant effects=== |
|||
The mechanism by which neurosteroid GABA<sub>A</sub> receptor PAMs like brexanolone have antidepressant effects is unknown.<ref name="ZorumskiPaul2019">{{cite journal | vauthors = Zorumski CF, Paul SM, Covey DF, Mennerick S | title = Neurosteroids as novel antidepressants and anxiolytics: GABA-A receptors and beyond | journal = Neurobiology of Stress | volume = 11 | pages = 100196 | date = November 2019 | pmid = 31649968 | pmc = 6804800 | doi = 10.1016/j.ynstr.2019.100196 }}</ref> Other GABA<sub>A</sub> receptor PAMs, such as [[benzodiazepine]]s, are not thought of as antidepressants and have no proven efficacy,<ref name="ZorumskiPaul2019" /> although [[alprazolam]] has historically been prescribed for depression.<ref>{{cite journal | vauthors = Warner MD, Peabody CA, Whiteford HA, Hollister LE | title = Alprazolam as an antidepressant | journal = The Journal of Clinical Psychiatry | volume = 49 | issue = 4 | pages = 148–150 | date = April 1988 | pmid = 3281931 }}</ref><ref>{{cite journal | vauthors = Srisurapanont M, Boonyanaruthee V | title = Alprazolam and standard antidepressants in the treatment of depression: a meta-analysis of the antidepressant effect | journal = Journal of the Medical Association of Thailand = Chotmaihet Thangphaet | volume = 80 | issue = 3 | pages = 183–188 | date = March 1997 | pmid = 9175386 | url = https://www.ncbi.nlm.nih.gov/books/NBK67062/ | publisher = Centre for Reviews and Dissemination (UK) }}</ref> Neurosteroid GABA<sub>A</sub> receptor PAMs are known to interact with GABA<sub>A</sub> receptors and subpopulations differently than benzodiazepines.<ref name="ZorumskiPaul2019" /> GABA<sub>A</sub> receptor-potentiating neurosteroids may preferentially target δ-subunit–containing GABA<sub>A</sub> receptors, and enhance both tonic and phasic inhibition mediated by GABA<sub>A</sub> receptors.<ref name="ZorumskiPaul2019" /> It is possible that neurosteroids like allopregnanolone may act on other [[biological target|target]]s, including membrane progesterone receptors, [[T-type calcium channel|T-type voltage-gated calcium channel]]s, and others, to mediate antidepressant effects.<ref name="ZorumskiPaul2019" /> |
|||
==Pharmacology== |
|||
===Pharmacokinetics=== |
|||
Brexanolone has low [[oral administration|oral]] [[bioavailability]] of less than 5%, necessitating [[parenteral administration|non-oral administration]].<ref name="Scott2019">{{cite journal | vauthors = Scott LJ | title = Brexanolone: First Global Approval | journal = Drugs | volume = 79 | issue = 7 | pages = 779–783 | date = May 2019 | pmid = 31006078 | doi = 10.1007/s40265-019-01121-0 | s2cid = 123095177 }}</ref> The [[volume of distribution]] of brexanolone is approximately 3 L/kg.<ref name="Scott2019" /> Its [[plasma protein binding]] is more than 99%.<ref name="Zulresso label" /><ref name="Scott2019" /> Brexanolone is [[metabolism|metabolized]] by [[ketone|keto]]-[[redox|reduction]] mediated via [[aldo-keto reductase]]s.<ref name="Zulresso label" /><ref name="Scott2019" /> The compound is [[conjugation (biochemistry)|conjugated]] by [[glucuronidation]] via [[glucuronosyltransferase]]s and [[sulfation]] via [[sulfotransferase]]s.<ref name="Zulresso label" /> It is not metabolized significantly by the [[cytochrome P450]] system.<ref name="Zulresso label" /><ref name="Scott2019" /> The three main [[metabolite]]s of brexanolone are inactive.<ref name="Scott2019" /> The [[elimination half-life]] of brexanolone is nine hours.<ref name="Zulresso label" /><ref name="Scott2019" /> Its total [[blood plasma|plasma]] [[clearance (pharmacology)|clearance]] is 1 L/h/kg.<ref name="Scott2019" /> It is [[excretion|excreted]] 47% in [[feces]] and 42% in [[urine]].<ref name="Zulresso label" /><ref name="Scott2019" /> Less than 1% is excreted as unchanged brexanolone.<ref name="Scott2019" /> |
|||
==Chemistry== |
==Chemistry== |
||
{{See also|List of neurosteroids}} |
|||
Allopregnanolone is a [[pregnane]] (C21) [[steroid]] and is also known as '''5α-pregnan-3α-ol-20-one''', '''3α-hydroxy-5α-pregnan-20-one''', or '''3α,5α-tetrahydroprogesterone''' ('''3α,5α-THP'''). It is very closely related structurally to [[5-pregnenolone]] (pregn-5-en-3β-ol-20-dione), [[progesterone]] (pregn-4-ene-3,20-dione), the [[isomer]]s of [[pregnanedione]] (5-dihydroprogesterone; 5-pregnane-3,20-dione), the isomers of [[4-pregnenolone]] (3-dihydroprogesterone; pregn-4-en-3-ol-20-one), and the isomers of [[pregnanediol]] (5-pregnane-3,20-diol). In addition, allopregnanolone is one of four [[isomer]]s of [[pregnanolone (disambiguation)|pregnanolone]] (3,5-tetrahydroprogesterone), with the other three isomers being [[pregnanolone]] (5β-pregnan-3α-ol-20-one), [[isopregnanolone]] (5α-pregnan-3β-ol-20-one), and [[epipregnanolone]] (5β-pregnan-3β-ol-20-one). |
|||
Allopregnanolone is a pregnane (C21) [[steroid]] and is also known as 5α-pregnan-3α-ol-20-one, 5α-pregnane-3α-ol-20-one,<ref name="doi1010970000054219900900100702"/><ref name="pmid697360"/><ref name="pmid9038248"/><ref name="pmid12175598"/><ref name="pmid15249131"/> 3α-hydroxy-5α-pregnan-20-one, or 3α,5α-tetrahydroprogesterone (3α,5α-THP). It is closely related structurally to [[5-pregnenolone]] (pregn-5-en-3β-ol-20-dione), progesterone (pregn-4-ene-3,20-dione), the isomers of [[pregnanedione]] (5-dihydroprogesterone; 5-pregnane-3,20-dione), the isomers of [[4-pregnenolone]] (3-dihydroprogesterone; pregn-4-en-3-ol-20-one), and the isomers of [[pregnanediol]] (5-pregnane-3,20-diol). In addition, allopregnanolone is one of four isomers of [[pregnanolone (disambiguation)|pregnanolone]] (3,5-tetrahydroprogesterone), with the other three isomers being [[pregnanolone]] (5β-pregnan-3α-ol-20-one), [[isopregnanolone]] (5α-pregnan-3β-ol-20-one), and [[epipregnanolone]] (5β-pregnan-3β-ol-20-one). |
|||
===Biosynthesis=== |
|||
The biosynthesis of allopregnanolone in the brain starts with the conversion of progesterone into 5α-dihydroprogesterone by [[5α-reductase]]. After that, [[3α-hydroxysteroid dehydrogenase]] converts this [[metabolic intermediate|intermediate]] into allopregnanolone.<ref name="Reddy_2010" /> Allopregnanolone in the brain is produced by cortical and [[hippocampus]] [[pyramidal neuron]]s and pyramidal-like neurons of the [[basolateral amygdala]].<ref>{{cite journal | vauthors = Agís-Balboa RC, Pinna G, Zhubi A, Maloku E, Veldic M, Costa E, Guidotti A | title = Characterization of brain neurons that express enzymes mediating neurosteroid biosynthesis | journal = Proceedings of the National Academy of Sciences of the United States of America | volume = 103 | issue = 39 | pages = 14602–14607 | date = September 2006 | pmid = 16984997 | pmc = 1600006 | doi = 10.1073/pnas.0606544103 | doi-access = free | bibcode = 2006PNAS..10314602A | title-link = doi }}</ref> |
|||
===Derivatives=== |
===Derivatives=== |
||
A variety of [[synthetic compound|synthetic]] [[chemical derivative| |
A variety of [[synthetic compound|synthetic]] [[chemical derivative|derivatives]] and [[structural analog|analogues]] of allopregnanolone with similar activity and effects exist, including [[alfadolone]] (3α,21-dihydroxy-5α-pregnane-11,20-dione), [[alfaxolone]] (3α-hydroxy-5α-pregnane-11,20-dione), [[ganaxolone]] (3α-hydroxy-3β-methyl-5α-pregnan-20-one), [[hydroxydione]] (21-hydroxy-5β-pregnane-3,20-dione), [[minaxolone]] (11α-(dimethylamino)-2β-ethoxy-3α-hydroxy-5α-pregnan-20-one), [[Org 20599]] (21-chloro-3α-hydroxy-2β-morpholin-4-yl-5β-pregnan-20-one), [[Org 21465]] (2β-(2,2-dimethyl-4-morpholinyl)-3α-hydroxy-11,20-dioxo-5α-pregnan-21-yl methanesulfonate), and [[renanolone]] (3α-hydroxy-5β-pregnan-11,20-dione). |
||
The 21-hydroxylated derivative of this compound, [[tetrahydrodeoxycorticosterone]], is an endogenous inhibitory neurosteroid with similar properties to those of allopregnanolone, and the 3β-methyl analogue of allopregnanolone, ganaxolone, is under development to treat [[epilepsy]] and other conditions, including [[post-traumatic stress disorder]].<ref name="Reddy_2010" /> |
|||
==History== |
|||
In March 2019, brexanolone was approved in the United States for the treatment of postpartum depression (PPD) in adult women,<ref name=FDA2019 /><ref name="FDA approval" /> the first drug approved by the U.S. [[Food and Drug Administration]] (FDA) specifically for PPD.<ref name=FDA2019 /> |
|||
The efficacy of brexanolone was shown in two clinical studies of participants who received a 60-hour continuous intravenous infusion of brexanolone or placebo and were then followed for four weeks.<ref name=FDA2019 /> The FDA approved allopregnanolone based on evidence from three clinical trials, conducted in the United States, (Trial 1/NCT02942004, Trial 3/NCT02614541, Trial 2/ NCT02942017) of 247 women with moderate or severe postpartum depression.<ref>{{cite web | title=Drug Trials Snapshots: Zulresso | website=U.S. [[Food and Drug Administration]] (FDA) | date=2 April 2019 | url=https://www.fda.gov/drugs/drug-approvals-and-databases/drug-trials-snapshots-zulresso | archive-url=https://web.archive.org/web/20190928054122/https://www.fda.gov/drugs/drug-approvals-and-databases/drug-trials-snapshots-zulresso | archive-date=28 September 2019 | url-status=live | access-date=24 November 2019}}</ref> |
|||
The FDA granted the application for brexanolone [[priority review]] and [[breakthrough therapy]] designations, and granted approval of Zulresso to Sage Therapeutics, Inc.<ref name=FDA2019 /> |
|||
==Society and culture== |
|||
===Names=== |
|||
Brexanolone is both the [[International Nonproprietary Name]] and the [[United States Adopted Name]] in the context of its use as a medication.<ref>{{cite web |title=INN Brexanolone |url=https://mednet-communities.net/inn/db/ViewINN.aspx?i=10446 |access-date=3 September 2019 |archive-date=4 November 2021 |archive-url=https://web.archive.org/web/20211104143210/https://extranet.who.int/soinn/?i=10446 |url-status=dead }}</ref><ref name="KEGG">{{cite web | url=https://www.genome.jp/dbget-bin/www_bget?dr:D11149 |title = KEGG DRUG: Brexanolone}}</ref> |
|||
Zulresso is a brand name of the medication.<ref name="Zulresso label" /> |
|||
===Legal status=== |
|||
In the United States, brexanolone is a [[Controlled Substances Act#Schedule IV controlled substances|Schedule IV]] [[controlled substance]].<ref name="MPR" /><ref name="Zulresso label" /> |
|||
===Available forms=== |
|||
Brexanolone is an [[aqueous solution|aqueous]] [[mixture]] of synthetic allopregnanolone and [[sulfobutyl ether β-cyclodextrin]] (betadex sulfobutyl ether sodium), a [[solubilizing agent]].<ref name="Zulresso label" /><ref name="Scott2019" /> It is provided at an allopregnanolone concentration of 100 mg/20 mL (5 mg/mL) in single-dose [[vial]]s for use by intravenous infusion.<ref name="Zulresso label" /> Each mL of brexanolone solution contains 5 mg allopregnanolone, 250 mg sulfobutyl ether β-cyclodextrin, 0.265 mg [[citric acid monohydrate]], 2.57 mg [[sodium citrate dihydrate]], and [[water for injection]].<ref name="Zulresso label" /> The solution is [[hypertonic]] and must be [[Concentration|diluted]] to a target concentration of 1 mg/mL with [[sterile water]] and [[sodium chloride]] prior to administration.<ref name="Zulresso label" /> Five [[infusion bag]]s are generally required for the full infusion.<ref name="Zulresso label" /> More than five infusion bags are necessary for patients weighing more than 90 kg (200 lbs).<ref name="Zulresso label" /> |
|||
==Research== |
==Research== |
||
Brexanolone was under development as an intravenously administered medication for the treatment of [[major depressive disorder]], [[super-refractory status epilepticus]], and [[essential tremor]], but development for these indications was discontinued.<ref name="AdisInsight">{{cite web|title=Brexanolone - Sage Therapeutics|url=http://adisinsight.springer.com/drugs/800039944|publisher=AdisInsight}}</ref> |
|||
Allopregnanolone and the other endogenous inhibitory neurosteroids have very short [[terminal half-life|terminal half-lives]], and for this reason, have not been pursued for [[clinical use]] themselves. Instead, [[Pharmaceutical drug|synthetic]] [[structural analog|analog]]s with improved [[pharmacokinetic]] profiles, such as [[ganaxolone]], have been synthesized and are being investigated. However, [[exogenous]] progesterone, such as oral micronized progesterone (OMP), reliably elevates allopregnanolone levels in the body with good dose-to-serum level correlations.<ref name="pmid16406399">{{cite journal |vauthors=Andréen L, Spigset O, Andersson A, Nyberg S, Bäckström T | title = Pharmacokinetics of progesterone and its metabolites allopregnanolone and pregnanolone after oral administration of low-dose progesterone | journal = Maturitas | volume = 54 | issue = 3 | pages = 238–44 |date=June 2006 | pmid = 16406399 | doi = 10.1016/j.maturitas.2005.11.005 | url = }}</ref> Due to this, it has been suggested that OMP could be described as a [[prodrug]] of sorts for allopregnanolone.<ref name="pmid16406399" /> As a result, there has been some interest in using OMP to treat [[catamenial epilepsy]],<ref name="DevinskySchachter2005">{{cite book | author1 = Orrin Devinsky | author2 = Steven Schachter | author3 = Steven Pacia | title = Complementary and Alternative Therapies for Epilepsy | url = https://books.google.com/books?id=WVUE-6Xdny4C&pg=PT378 | date = 1 January 2005 | publisher = Demos Medical Publishing | isbn = 978-1-934559-08-6 | pages = 378–}}</ref> as well as other menstrual cycle-related and neurosteroid-associated conditions. In addition to OMP, oral [[pregnenolone]] has also been found to act as a prodrug of allopregnanolone,<ref name="pmid15763596">{{cite journal | vauthors = Saudan C, Desmarchelier A, Sottas PE, Mangin P, Saugy M | title = Urinary marker of oral pregnenolone administration | journal = Steroids | volume = 70 | issue = 3 | pages = 179–83 | year = 2005 | pmid = 15763596 | doi = 10.1016/j.steroids.2004.12.007 | url = }}</ref><ref name="pmid21538944">{{cite journal | vauthors = Piper T, Schlug C, Mareck U, Schänzer W | title = Investigations on changes in ¹³C/¹²C ratios of endogenous urinary steroids after pregnenolone administration | journal = Drug Test Anal | volume = 3 | issue = 5 | pages = 283–90 | year = 2011 | pmid = 21538944 | doi = 10.1002/dta.281 | url = }}</ref><ref name="pmid23348009">{{cite journal | vauthors = Sripada RK, Marx CE, King AP, Rampton JC, Ho SS, Liberzon I | title = Allopregnanolone elevations following pregnenolone administration are associated with enhanced activation of emotion regulation neurocircuits | journal = Biol. Psychiatry | volume = 73 | issue = 11 | pages = 1045–53 | year = 2013 | pmid = 23348009 | pmc = 3648625 | doi = 10.1016/j.biopsych.2012.12.008 | url = }}</ref> though also of [[pregnenolone sulfate]].<ref name="pmid20570588">{{cite journal | vauthors = Ducharme N, Banks WA, Morley JE, Robinson SM, Niehoff ML, Mattern C, Farr SA | title = Brain distribution and behavioral effects of progesterone and pregnenolone after intranasal or intravenous administration | journal = Eur. J. Pharmacol. | volume = 641 | issue = 2-3 | pages = 128–34 | year = 2010 | pmid = 20570588 | pmc = 3008321 | doi = 10.1016/j.ejphar.2010.05.033 | url = }}</ref> |
|||
It has been suggested that allopregnanolone and its precursor pregnenolone may have therapeutic potential for treatment of various symptoms of alcohol use disorders by restoring deficits in GABAergic inhibition, moderating [[Corticotropin-releasing hormone|corticotropin releasing factor (CRF)]] signaling, and inhibiting excessive neuroimmune activation. Many co-occurring symptoms of [[Alcoholism|ethanol addiction]] (e.g., anxiety, depression, seizures, sleep disturbance, pain) that are believed to contribute to the downward spiral of the addiction may also be controlled with [[Neurosteroid|neuroactive steroids]].<ref>{{cite journal | vauthors = Morrow AL, Boero G, Porcu P | title = A Rationale for Allopregnanolone Treatment of Alcohol Use Disorders: Basic and Clinical Studies | journal = Alcoholism: Clinical and Experimental Research | volume = 44 | issue = 2 | pages = 320–339 | date = February 2020 | pmid = 31782169 | pmc = 7018555 | doi = 10.1111/acer.14253 }}</ref> |
|||
Under the names '''brexanolone''' and '''SAGE-547''', allopregnanolone is under development by [[SAGE Therapeutics]] as an [[intravenous]]ly administered [[drug]] for the treatment of [[super-refractory status epilepticus]], [[postpartum depression]], and [[essential tremor]].<ref name="SageTherapeutics" /> As of June 2016, it is in [[Phases of clinical research#Phase III|phase III]] [[clinical trial]]s for the former indication and [[Phases of clinical research#Phase II|phase II]] trials for the latter two.[http://adisinsight.springer.com/drugs/800039944][http://www.sagerx.com/programs.php#547] |
|||
[[Exogenous]] progesterone, such as [[oral administration|oral]] progesterone, elevates allopregnanolone levels in the body with good dose-to-serum level correlations.<ref name="pmid16406399">{{cite journal | vauthors = Andréen L, Spigset O, Andersson A, Nyberg S, Bäckström T | title = Pharmacokinetics of progesterone and its metabolites allopregnanolone and pregnanolone after oral administration of low-dose progesterone | journal = Maturitas | volume = 54 | issue = 3 | pages = 238–244 | date = June 2006 | pmid = 16406399 | doi = 10.1016/j.maturitas.2005.11.005 }}</ref> Due to this, it has been suggested that oral progesterone could be described as a [[prodrug]] of sorts for allopregnanolone.<ref name="pmid16406399" /> As a result, there has been some interest in using oral progesterone to treat catamenial epilepsy,<ref name="DevinskySchachter2005">{{cite book | vauthors = Devinsky O, Schachter S, Pacia S | title = Complementary and Alternative Therapies for Epilepsy | url = https://books.google.com/books?id=WVUE-6Xdny4C&pg=PT378 | date = 1 January 2005 | publisher = Demos Medical Publishing | isbn = 978-1-934559-08-6 | pages = 378–}}</ref> as well as other menstrual cycle-related and neurosteroid-associated conditions. In addition to oral progesterone, oral [[pregnenolone]] has also been found to act as a prodrug of allopregnanolone,<ref name="pmid15763596">{{cite journal | vauthors = Saudan C, Desmarchelier A, Sottas PE, Mangin P, Saugy M | title = Urinary marker of oral pregnenolone administration | journal = Steroids | volume = 70 | issue = 3 | pages = 179–183 | date = March 2005 | pmid = 15763596 | doi = 10.1016/j.steroids.2004.12.007 | s2cid = 25490229 }}</ref><ref name="pmid21538944">{{cite journal | vauthors = Piper T, Schlug C, Mareck U, Schänzer W | title = Investigations on changes in ¹³C/¹²C ratios of endogenous urinary steroids after pregnenolone administration | journal = Drug Testing and Analysis | volume = 3 | issue = 5 | pages = 283–290 | date = May 2011 | pmid = 21538944 | doi = 10.1002/dta.281 }}</ref><ref name="pmid23348009">{{cite journal | vauthors = Sripada RK, Marx CE, King AP, Rampton JC, Ho SS, Liberzon I | title = Allopregnanolone elevations following pregnenolone administration are associated with enhanced activation of emotion regulation neurocircuits | journal = Biological Psychiatry | volume = 73 | issue = 11 | pages = 1045–1053 | date = June 2013 | pmid = 23348009 | pmc = 3648625 | doi = 10.1016/j.biopsych.2012.12.008 }}</ref> though also of [[pregnenolone sulfate]].<ref name="pmid20570588">{{cite journal | vauthors = Ducharme N, Banks WA, Morley JE, Robinson SM, Niehoff ML, Mattern C, Farr SA | title = Brain distribution and behavioral effects of progesterone and pregnenolone after intranasal or intravenous administration | journal = European Journal of Pharmacology | volume = 641 | issue = 2–3 | pages = 128–134 | date = September 2010 | pmid = 20570588 | pmc = 3008321 | doi = 10.1016/j.ejphar.2010.05.033 }}</ref> |
|||
==References== |
|||
{{Reflist|2}} |
|||
In animal models of [[traumatic brain injury]], allopregnanolone has been shown to reduce inflammation by attenuating the production of proinflammatory cytokines (IL-1β and TNF-α) at 3 h after the injury. It has also been shown to reduce the severity of brain damage and improve cognitive function and recovery.<ref>{{cite journal | vauthors = He J, Evans CO, Hoffman SW, Oyesiku NM, Stein DG | title = Progesterone and allopregnanolone reduce inflammatory cytokines after traumatic brain injury | journal = Experimental Neurology | volume = 189 | issue = 2 | pages = 404–412 | date = October 2004 | pmid = 15380490 | doi = 10.1016/j.expneurol.2004.06.008 | s2cid = 10008079 }}</ref> |
|||
==Additional reading== |
|||
* {{cite book | last1=Herd | first1=MB | last2= Belelli |first2=D |last3=Lambert |first3=JJ |title= Neurosteroid modulation of synaptic and extrasynaptic GABA(A) receptors |url= |format= |accessdate= |edition= |year= 2007 |publisher= Pharmacol. Ther. 116(1):20-34 |location= |language= |id= |doi= 10.1016/j.pharmthera.2007.03.007 }} |
|||
== References == |
|||
{{Reflist}} |
|||
== Further reading == |
|||
{{Steroid hormones}} |
|||
{{ |
{{refbegin}} |
||
* {{cite journal | vauthors = Herd MB, Belelli D, Lambert JJ | title = Neurosteroid modulation of synaptic and extrasynaptic GABA(A) receptors | journal = Pharmacology & Therapeutics | volume = 116 | issue = 1 | pages = 20–34 | date = October 2007 | pmid = 17531325 | doi = 10.1016/j.pharmthera.2007.03.007 | arxiv = 1607.02870 }} |
|||
{{GABAAR PAMs}} |
|||
* {{cite journal | vauthors = Zorumski CF, Paul SM, Covey DF, Mennerick S | title = Neurosteroids as novel antidepressants and anxiolytics: GABA-A receptors and beyond | journal = Neurobiology of Stress | volume = 11 | pages = 100196 | date = November 2019 | pmid = 31649968 | pmc = 6804800 | doi = 10.1016/j.ynstr.2019.100196 }} |
|||
{{Progestogenics}} |
|||
{{ |
{{refend}} |
||
== External links == |
|||
* {{ClinicalTrialsGov|NCT02942004|A Study to Evaluate Efficacy and Safety of SAGE-547 in Participants With Severe Postpartum Depression (547-PPD-202B)}} |
|||
* {{ClinicalTrialsGov|NCT02614547|A Study to Evaluate SAGE-547 in Patients With Severe Postpartum Depression}} |
|||
* {{ClinicalTrialsGov|NCT02942017|A Study to Evaluate Safety and Efficacy of SAGE-547 in Participants With Moderate Postpartum Depression (547-PPD-202C)}} |
|||
{{Progesterone}} |
|||
{{Endogenous steroids}} |
|||
{{Antidepressants}} |
|||
{{Navboxes |
|||
| title = [[Biological activity]] |
|||
| titlestyle = background:#ccccff |
|||
| list1 = |
|||
{{GABAA receptor positive allosteric modulators}} |
|||
{{Glycine receptor modulators}} |
|||
{{Nicotinic acetylcholine receptor modulators}} |
|||
{{Progesterone receptor modulators}} |
|||
{{Xenobiotic-sensing receptor modulators}} |
|||
}} |
|||
{{Portal bar | Medicine}} |
|||
[[Category:Anticonvulsants]] |
[[Category:Anticonvulsants]] |
||
[[Category:Antidepressants]] |
[[Category:Antidepressants]] |
||
[[Category:Neurosteroids]] |
|||
[[Category:Alcohols]] |
|||
[[Category:Ketones]] |
|||
[[Category:GABAA receptor positive allosteric modulators]] |
[[Category:GABAA receptor positive allosteric modulators]] |
||
[[Category:GABAA-rho receptor positive allosteric modulators]] |
[[Category:GABAA-rho receptor positive allosteric modulators]] |
||
[[Category:Glycine receptor agonists]] |
[[Category:Glycine receptor agonists]] |
||
[[Category: |
[[Category:Acetyl compounds]] |
||
[[Category:Neurosteroids]] |
|||
[[Category:Nicotinic antagonists]] |
|||
[[Category:Pregnane X receptor agonists]] |
[[Category:Pregnane X receptor agonists]] |
||
[[Category:Pregnanes]] |
[[Category:Pregnanes]] |
||
[[Category:5α-Pregnanes]] |
|||
[[Category:Progestogens]] |
|||
[[Category:Sterols]] |
|||
Latest revision as of 03:36, 8 December 2024
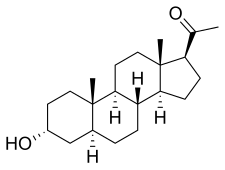 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Zulresso |
| Other names | ALLO; ALLOP; SAGE-547; SGE-102; 5α-Pregnan-3α-ol-20-one; 5α-Pregnane-3α-ol-20-one;[1][2][3][4][5] 3α-Hydroxy-5α-pregnan-20-one; 3α,5α-Tetrahydroprogesterone; 3α,5α-THP, brexanolone (USAN US) |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a619037 |
| License data |
|
| Routes of administration | Intravenous[6] |
| Drug class | Neurosteroids; Antidepressants |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Oral: <5%[9] |
| Protein binding | >99%[6][9] |
| Metabolism | Non-CYP450 (keto-reduction via aldo-keto reductases (AKR), glucuronidation via glucuronosyltransferases (UGT), sulfation via sulfotransferases (SULT))[6][9] |
| Elimination half-life | 9 hours[6][9] |
| Excretion | Feces: 47%[6][9] Urine: 42%[6][9] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C21H34O2 |
| Molar mass | 318.501 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Allopregnanolone is a naturally occurring neurosteroid which is made in the body from the hormone progesterone.[10][11] As a medication, allopregnanolone is referred to as brexanolone, sold under the brand name Zulresso,[6][12] and used to treat postpartum depression.[11][13][14] It is given by injection into a vein.[11][6]
Side effects of brexanolone may include sedation, sleepiness, dry mouth, hot flashes, and loss of consciousness.[6][11] It is a neurosteroid and acts as a positive allosteric modulator of the GABAA receptor, the major biological target of the inhibitory neurotransmitter γ-aminobutyric acid (GABA).[6]
Brexanolone was approved for medical use in the United States in 2019.[11][15] The U.S. Food and Drug Administration (FDA) considers it to be a first-in-class medication.[16] The long administration time, as well as the cost for a one-time treatment, have raised concerns about accessibility for many women.[17]
Medical uses
[edit]Brexanolone is used to treat postpartum depression in adult women, administered as a continuous intravenous infusion over a period of 60 hours and essential tremor.[11][18]
Clinical efficacy
[edit]Women experiencing moderate to severe postpartum depression when treated with a single dose of intravenous brexanolone display a significant reduction in HAM-D scores which persisted 30 days post-treatment.[19]
Side effects
[edit]Side effects of brexanolone include dizziness (10–20%), sedation (13–21%), headache (18%), nausea (10%), dry mouth (3–11%), loss of consciousness (3–5%), and flushing (2–5%).[6][11][9][20] It can produce euphoria to a degree similar to that of alprazolam (3–13% at infusion doses of 90–270 μg over a one-hour period).[6] Serious or severe adverse effects are rare but may include altered state of consciousness, syncope, presyncope, fatigue, and insomnia.[20]
Biological function
[edit]Allopregnanolone possesses a wide variety of effects, including, in no particular order, antidepressant, anxiolytic, stress-reducing, rewarding,[21] prosocial,[22] antiaggressive,[23] prosexual,[22] sedative, pro-sleep,[24] cognitive, memory-impairment, analgesic,[25] anesthetic, anticonvulsant, neuroprotective, and neurogenic effects.[10] Fluctuations in the levels of allopregnanolone and the other neurosteroids seem to play an important role in the pathophysiology of mood, anxiety, premenstrual syndrome, catamenial epilepsy, and various other neuropsychiatric conditions.[26][27][28]
During pregnancy, allopregnanolone and pregnanolone are involved in sedation and anesthesia of the fetus.[29][30]
Allopregnanolone is a metabolic intermediate in an androgen backdoor pathway from progesterone to dihydrotestosterone, which occurs during normal male fetus development; placental progesterone in the male fetus is the feedstock of this pathway; deficiencies in this pathway lead to insufficient virilization of the male fetus.[31]
Mechanism of action
[edit]Molecular interactions
[edit]Allopregnanolone is an endogenous inhibitory pregnane neurosteroid.[10] It is made from pregnenolone, and is a positive allosteric modulator of the action of γ-aminobutyric acid (GABA) at GABAA receptor.[10] Allopregnanolone has effects similar to those of other positive allosteric modulators of the GABA action at GABAA receptor such as the benzodiazepines, including anxiolytic, sedative, and anticonvulsant activity.[10][32][33] Endogenously produced allopregnanolone exerts a neurophysiological role by fine-tuning of GABAA receptor and modulating the action of several positive allosteric modulators and agonists at GABAA receptor.[34]
Allopregnanolone acts as a highly potent positive allosteric modulator of the GABAA receptor.[10] While allopregnanolone, like other inhibitory neurosteroids such as THDOC, positively modulates all GABAA receptor isoforms, those isoforms containing δ subunits exhibit the greatest potentiation.[35] Allopregnanolone has also been found to act as a positive allosteric modulator of the GABAA-ρ receptor, though the implications of this action are unclear.[36][37] In addition to its actions on GABA receptors, allopregnanolone, like progesterone, is known to be a negative allosteric modulator of nACh receptors,[38] and also appears to act as a negative allosteric modulator of the 5-HT3 receptor.[39] Along with the other inhibitory neurosteroids, allopregnanolone appears to have little or no action at other ligand-gated ion channels, including the NMDA, AMPA, kainate, and glycine receptors.[40]
Unlike progesterone, allopregnanolone is inactive at the classical nuclear progesterone receptor (PR).[40] However, allopregnanolone can be intracellularly oxidized into 5α-dihydroprogesterone, which does act as an agonist of the PR, and for this reason, allopregnanolone can produce PR-mediated progestogenic effects.[41][42] (5α-dihydroprogesterone is reduced to produce allopregnanolone, and progesterone is reduced to produce 5α-dihydroprogesterone). In addition, allopregnanolone was reported in 2012 to be an agonist of the membrane progesterone receptors (mPRs) discovered shortly before, including mPRδ, mPRα, and mPRβ, with its activity at these receptors about a magnitude more potent than at the GABAA receptor.[43][44] The action of allopregnanolone at these receptors may be related, in part, to its neuroprotective and antigonadotropic properties.[43][45] Also like progesterone, recent evidence has shown that allopregnanolone is an activator of the pregnane X receptor.[40][46]
Similarly to many other GABAA receptor positive allosteric modulators, allopregnanolone has been found to act as an inhibitor of L-type voltage-gated calcium channels (L-VGCCs),[47] including α1 subtypes Cav1.2 and Cav1.3.[48] However, the threshold concentration of allopregnanolone to inhibit L-VGCCs was determined to be 3 μM (3,000 nM), which is far greater than the concentration of 5 nM that has been estimated to be naturally produced in the human brain.[48] Thus, inhibition of L-VGCCs is unlikely of any actual significance in the effects of endogenous allopregnanolone.[48] Also, allopregnanolone, along with several other neurosteroids, has been found to activate the G protein-coupled bile acid receptor (GPBAR1, or TGR5).[49] However, it is only able to do so at micromolar concentrations, which, similarly to the case of the L-VGCCs, are far greater than the low nanomolar concentrations of allopregnanolone estimated to be present in the brain.[49]
Biphasic actions at the GABAA receptor
[edit]Increased levels of allopregnanolone can produce paradoxical effects, including negative mood, anxiety, irritability, and aggression.[50][51][52] This appears to be because allopregnanolone possesses biphasic, U-shaped actions at the GABAA receptor – moderate level increases (in the range of 1.5–2 nmol/L total allopregnanolone, which are approximately equivalent to luteal phase levels) inhibit the activity of the receptor, while lower and higher concentration increases stimulate it.[50][51] This seems to be a common effect of many GABAA receptor positive allosteric modulators.[26][52] In accordance, acute administration of low doses of micronized progesterone (which reliably elevates allopregnanolone levels) has been found to have negative effects on mood, while higher doses have a neutral effect.[53]
Antidepressant effects
[edit]The mechanism by which neurosteroid GABAA receptor PAMs like brexanolone have antidepressant effects is unknown.[54] Other GABAA receptor PAMs, such as benzodiazepines, are not thought of as antidepressants and have no proven efficacy,[54] although alprazolam has historically been prescribed for depression.[55][56] Neurosteroid GABAA receptor PAMs are known to interact with GABAA receptors and subpopulations differently than benzodiazepines.[54] GABAA receptor-potentiating neurosteroids may preferentially target δ-subunit–containing GABAA receptors, and enhance both tonic and phasic inhibition mediated by GABAA receptors.[54] It is possible that neurosteroids like allopregnanolone may act on other targets, including membrane progesterone receptors, T-type voltage-gated calcium channels, and others, to mediate antidepressant effects.[54]
Pharmacology
[edit]Pharmacokinetics
[edit]Brexanolone has low oral bioavailability of less than 5%, necessitating non-oral administration.[9] The volume of distribution of brexanolone is approximately 3 L/kg.[9] Its plasma protein binding is more than 99%.[6][9] Brexanolone is metabolized by keto-reduction mediated via aldo-keto reductases.[6][9] The compound is conjugated by glucuronidation via glucuronosyltransferases and sulfation via sulfotransferases.[6] It is not metabolized significantly by the cytochrome P450 system.[6][9] The three main metabolites of brexanolone are inactive.[9] The elimination half-life of brexanolone is nine hours.[6][9] Its total plasma clearance is 1 L/h/kg.[9] It is excreted 47% in feces and 42% in urine.[6][9] Less than 1% is excreted as unchanged brexanolone.[9]
Chemistry
[edit]Allopregnanolone is a pregnane (C21) steroid and is also known as 5α-pregnan-3α-ol-20-one, 5α-pregnane-3α-ol-20-one,[1][2][3][4][5] 3α-hydroxy-5α-pregnan-20-one, or 3α,5α-tetrahydroprogesterone (3α,5α-THP). It is closely related structurally to 5-pregnenolone (pregn-5-en-3β-ol-20-dione), progesterone (pregn-4-ene-3,20-dione), the isomers of pregnanedione (5-dihydroprogesterone; 5-pregnane-3,20-dione), the isomers of 4-pregnenolone (3-dihydroprogesterone; pregn-4-en-3-ol-20-one), and the isomers of pregnanediol (5-pregnane-3,20-diol). In addition, allopregnanolone is one of four isomers of pregnanolone (3,5-tetrahydroprogesterone), with the other three isomers being pregnanolone (5β-pregnan-3α-ol-20-one), isopregnanolone (5α-pregnan-3β-ol-20-one), and epipregnanolone (5β-pregnan-3β-ol-20-one).
Biosynthesis
[edit]The biosynthesis of allopregnanolone in the brain starts with the conversion of progesterone into 5α-dihydroprogesterone by 5α-reductase. After that, 3α-hydroxysteroid dehydrogenase converts this intermediate into allopregnanolone.[10] Allopregnanolone in the brain is produced by cortical and hippocampus pyramidal neurons and pyramidal-like neurons of the basolateral amygdala.[57]
Derivatives
[edit]A variety of synthetic derivatives and analogues of allopregnanolone with similar activity and effects exist, including alfadolone (3α,21-dihydroxy-5α-pregnane-11,20-dione), alfaxolone (3α-hydroxy-5α-pregnane-11,20-dione), ganaxolone (3α-hydroxy-3β-methyl-5α-pregnan-20-one), hydroxydione (21-hydroxy-5β-pregnane-3,20-dione), minaxolone (11α-(dimethylamino)-2β-ethoxy-3α-hydroxy-5α-pregnan-20-one), Org 20599 (21-chloro-3α-hydroxy-2β-morpholin-4-yl-5β-pregnan-20-one), Org 21465 (2β-(2,2-dimethyl-4-morpholinyl)-3α-hydroxy-11,20-dioxo-5α-pregnan-21-yl methanesulfonate), and renanolone (3α-hydroxy-5β-pregnan-11,20-dione).
The 21-hydroxylated derivative of this compound, tetrahydrodeoxycorticosterone, is an endogenous inhibitory neurosteroid with similar properties to those of allopregnanolone, and the 3β-methyl analogue of allopregnanolone, ganaxolone, is under development to treat epilepsy and other conditions, including post-traumatic stress disorder.[10]
History
[edit]In March 2019, brexanolone was approved in the United States for the treatment of postpartum depression (PPD) in adult women,[11][15] the first drug approved by the U.S. Food and Drug Administration (FDA) specifically for PPD.[11]
The efficacy of brexanolone was shown in two clinical studies of participants who received a 60-hour continuous intravenous infusion of brexanolone or placebo and were then followed for four weeks.[11] The FDA approved allopregnanolone based on evidence from three clinical trials, conducted in the United States, (Trial 1/NCT02942004, Trial 3/NCT02614541, Trial 2/ NCT02942017) of 247 women with moderate or severe postpartum depression.[58]
The FDA granted the application for brexanolone priority review and breakthrough therapy designations, and granted approval of Zulresso to Sage Therapeutics, Inc.[11]
Society and culture
[edit]Names
[edit]Brexanolone is both the International Nonproprietary Name and the United States Adopted Name in the context of its use as a medication.[59][60]
Zulresso is a brand name of the medication.[6]
Legal status
[edit]In the United States, brexanolone is a Schedule IV controlled substance.[8][6]
Available forms
[edit]Brexanolone is an aqueous mixture of synthetic allopregnanolone and sulfobutyl ether β-cyclodextrin (betadex sulfobutyl ether sodium), a solubilizing agent.[6][9] It is provided at an allopregnanolone concentration of 100 mg/20 mL (5 mg/mL) in single-dose vials for use by intravenous infusion.[6] Each mL of brexanolone solution contains 5 mg allopregnanolone, 250 mg sulfobutyl ether β-cyclodextrin, 0.265 mg citric acid monohydrate, 2.57 mg sodium citrate dihydrate, and water for injection.[6] The solution is hypertonic and must be diluted to a target concentration of 1 mg/mL with sterile water and sodium chloride prior to administration.[6] Five infusion bags are generally required for the full infusion.[6] More than five infusion bags are necessary for patients weighing more than 90 kg (200 lbs).[6]
Research
[edit]Brexanolone was under development as an intravenously administered medication for the treatment of major depressive disorder, super-refractory status epilepticus, and essential tremor, but development for these indications was discontinued.[61]
It has been suggested that allopregnanolone and its precursor pregnenolone may have therapeutic potential for treatment of various symptoms of alcohol use disorders by restoring deficits in GABAergic inhibition, moderating corticotropin releasing factor (CRF) signaling, and inhibiting excessive neuroimmune activation. Many co-occurring symptoms of ethanol addiction (e.g., anxiety, depression, seizures, sleep disturbance, pain) that are believed to contribute to the downward spiral of the addiction may also be controlled with neuroactive steroids.[62]
Exogenous progesterone, such as oral progesterone, elevates allopregnanolone levels in the body with good dose-to-serum level correlations.[63] Due to this, it has been suggested that oral progesterone could be described as a prodrug of sorts for allopregnanolone.[63] As a result, there has been some interest in using oral progesterone to treat catamenial epilepsy,[64] as well as other menstrual cycle-related and neurosteroid-associated conditions. In addition to oral progesterone, oral pregnenolone has also been found to act as a prodrug of allopregnanolone,[65][66][67] though also of pregnenolone sulfate.[68]
In animal models of traumatic brain injury, allopregnanolone has been shown to reduce inflammation by attenuating the production of proinflammatory cytokines (IL-1β and TNF-α) at 3 h after the injury. It has also been shown to reduce the severity of brain damage and improve cognitive function and recovery.[69]
References
[edit]- ^ a b Krieger NR, Mok WM, Herschkowitz S (September 1990). "5α-Pregnane-3α-ol-20-one Identified as an Active Molecular Species of Steroid Anesthetic in Brain". Anesthesiology. 73. doi:10.1097/00000542-199009001-00702.
- ^ a b Yagen B, Gallili GE, Mateles RI (August 1978). "Progesterone biotransformation by plant cell suspension cultures". Applied and Environmental Microbiology. 36 (2): 213–216. Bibcode:1978ApEnM..36..213Y. doi:10.1128/AEM.36.2.213-216.1978. PMC 291203. PMID 697360.
- ^ a b Meyer HH, Jewgenow K, Hodges JK (February 1997). "Binding activity of 5alpha-reduced gestagens to the progestin receptor from African elephant (Loxodonta africana)". General and Comparative Endocrinology. 105 (2): 164–167. doi:10.1006/gcen.1996.6813. PMID 9038248.
- ^ a b Frye C, Seliga A (May 2002). "Olanzapine and progesterone have dose-dependent and additive effects to enhance lordosis and progestin concentrations of rats". Physiology & Behavior. 76 (1): 151–158. doi:10.1016/s0031-9384(02)00689-3. PMID 12175598. S2CID 38249308.
- ^ a b Mahendroo M, Wilson JD, Richardson JA, Auchus RJ (July 2004). "Steroid 5alpha-reductase 1 promotes 5alpha-androstane-3alpha,17beta-diol synthesis in immature mouse testes by two pathways". Molecular and Cellular Endocrinology. 222 (1–2): 113–120. doi:10.1016/j.mce.2004.04.009. PMID 15249131. S2CID 54297812.
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa "Zulresso- brexanolone injection, solution". DailyMed. 18 November 2019. Retrieved 23 November 2019.
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 October 2023.
- ^ a b "DEA Schedules Postpartum Depression Treatment Zulresso". Monthly Prescribing Reference. 17 June 2019. Archived from the original on 3 September 2019. Retrieved 24 November 2019.
- ^ a b c d e f g h i j k l m n o p q r Scott LJ (May 2019). "Brexanolone: First Global Approval". Drugs. 79 (7): 779–783. doi:10.1007/s40265-019-01121-0. PMID 31006078. S2CID 123095177.
- ^ a b c d e f g h Reddy DS (2010). "Neurosteroids". Sex Differences in the Human Brain, their Underpinnings and Implications. Progress in Brain Research. Vol. 186. Elsevier. pp. 113–137. doi:10.1016/B978-0-444-53630-3.00008-7. ISBN 9780444536303. PMC 3139029. PMID 21094889.
- ^ a b c d e f g h i j k "FDA approves first treatment for post-partum depression". U.S. Food and Drug Administration (FDA) (Press release). 19 March 2019. Archived from the original on 11 October 2019. Retrieved 21 March 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "ChemIDplus - 516-54-1 - AURFZBICLPNKBZ-SYBPFIFISA-N - Brexanolone [USAN] - Similar structures search, synonyms, formulas, resource links, and other chemical information". NIH Toxnet. Retrieved 26 December 2017.
- ^ Frieder A, Fersh M, Hainline R, Deligiannidis KM (March 2019). "Pharmacotherapy of Postpartum Depression: Current Approaches and Novel Drug Development". CNS Drugs. 33 (3): 265–282. doi:10.1007/s40263-019-00605-7. PMC 6424603. PMID 30790145.
- ^ Wilkinson ST, Sanacora G (February 2019). "A new generation of antidepressants: an update on the pharmaceutical pipeline for novel and rapid-acting therapeutics in mood disorders based on glutamate/GABA neurotransmitter systems". Drug Discovery Today. 24 (2): 606–615. doi:10.1016/j.drudis.2018.11.007. PMC 6397075. PMID 30447328.
- ^ a b "Drug Approval Package: Zulresso". U.S. Food and Drug Administration (FDA). 7 February 2019. Retrieved 6 August 2020.
- ^ "New Drug Therapy Approvals 2019". U.S. Food and Drug Administration. 31 December 2019. Retrieved 15 September 2020.
- ^ Chatterjee R (21 March 2019). "New Postpartum Depression Drug Could Be Hard To Access For Moms Most In Need". NPR. Retrieved 22 March 2019.
- ^ "Zulresso (brexanolone) dosing, indications, interactions, adverse effects, and more". reference.medscape.com. Retrieved 30 September 2023.
- ^ Edinoff AN, Odisho AS, Lewis K, Kaskas A, Hunt G, Cornett EM, et al. (2021). "Brexanolone, a GABAA Modulator, in the Treatment of Postpartum Depression in Adults: A Comprehensive Review". Frontiers in Psychiatry. 12: 699740. doi:10.3389/fpsyt.2021.699740. PMC 8477036. PMID 34594247.
- ^ a b Walkery A, Leader LD, Cooke E, VandenBerg A (9 July 2021). "Review of Allopregnanolone Agonist Therapy for the Treatment of Depressive Disorders". Drug Design, Development and Therapy. 15: 3017–3026. doi:10.2147/DDDT.S240856. PMC 8276990. PMID 34267503.
- ^ Rougé-Pont F, Mayo W, Marinelli M, Gingras M, Le Moal M, Piazza PV (July 2002). "The neurosteroid allopregnanolone increases dopamine release and dopaminergic response to morphine in the rat nucleus accumbens". The European Journal of Neuroscience. 16 (1): 169–173. doi:10.1046/j.1460-9568.2002.02084.x. PMID 12153544. S2CID 9953445.
- ^ a b Frye CA (December 2009). "Neurosteroids' effects and mechanisms for social, cognitive, emotional, and physical functions". Psychoneuroendocrinology. 34 (Suppl 1): S143 – S161. doi:10.1016/j.psyneuen.2009.07.005. PMC 2898141. PMID 19656632.
- ^ Pinna G, Costa E, Guidotti A (February 2005). "Changes in brain testosterone and allopregnanolone biosynthesis elicit aggressive behavior". Proceedings of the National Academy of Sciences of the United States of America. 102 (6): 2135–2140. Bibcode:2005PNAS..102.2135P. doi:10.1073/pnas.0409643102. PMC 548579. PMID 15677716.
- ^ Terán-Pérez G, Arana-Lechuga Y, Esqueda-León E, Santana-Miranda R, Rojas-Zamorano JÁ, Velázquez Moctezuma J (October 2012). "Steroid hormones and sleep regulation". Mini Reviews in Medicinal Chemistry. 12 (11): 1040–1048. doi:10.2174/138955712802762167. PMID 23092405.
- ^ Patte-Mensah C, Meyer L, Taleb O, Mensah-Nyagan AG (February 2014). "Potential role of allopregnanolone for a safe and effective therapy of neuropathic pain". Progress in Neurobiology. 113: 70–78. doi:10.1016/j.pneurobio.2013.07.004. PMID 23948490. S2CID 207407077.
- ^ a b Bäckström T, Andersson A, Andreé L, Birzniece V, Bixo M, Björn I, et al. (December 2003). "Pathogenesis in menstrual cycle-linked CNS disorders". Annals of the New York Academy of Sciences. 1007 (1): 42–53. Bibcode:2003NYASA1007...42B. doi:10.1196/annals.1286.005. PMID 14993039. S2CID 20995334.
- ^ Guille C, Spencer S, Cavus I, Epperson CN (July 2008). "The role of sex steroids in catamenial epilepsy and premenstrual dysphoric disorder: implications for diagnosis and treatment". Epilepsy & Behavior. 13 (1): 12–24. doi:10.1016/j.yebeh.2008.02.004. PMC 4112568. PMID 18346939.
- ^ Finocchi C, Ferrari M (May 2011). "Female reproductive steroids and neuronal excitability". Neurological Sciences. 32 (Suppl 1): S31 – S35. doi:10.1007/s10072-011-0532-5. PMID 21533709. S2CID 8885335.
- ^ Mellor DJ, Diesch TJ, Gunn AJ, Bennet L (November 2005). "The importance of 'awareness' for understanding fetal pain". Brain Research. Brain Research Reviews. 49 (3): 455–471. doi:10.1016/j.brainresrev.2005.01.006. PMID 16269314. S2CID 9833426.
- ^ Lagercrantz H, Changeux JP (March 2009). "The emergence of human consciousness: from fetal to neonatal life". Pediatric Research. 65 (3): 255–260. doi:10.1203/PDR.0b013e3181973b0d. PMID 19092726. S2CID 39391626.
[...] the fetus is sedated by the low oxygen tension of the fetal blood and the neurosteroid anesthetics pregnanolone and the sleep-inducing prostaglandin D2 provided by the placenta (36).
- ^ Masiutin M, Yadav M (2023). "Alternative androgen pathways". WikiJournal of Medicine. 10: X. doi:10.15347/WJM/2023.003. S2CID 257943362.
- ^ Reddy DS, Rogawski MA, Rogawski MA, Olsen RW, Delgado-Escueta AV, Reddy DS, Rogawski MA (2012). "Neurosteroids — Endogenous Regulators of Seizure Susceptibility and Role in the Treatment of Epilepsy". Jasper's Basic Mechanisms of the Epilepsies, 4th Edition: 984–1002. doi:10.1093/med/9780199746545.003.0077. ISBN 9780199746545. PMID 22787590.
- ^ Kokate TG, Svensson BE, Rogawski MA (September 1994). "Anticonvulsant activity of neurosteroids: correlation with gamma-aminobutyric acid-evoked chloride current potentiation". The Journal of Pharmacology and Experimental Therapeutics. 270 (3): 1223–1229. PMID 7932175.
- ^ Pinna G, Uzunova V, Matsumoto K, Puia G, Mienville JM, Costa E, Guidotti A (January 2000). "Brain allopregnanolone regulates the potency of the GABA(A) receptor agonist muscimol". Neuropharmacology. 39 (3): 440–448. doi:10.1016/S0028-3908(99)00149-5. PMID 10698010. S2CID 42753647.
- ^ Nik AM, Pressly B, Singh V, Antrobus S, Hulsizer S, Rogawski MA, et al. (July 2017). "Rapid Throughput Analysis of GABAA Receptor Subtype Modulators and Blockers Using DiSBAC1(3) Membrane Potential Red Dye". Molecular Pharmacology. 92 (1): 88–99. doi:10.1124/mol.117.108563. PMC 5452057. PMID 28428226.
- ^ Morris KD, Moorefield CN, Amin J (October 1999). "Differential modulation of the gamma-aminobutyric acid type C receptor by neuroactive steroids". Molecular Pharmacology. 56 (4): 752–759. PMID 10496958.
- ^ Li W, Jin X, Covey DF, Steinbach JH (October 2007). "Neuroactive steroids and human recombinant rho1 GABAC receptors". The Journal of Pharmacology and Experimental Therapeutics. 323 (1): 236–247. doi:10.1124/jpet.107.127365. PMC 3905684. PMID 17636008. S2CID 12294587.
- ^ Bullock AE, Clark AL, Grady SR, Robinson SF, Slobe BS, Marks MJ, Collins AC (June 1997). "Neurosteroids modulate nicotinic receptor function in mouse striatal and thalamic synaptosomes". Journal of Neurochemistry. 68 (6): 2412–2423. doi:10.1046/j.1471-4159.1997.68062412.x. PMID 9166735. S2CID 26195479.
- ^ Wetzel CH, Hermann B, Behl C, Pestel E, Rammes G, Zieglgänsberger W, et al. (September 1998). "Functional antagonism of gonadal steroids at the 5-hydroxytryptamine type 3 receptor". Molecular Endocrinology. 12 (9): 1441–1451. doi:10.1210/mend.12.9.0163. PMID 9731711.
- ^ a b c Mellon SH (October 2007). "Neurosteroid regulation of central nervous system development". Pharmacology & Therapeutics. 116 (1): 107–124. doi:10.1016/j.pharmthera.2007.04.011. PMC 2386997. PMID 17651807.
- ^ Rupprecht R, Reul JM, Trapp T, van Steensel B, Wetzel C, Damm K, et al. (September 1993). "Progesterone receptor-mediated effects of neuroactive steroids". Neuron. 11 (3): 523–530. doi:10.1016/0896-6273(93)90156-l. PMID 8398145. S2CID 11205767.
- ^ Reddy DS, Estes WA (July 2016). "Clinical Potential of Neurosteroids for CNS Disorders". Trends in Pharmacological Sciences. 37 (7): 543–561. doi:10.1016/j.tips.2016.04.003. PMC 5310676. PMID 27156439.
- ^ a b Thomas P, Pang Y (2012). "Membrane progesterone receptors: evidence for neuroprotective, neurosteroid signaling and neuroendocrine functions in neuronal cells". Neuroendocrinology. 96 (2): 162–171. doi:10.1159/000339822. PMC 3489003. PMID 22687885.
- ^ Pang Y, Dong J, Thomas P (January 2013). "Characterization, neurosteroid binding and brain distribution of human membrane progesterone receptors δ and {epsilon} (mPRδ and mPR{epsilon}) and mPRδ involvement in neurosteroid inhibition of apoptosis". Endocrinology. 154 (1): 283–295. doi:10.1210/en.2012-1772. PMC 3529379. PMID 23161870.
- ^ Sleiter N, Pang Y, Park C, Horton TH, Dong J, Thomas P, Levine JE (August 2009). "Progesterone receptor A (PRA) and PRB-independent effects of progesterone on gonadotropin-releasing hormone release". Endocrinology. 150 (8): 3833–3844. doi:10.1210/en.2008-0774. PMC 2717864. PMID 19423765.
- ^ Lamba V, Yasuda K, Lamba JK, Assem M, Davila J, Strom S, Schuetz EG (September 2004). "PXR (NR1I2): splice variants in human tissues, including brain, and identification of neurosteroids and nicotine as PXR activators". Toxicology and Applied Pharmacology. 199 (3): 251–265. Bibcode:2004ToxAP.199..251L. doi:10.1016/j.taap.2003.12.027. PMID 15364541.
- ^ Hu AQ, Wang ZM, Lan DM, Fu YM, Zhu YH, Dong Y, Zheng P (July 2007). "Inhibition of evoked glutamate release by neurosteroid allopregnanolone via inhibition of L-type calcium channels in rat medial prefrontal cortex". Neuropsychopharmacology. 32 (7): 1477–1489. doi:10.1038/sj.npp.1301261. PMID 17151597.
- ^ a b c Earl DE, Tietz EI (April 2011). "Inhibition of recombinant L-type voltage-gated calcium channels by positive allosteric modulators of GABAA receptors". The Journal of Pharmacology and Experimental Therapeutics. 337 (1): 301–311. doi:10.1124/jpet.110.178244. PMC 3063747. PMID 21262851.
- ^ a b Keitel V, Görg B, Bidmon HJ, Zemtsova I, Spomer L, Zilles K, Häussinger D (November 2010). "The bile acid receptor TGR5 (Gpbar-1) acts as a neurosteroid receptor in brain". Glia. 58 (15): 1794–1805. doi:10.1002/glia.21049. PMID 20665558. S2CID 37368754.
- ^ a b Bäckström T, Haage D, Löfgren M, Johansson IM, Strömberg J, Nyberg S, et al. (September 2011). "Paradoxical effects of GABA-A modulators may explain sex steroid induced negative mood symptoms in some persons". Neuroscience. 191: 46–54. doi:10.1016/j.neuroscience.2011.03.061. PMID 21600269. S2CID 38928854.
- ^ a b Andréen L, Nyberg S, Turkmen S, van Wingen G, Fernández G, Bäckström T (September 2009). "Sex steroid induced negative mood may be explained by the paradoxical effect mediated by GABAA modulators". Psychoneuroendocrinology. 34 (8): 1121–1132. doi:10.1016/j.psyneuen.2009.02.003. PMID 19272715. S2CID 22259026.
- ^ a b Bäckström T, Bixo M, Johansson M, Nyberg S, Ossewaarde L, Ragagnin G, et al. (February 2014). "Allopregnanolone and mood disorders". Progress in Neurobiology. 113: 88–94. doi:10.1016/j.pneurobio.2013.07.005. PMID 23978486. S2CID 207407084.
- ^ Andréen L, Sundström-Poromaa I, Bixo M, Nyberg S, Bäckström T (August 2006). "Allopregnanolone concentration and mood--a bimodal association in postmenopausal women treated with oral progesterone". Psychopharmacology. 187 (2): 209–221. doi:10.1007/s00213-006-0417-0. PMID 16724185. S2CID 1933116.
- ^ a b c d e Zorumski CF, Paul SM, Covey DF, Mennerick S (November 2019). "Neurosteroids as novel antidepressants and anxiolytics: GABA-A receptors and beyond". Neurobiology of Stress. 11: 100196. doi:10.1016/j.ynstr.2019.100196. PMC 6804800. PMID 31649968.
- ^ Warner MD, Peabody CA, Whiteford HA, Hollister LE (April 1988). "Alprazolam as an antidepressant". The Journal of Clinical Psychiatry. 49 (4): 148–150. PMID 3281931.
- ^ Srisurapanont M, Boonyanaruthee V (March 1997). "Alprazolam and standard antidepressants in the treatment of depression: a meta-analysis of the antidepressant effect". Journal of the Medical Association of Thailand = Chotmaihet Thangphaet. 80 (3). Centre for Reviews and Dissemination (UK): 183–188. PMID 9175386.
- ^ Agís-Balboa RC, Pinna G, Zhubi A, Maloku E, Veldic M, Costa E, Guidotti A (September 2006). "Characterization of brain neurons that express enzymes mediating neurosteroid biosynthesis". Proceedings of the National Academy of Sciences of the United States of America. 103 (39): 14602–14607. Bibcode:2006PNAS..10314602A. doi:10.1073/pnas.0606544103. PMC 1600006. PMID 16984997.
- ^ "Drug Trials Snapshots: Zulresso". U.S. Food and Drug Administration (FDA). 2 April 2019. Archived from the original on 28 September 2019. Retrieved 24 November 2019.
- ^ "INN Brexanolone". Archived from the original on 4 November 2021. Retrieved 3 September 2019.
- ^ "KEGG DRUG: Brexanolone".
- ^ "Brexanolone - Sage Therapeutics". AdisInsight.
- ^ Morrow AL, Boero G, Porcu P (February 2020). "A Rationale for Allopregnanolone Treatment of Alcohol Use Disorders: Basic and Clinical Studies". Alcoholism: Clinical and Experimental Research. 44 (2): 320–339. doi:10.1111/acer.14253. PMC 7018555. PMID 31782169.
- ^ a b Andréen L, Spigset O, Andersson A, Nyberg S, Bäckström T (June 2006). "Pharmacokinetics of progesterone and its metabolites allopregnanolone and pregnanolone after oral administration of low-dose progesterone". Maturitas. 54 (3): 238–244. doi:10.1016/j.maturitas.2005.11.005. PMID 16406399.
- ^ Devinsky O, Schachter S, Pacia S (1 January 2005). Complementary and Alternative Therapies for Epilepsy. Demos Medical Publishing. pp. 378–. ISBN 978-1-934559-08-6.
- ^ Saudan C, Desmarchelier A, Sottas PE, Mangin P, Saugy M (March 2005). "Urinary marker of oral pregnenolone administration". Steroids. 70 (3): 179–183. doi:10.1016/j.steroids.2004.12.007. PMID 15763596. S2CID 25490229.
- ^ Piper T, Schlug C, Mareck U, Schänzer W (May 2011). "Investigations on changes in ¹³C/¹²C ratios of endogenous urinary steroids after pregnenolone administration". Drug Testing and Analysis. 3 (5): 283–290. doi:10.1002/dta.281. PMID 21538944.
- ^ Sripada RK, Marx CE, King AP, Rampton JC, Ho SS, Liberzon I (June 2013). "Allopregnanolone elevations following pregnenolone administration are associated with enhanced activation of emotion regulation neurocircuits". Biological Psychiatry. 73 (11): 1045–1053. doi:10.1016/j.biopsych.2012.12.008. PMC 3648625. PMID 23348009.
- ^ Ducharme N, Banks WA, Morley JE, Robinson SM, Niehoff ML, Mattern C, Farr SA (September 2010). "Brain distribution and behavioral effects of progesterone and pregnenolone after intranasal or intravenous administration". European Journal of Pharmacology. 641 (2–3): 128–134. doi:10.1016/j.ejphar.2010.05.033. PMC 3008321. PMID 20570588.
- ^ He J, Evans CO, Hoffman SW, Oyesiku NM, Stein DG (October 2004). "Progesterone and allopregnanolone reduce inflammatory cytokines after traumatic brain injury". Experimental Neurology. 189 (2): 404–412. doi:10.1016/j.expneurol.2004.06.008. PMID 15380490. S2CID 10008079.
Further reading
[edit]- Herd MB, Belelli D, Lambert JJ (October 2007). "Neurosteroid modulation of synaptic and extrasynaptic GABA(A) receptors". Pharmacology & Therapeutics. 116 (1): 20–34. arXiv:1607.02870. doi:10.1016/j.pharmthera.2007.03.007. PMID 17531325.
- Zorumski CF, Paul SM, Covey DF, Mennerick S (November 2019). "Neurosteroids as novel antidepressants and anxiolytics: GABA-A receptors and beyond". Neurobiology of Stress. 11: 100196. doi:10.1016/j.ynstr.2019.100196. PMC 6804800. PMID 31649968.
External links
[edit]- Clinical trial number NCT02942004 for "A Study to Evaluate Efficacy and Safety of SAGE-547 in Participants With Severe Postpartum Depression (547-PPD-202B)" at ClinicalTrials.gov
- Clinical trial number NCT02614547 for "A Study to Evaluate SAGE-547 in Patients With Severe Postpartum Depression" at ClinicalTrials.gov
- Clinical trial number NCT02942017 for "A Study to Evaluate Safety and Efficacy of SAGE-547 in Participants With Moderate Postpartum Depression (547-PPD-202C)" at ClinicalTrials.gov
