Hydrogen isocyanide: Difference between revisions
m →Chemistry in the interstellar medium: Task 16: replaced (0×) / removed (1×) deprecated |dead-url= and |deadurl= with |url-status=; |
CodeTalker (talk | contribs) Reverted 2 edits by 100.35.210.130 (talk): Rvv |
||
| (31 intermediate revisions by 18 users not shown) | |||
| Line 2: | Line 2: | ||
| Watchedfields = changed |
| Watchedfields = changed |
||
| verifiedrevid = 443863009 |
| verifiedrevid = 443863009 |
||
| ImageFileL1 = Hydrogen |
| ImageFileL1 = Hydrogen Isocyanide.svg |
||
| ImageNameL1 = Hydrogen cyanide bonding |
| ImageNameL1 = Hydrogen cyanide bonding |
||
| ImageFileR1 = Hydrogen-isocyanide-3D-vdW.png |
| ImageFileR1 = Hydrogen-isocyanide-3D-vdW.png |
||
| Line 13: | Line 13: | ||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} |
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} |
||
| StdInChIKey = QIUBLANJVAOHHY-UHFFFAOYSA-N |
| StdInChIKey = QIUBLANJVAOHHY-UHFFFAOYSA-N |
||
| CASNo_Ref = {{cascite| |
| CASNo_Ref = {{cascite|changed|CAS}} |
||
| CASNo = |
| CASNo = 6914-07-4 |
||
| Beilstein = 2069401 |
|||
| EINECS = |
| EINECS = |
||
| Gmelin = 113 |
|||
| PubChem = 6432654 |
| PubChem = 6432654 |
||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
||
| Line 23: | Line 25: | ||
| SMILES = [C-]#[NH+] |
| SMILES = [C-]#[NH+] |
||
| InChI = 1/CHN/c1-2/h2H |
| InChI = 1/CHN/c1-2/h2H |
||
| RTECS = |
|||
}} |
}} |
||
|Section2={{Chembox Properties |
|Section2={{Chembox Properties |
||
| Line 48: | Line 49: | ||
== Nomenclature == |
== Nomenclature == |
||
Both ''hydrogen isocyanide'' and ''azanylidyniummethanide'' are correct [[IUPAC name]]s for HNC. There is no [[preferred IUPAC name]]. The second one is according to the ''[[substitutive nomenclature]] rules'', derived from the ''[[parent hydride]]'' [[azane]] ( |
Both ''hydrogen isocyanide'' and ''azanylidyniummethanide'' are correct [[IUPAC name]]s for HNC. There is no [[preferred IUPAC name]]. The second one is according to the ''[[substitutive nomenclature]] rules'', derived from the ''[[parent hydride]]'' [[azane]] ({{chem2|NH3}}) and the anion [[methanide]] ({{chem2|CH3−}}).<ref>The suffix ''ylidyne'' refers to the loss of three hydrogen atoms from the nitrogen atom in [[azanium]] ({{chem2|[NH4]+}}) See the [http://www.iupac.org/publications/books/rbook/Red_Book_2005.pdf ''IUPAC Red Book'' 2005] Table III, "Suffixes and endings", p. 257.</ref> |
||
==Molecular properties== |
==Molecular properties== |
||
Hydrogen isocyanide (HNC) is a linear triatomic molecule with C<sub>∞v</sub> [[Molecular symmetry|point group symmetry]]. It is a [[zwitterion]] and an [[isomer]] of [[hydrogen cyanide]] (HCN).<ref>{{Cite journal| |
Hydrogen isocyanide (HNC) is a linear triatomic molecule with C<sub>∞v</sub> [[Molecular symmetry|point group symmetry]]. It is a [[zwitterion]] and an [[isomer]] of [[hydrogen cyanide]] (HCN).<ref>{{Cite journal|last1=Pau|first1=Chin Fong|last2=Hehre|first2=Warren J.|date=1982-02-01|title=Heat of formation of hydrogen isocyanide by ion cyclotron double resonance spectroscopy|journal=The Journal of Physical Chemistry|volume=86|issue=3|pages=321–322|doi=10.1021/j100392a006|issn=0022-3654}}</ref> Both HNC and HCN have large, similar [[Molecular dipole moment|dipole moment]]s, with ''μ''<sub>HNC</sub> = 3.05 [[Debye]] and ''μ''<sub>HCN</sub> = 2.98 Debye respectively.<ref name="Tennekes2006">{{cite journal|author=Tennekes, P. P.|display-authors=etal|year=2006|title=HCN and HNC mapping of the protostellar core Chamaeleon-MMS1|journal=Astronomy and Astrophysics|volume=456|issue=3|pages=1037–1043|arxiv=astro-ph/0606547|bibcode=2006A&A...456.1037T|doi=10.1051/0004-6361:20040294|s2cid=54492819}}</ref> These large dipole moments facilitate the easy observation of these species in the [[interstellar medium]]. |
||
=== HNC−HCN tautomerism === |
=== HNC−HCN tautomerism === |
||
As HNC is higher in energy than HCN by 3920 cm<sup>−1</sup> (46.9 |
As HNC is higher in energy than HCN by 3920 cm<sup>−1</sup> (46.9 kJ/mol), one might assume that the two would have an equilibrium ratio<math display="inline">\left ( \frac{[HNC]}{[HCN]} \right )_{eq}</math> at temperatures below 100 Kelvin of 10<sup>−25</sup>.<ref>{{cite journal|author=Hirota, T.|display-authors=etal|year=1998|title=Abundances of HCN and HNC in Dark Cloud Cores|journal=Astrophysical Journal|volume=503|issue=2|pages=717–728|bibcode=1998ApJ...503..717H|doi=10.1086/306032|doi-access=free}}</ref> However, observations show a very different conclusion; <math display="inline">\left ( \frac{[HNC]}{[HCN]} \right )_{observed}</math> is much higher than 10<sup>−25</sup>, and is in fact on the order of unity in cold environments. This is because of the potential energy path of the tautomerization reaction; there is an activation barrier on the order of roughly 12,000 cm<sup>−1</sup> for the tautomerization to occur, which corresponds to a temperature at which HNC would already have been destroyed by neutral-neutral reactions.<ref name="Bentley1993">{{cite journal|author=Bentley, J. A.|display-authors=etal|year=1993|title=Highly vibrationally excited HCN/HNC: Eigenvalues, wave functions, and stimulated emission pumping spectra|journal=J. Chem. Phys.|volume=98|issue=7|page=5209|bibcode=1993JChPh..98.5207B|doi=10.1063/1.464921|doi-access=free}}</ref> |
||
==Spectral properties== |
==Spectral properties== |
||
In practice, HNC is almost exclusively observed astronomically using the ''J'' = 1→0 transition. This transition occurs at ~90.66 GHz, which is a point of good visibility in the [[Radio window|atmospheric window]], thus making astronomical observations of HNC particularly simple. Many other related species (including HCN) are observed in roughly the same window.<ref name=Schilke1992>{{cite journal|author=Schilke, P.|display-authors=etal|year=1992|title=A study of HCN, HNC and their isotopomers in OMC-1. I. Abundances and chemistry|journal=Astronomy and Astrophysics|volume=256 |
In practice, HNC is almost exclusively observed astronomically using the ''J'' = 1→0 transition. This transition occurs at ~90.66 GHz, which is a point of good visibility in the [[Radio window|atmospheric window]], thus making astronomical observations of HNC particularly simple. Many other related species (including HCN) are observed in roughly the same window.<ref name=Schilke1992>{{cite journal|author=Schilke, P.|display-authors=etal|year=1992|title=A study of HCN, HNC and their isotopomers in OMC-1. I. Abundances and chemistry|journal=Astronomy and Astrophysics|volume=256|pages=595–612|bibcode=1992A&A...256..595S}}</ref><ref name="Pratap1997">{{cite journal|author=Pratap, P.|display-authors=etal|year=1997|title=A Study of the Physics and Chemistry of TMC-1|journal=Astrophysical Journal|volume=486|issue=2|pages=862–885|bibcode=1997ApJ...486..862P|doi=10.1086/304553|pmid=11540493|doi-access=free}}</ref> |
||
==Significance in the interstellar medium== |
==Significance in the interstellar medium== |
||
| Line 67: | Line 68: | ||
Furthermore, HNC (alongside HCN) is a commonly used tracer of dense gas in molecular clouds. Aside from the potential to use HNC to investigate [[gravitational collapse]] as the means of star formation, HNC abundance (relative to the abundance of other nitrogenous molecules) can be used to determine the evolutionary stage of protostellar cores.<ref name="Tennekes2006" /> |
Furthermore, HNC (alongside HCN) is a commonly used tracer of dense gas in molecular clouds. Aside from the potential to use HNC to investigate [[gravitational collapse]] as the means of star formation, HNC abundance (relative to the abundance of other nitrogenous molecules) can be used to determine the evolutionary stage of protostellar cores.<ref name="Tennekes2006" /> |
||
The HCO<sup>+</sup>/HNC line ratio is used to good effect as a measure of density of gas.<ref>{{cite journal|author=Loenen, A. F.|display-authors=etal|year=2007|title=Molecular properties of (U)LIRGs: CO, HCN, HNC and HCO<sup>+</sup>|journal=Proceedings IAU Symposium|volume=242 |
The HCO<sup>+</sup>/HNC line ratio is used to good effect as a measure of density of gas.<ref>{{cite journal|author=Loenen, A. F.|display-authors=etal|year=2007|title=Molecular properties of (U)LIRGs: CO, HCN, HNC and HCO<sup>+</sup>|journal=Proceedings IAU Symposium|volume=242|pages=1–5|doi=10.1017/S1743921307013609|arxiv=0709.3423|bibcode=2007IAUS..242..462L|s2cid=14398456}}</ref> This information provides great insight into the mechanisms of the formation of (Ultra-)Luminous Infrared Galaxies ((U)LIRGs), as it provides data on the nuclear environment, [[star formation]], and even [[black hole]] fueling. Furthermore, the HNC/HCN line ratio is used to distinguish between [[Photodissociation region|photodissociation regions]] and X-ray-dissociation regions on the basis that [HNC]/[HCN] is roughly unity in the former, but greater than unity in the latter. |
||
The study of HNC is |
The study of HNC is relatively straightforward, which is a major motivation for its research. Its J = 1→0 transition occurs in a clear portion of the atmospheric window, and it has numerous isotopomers that are easily studied. Additionally, its large dipole moment makes observations particularly simple. Moreover, HNC is a fundamentally simple molecule in its molecular nature. This makes the study of the reaction pathways that lead to its formation and destruction a good means of obtaining insight to the workings of these reactions in space. Furthermore, the study of the tautomerization of HNC to HCN (and vice versa), which has been studied extensively, has been suggested as a model by which more complicated isomerization reactions can be studied.<ref name="Bentley1993" /><ref>{{cite journal|author=Skurski, P.|display-authors=etal|year=2001|title=''Ab initio'' electronic structure of HCN<sup>−</sup> and HNC<sup>−</sup> dipole-bound anions and a description of electron loss upon tautomerization|journal=J. Chem. Phys.|volume=114|issue=17|page=7446|bibcode=2001JChPh.114.7443S|doi=10.1063/1.1358863}}</ref><ref>{{cite journal|author1=Jakubetz, W.|author2=Lan, B. L.|year=1997|title=A simulation of ultrafast state-selective IR-laser-controlled isomerization of hydrogen cyanide based on global 3D ab initio potential and dipole surfaces|journal=Chem. Phys.|volume=217|issue=2–3|pages=375–388|bibcode=1997CP....217..375J|doi=10.1016/S0301-0104(97)00056-6}}</ref> |
||
==Chemistry in the interstellar medium== |
==Chemistry in the interstellar medium== |
||
HNC is found primarily in dense molecular clouds, though it is ubiquitous in the interstellar medium. Its abundance is closely linked to the abundances of other nitrogen-containing compounds.<ref name="Turner1997">{{cite journal|author=Turner, B. E.|display-authors=etal|year=1997|title=The Physics and Chemistry of Small Translucent Molecular Clouds. VIII. HCN and HNC|journal=Astrophysical Journal|volume=483|issue=1|pages=235–261|bibcode=1997ApJ...483..235T|doi=10.1086/304228}}</ref> HNC is formed primarily through the [[dissociative recombination]] of [[HCNH+|HNCH<sup>+</sup>]] and H<sub>2</sub>NC<sup>+</sup>, and it is destroyed primarily through ion-neutral reactions with {{chem|H|3|+}} and C<sup>+</sup>.<ref name="Hiraoka2006">{{cite journal|author=Hiraoka, K.|display-authors=etal|year=2006|title=How are CH<sub>3</sub>OH, HNC/HCN, and NH<sub>3</sub> Formed in the Interstellar Medium? |
HNC is found primarily in dense molecular clouds, though it is ubiquitous in the interstellar medium. Its abundance is closely linked to the abundances of other nitrogen-containing compounds.<ref name="Turner1997">{{cite journal|author=Turner, B. E.|display-authors=etal|year=1997|title=The Physics and Chemistry of Small Translucent Molecular Clouds. VIII. HCN and HNC|journal=Astrophysical Journal|volume=483|issue=1|pages=235–261|bibcode=1997ApJ...483..235T|doi=10.1086/304228|doi-access=free}}</ref> HNC is formed primarily through the [[dissociative recombination]] of [[HCNH+|HNCH<sup>+</sup>]] and H<sub>2</sub>NC<sup>+</sup>, and it is destroyed primarily through ion-neutral reactions with [[Trihydrogen cation|{{chem|H|3|+}}]] and C<sup>+</sup>.<ref name="Hiraoka2006">{{cite journal|author=Hiraoka, K.|display-authors=etal|year=2006|title=How are CH<sub>3</sub>OH, HNC/HCN, and NH<sub>3</sub> Formed in the Interstellar Medium?|journal=AIP Conf. Proc.|volume=855|pages=86–99|doi=10.1063/1.2359543|bibcode=2006AIPC..855...86H}}</ref><ref>{{cite journal|author=Doty, S. D.|display-authors=etal|year=2004|title=Physical-chemical modeling of the low-mass protostar IRAS 16293-2422|journal=Astronomy and Astrophysics|volume=418|issue=3|pages=1021–1034|arxiv=astro-ph/0402610|bibcode=2004A&A...418.1021D|doi=10.1051/0004-6361:20034476|s2cid=2960790}}</ref> Rate calculations were done at 3.16 × 10<sup>5</sup> years, which is considered early time, and at 20 K, which is a typical temperature for dense molecular clouds.<ref>{{Cite web|url=http://udfa.net/|title=The UMIST Database for Astrochemistry}}</ref><ref>{{cite journal|author=Millar, T. J.|display-authors=etal|year=1997|title=The UMIST database for astrochemistry 1995|journal=Astronomy and Astrophysics Supplement Series|volume=121|pages=139–185|doi=10.1051/aas:1997118|arxiv=1212.6362|bibcode=1997A&AS..121..139M}}</ref> |
||
{| class="wikitable" |
|||
{| border="1" cellpadding="5" cellspacing="0" |
|||
|- |
|||
! colspan="7" style="text-align:center;"| '''Formation Reactions''' |
! colspan="7" style="text-align:center;"| '''Formation Reactions''' |
||
|- |
|- |
||
| Line 89: | Line 89: | ||
| style="text-align:center;"| '''Reactant 1''' || '''Reactant 2''' || '''Product 1''' || '''Product 2''' || '''Rate constant''' || '''Rate/[H<sub>2</sub>]<sup>2</sup>''' || '''Relative Rate''' |
| style="text-align:center;"| '''Reactant 1''' || '''Reactant 2''' || '''Product 1''' || '''Product 2''' || '''Rate constant''' || '''Rate/[H<sub>2</sub>]<sup>2</sup>''' || '''Relative Rate''' |
||
|- |
|- |
||
| {{ |
| {{chem2|H3+}} || HNC || HCNH<sup>+</sup> || H<sub>2</sub> || {{val|8.10e-9}} || {{val|1.26e-24}} || 1.7 |
||
|- |
|- |
||
| C<sup>+</sup> || HNC || C<sub>2</sub>N<sup>+</sup> || H || {{val|3.10e-9}} || {{val|7.48e-25}} || 1.0 |
| C<sup>+</sup> || HNC || C<sub>2</sub>N<sup>+</sup> || H || {{val|3.10e-9}} || {{val|7.48e-25}} || 1.0 |
||
| Line 98: | Line 98: | ||
==Astronomical detections== |
==Astronomical detections== |
||
HCN (not HNC) was first detected in June 1970 by L. E. Snyder and D. Buhl using the 36-foot radio telescope of the National Radio Astronomy Observatory.<ref>{{cite journal|author1=Snyder, L. E.|author2=Buhl, D.|year=1971|title=Observations of Radio Emission from Interstellar Hydrogen Cyanide|journal=Astrophysical Journal|volume=163 |
HCN (not HNC) was first detected in June 1970 by L. E. Snyder and D. Buhl using the 36-foot radio telescope of the National Radio Astronomy Observatory.<ref>{{cite journal|author1=Snyder, L. E.|author2=Buhl, D.|year=1971|title=Observations of Radio Emission from Interstellar Hydrogen Cyanide|journal=Astrophysical Journal|volume=163|pages=L47–L52|bibcode=1971ApJ...163L..47S|doi=10.1086/180664}}</ref> The main molecular isotope, H<sup>12</sup>C<sup>14</sup>N, was observed via its ''J'' = 1→0 transition at 88.6 GHz in six different sources: W3 (OH), Orion A, Sgr A(NH3A), W49, W51, DR 21(OH). A secondary molecular isotope, H<sup>13</sup>C<sup>14</sup>N, was observed via its ''J'' = 1→0 transition at 86.3 GHz in only two of these sources: Orion A and Sgr A(NH3A). HNC was then later detected extragalactically in 1988 using the [[IRAM 30-m]] telescope at the [[Veleta (Sierra Nevada)|Pico de Veleta]] in Spain.<ref>{{cite journal|author=Henkel, C.|display-authors=etal|year=1988|title=Molecules in external galaxies: the detection of CN, C<sub>2</sub>H, and HNC, and the tentative detection of HC<sub>3</sub>N|journal=Astronomy and Astrophysics|volume=201|pages=L23–L26|bibcode=1988A&A...201L..23H}}</ref> It was observed via its ''J'' = 1→0 transition at 90.7 GHz toward IC 342. |
||
A number of detections have been made towards the end of confirming the temperature dependence of the abundance ratio of [HNC]/[HCN]. A strong fit between temperature and the abundance ratio would allow observers to [[Spectroscopy|spectroscopically]] detect the ratio and then extrapolate the temperature of the environment, thus gaining great insight into the environment of the species. The abundance ratio of rare isotopes of HNC and HCN along the OMC-1 varies by more than an order of magnitude in warm regions versus cold regions.<ref>{{cite journal|author=Goldsmith, P. F.|display-authors=etal|year=1986|title=Variations in the HCN/HNC Abundance Ratio in the Orion Molecular Cloud|journal=Astrophysical Journal|volume=310|issue=|pages=383–391|bibcode=1986ApJ...310..383G|doi=10.1086/164692}}</ref> In 1992, the abundances of HNC, HCN, and deuterated analogs along the OMC-1 ridge and core were measured and the temperature dependence of the abundance ratio was confirmed.<ref name=Schilke1992 /> A survey of the W |
A number of detections have been made towards the end of confirming the temperature dependence of the abundance ratio of [HNC]/[HCN]. A strong fit between temperature and the abundance ratio would allow observers to [[Spectroscopy|spectroscopically]] detect the ratio and then extrapolate the temperature of the environment, thus gaining great insight into the environment of the species. The abundance ratio of rare isotopes of HNC and HCN along the OMC-1 varies by more than an order of magnitude in warm regions versus cold regions.<ref>{{cite journal|author=Goldsmith, P. F.|display-authors=etal|year=1986|title=Variations in the HCN/HNC Abundance Ratio in the Orion Molecular Cloud|journal=Astrophysical Journal|volume=310|issue=1|pages=383–391|bibcode=1986ApJ...310..383G|doi=10.1086/164692|pmid=11539669}}</ref> In 1992, the abundances of HNC, HCN, and deuterated analogs along the OMC-1 ridge and core were measured and the temperature dependence of the abundance ratio was confirmed.<ref name=Schilke1992 /> A survey of the W 3 Giant Molecular Cloud in 1997 showed over 24 different molecular isotopes, comprising over 14 distinct chemical species, including HNC, HN<sup>13</sup>C, and H<sup>15</sup>NC. This survey further confirmed the temperature dependence of the abundance ratio, [HNC]/[HCN], this time even confirming the dependence of the isotopomers.<ref>{{cite journal|author1=Helmich, F. P.|author2=van Dishoeck, E. F.|year=1997|title=Physical and chemical variations within the W3 star-forming region|journal=Astronomy and Astrophysics|volume=124|issue=2|pages=205–253|bibcode=1997A&AS..124..205H|doi=10.1051/aas:1997357|doi-access=free|hdl=1887/2219|hdl-access=free}}</ref> |
||
These are not the only detections of importance of HNC in the interstellar medium. In 1997, HNC was observed along the TMC-1 ridge and its abundance relative to HCO<sup>+</sup> was found to be constant along the ridge—this led credence to the reaction pathway that posits that HNC is derived initially from HCO<sup>+</sup>.<ref name="Pratap1997" /> One significant astronomical detection that demonstrated the practical use of observing HNC occurred in 2006, when abundances of various nitrogenous compounds (including HN<sup>13</sup>C and H<sup>15</sup>NC) were used to determine the stage of evolution of the protostellar core Cha-MMS1 based on the relative magnitudes of the abundances.<ref name="Tennekes2006" /> |
These are not the only detections of importance of HNC in the interstellar medium. In 1997, HNC was observed along the TMC-1 ridge and its abundance relative to HCO<sup>+</sup> was found to be constant along the ridge—this led credence to the reaction pathway that posits that HNC is derived initially from HCO<sup>+</sup>.<ref name="Pratap1997" /> One significant astronomical detection that demonstrated the practical use of observing HNC occurred in 2006, when abundances of various nitrogenous compounds (including HN<sup>13</sup>C and H<sup>15</sup>NC) were used to determine the stage of evolution of the protostellar core Cha-MMS1 based on the relative magnitudes of the abundances.<ref name="Tennekes2006" /> |
||
On 11 August 2014, astronomers released studies, using the [[Atacama Large Millimeter Array|Atacama Large Millimeter/Submillimeter Array (ALMA)]] for the first time, that detailed the distribution of [[Hydrogen cyanide|HCN]], HNC, [[Formaldehyde|H<sub>2</sub>CO]], and [[dust]] inside the [[Coma (cometary)|comae]] of [[comet]]s [[C/2012 F6 (Lemmon)]] and [[Comet ISON|C/2012 S1 (ISON)]].<ref name="NASA-20140811">{{cite web | |
On 11 August 2014, astronomers released studies, using the [[Atacama Large Millimeter Array|Atacama Large Millimeter/Submillimeter Array (ALMA)]] for the first time, that detailed the distribution of [[Hydrogen cyanide|HCN]], HNC, [[Formaldehyde|H<sub>2</sub>CO]], and [[dust]] inside the [[Coma (cometary)|comae]] of [[comet]]s [[C/2012 F6 (Lemmon)]] and [[Comet ISON|C/2012 S1 (ISON)]].<ref name="NASA-20140811">{{cite web |last1=Zubritsky |first1=Elizabeth |last2=Neal-Jones |first2=Nancy |title=RELEASE 14-038 - NASA's 3-D Study of Comets Reveals Chemical Factory at Work |url=http://www.nasa.gov/press/2014/august/goddard/nasa-s-3-d-study-of-comets-reveals-chemical-factory-at-work |date=11 August 2014 |work=[[NASA]] |access-date=12 August 2014 }}</ref><ref name="AJL-20140811">{{cite journal |author=Cordiner, M.A.|title=Mapping the Release of Volatiles in the Inner Comae of Comets C/2012 F6 (Lemmon) and C/2012 S1 (ISON) Using the Atacama Large Millimeter/Submillimeter Array |date=11 August 2014 |journal=[[The Astrophysical Journal]] |volume=792 |pages=L2 |number=1 |doi=10.1088/2041-8205/792/1/L2 |display-authors=etal|arxiv=1408.2458|bibcode=2014ApJ...792L...2C|s2cid=26277035 }}</ref> |
||
== See also == |
== See also == |
||
| Line 120: | Line 120: | ||
{{DEFAULTSORT:Hydrogen Isocyanide}} |
{{DEFAULTSORT:Hydrogen Isocyanide}} |
||
[[Category:Hydrogen compounds]] |
[[Category:Hydrogen compounds]] |
||
[[Category:Triatomic molecules]] |
|||
[[Category:Isocyanides]] |
[[Category:Isocyanides]] |
||
[[Category:Zwitterions]] |
[[Category:Zwitterions]] |
||
Latest revision as of 22:05, 5 December 2024
| |||
| Names | |||
|---|---|---|---|
| IUPAC names
hydrogen isocyanide
azanylidyniummethanide | |||
| Other names
isohydrocyanic acid
hydroisocyanic acid isoprussic acid | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 2069401 | |||
| ChEBI | |||
| ChemSpider | |||
| 113 | |||
PubChem CID
|
|||
| |||
| |||
| Properties | |||
| HNC | |||
| Molar mass | 27.03 g/mol | ||
| Conjugate acid | Hydrocyanonium | ||
| Conjugate base | Cyanide | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Hydrogen isocyanide is a chemical with the molecular formula HNC. It is a minor tautomer of hydrogen cyanide (HCN). Its importance in the field of astrochemistry is linked to its ubiquity in the interstellar medium.
Nomenclature
[edit]Both hydrogen isocyanide and azanylidyniummethanide are correct IUPAC names for HNC. There is no preferred IUPAC name. The second one is according to the substitutive nomenclature rules, derived from the parent hydride azane (NH3) and the anion methanide (CH−3).[1]
Molecular properties
[edit]Hydrogen isocyanide (HNC) is a linear triatomic molecule with C∞v point group symmetry. It is a zwitterion and an isomer of hydrogen cyanide (HCN).[2] Both HNC and HCN have large, similar dipole moments, with μHNC = 3.05 Debye and μHCN = 2.98 Debye respectively.[3] These large dipole moments facilitate the easy observation of these species in the interstellar medium.
HNC−HCN tautomerism
[edit]As HNC is higher in energy than HCN by 3920 cm−1 (46.9 kJ/mol), one might assume that the two would have an equilibrium ratio at temperatures below 100 Kelvin of 10−25.[4] However, observations show a very different conclusion; is much higher than 10−25, and is in fact on the order of unity in cold environments. This is because of the potential energy path of the tautomerization reaction; there is an activation barrier on the order of roughly 12,000 cm−1 for the tautomerization to occur, which corresponds to a temperature at which HNC would already have been destroyed by neutral-neutral reactions.[5]
Spectral properties
[edit]In practice, HNC is almost exclusively observed astronomically using the J = 1→0 transition. This transition occurs at ~90.66 GHz, which is a point of good visibility in the atmospheric window, thus making astronomical observations of HNC particularly simple. Many other related species (including HCN) are observed in roughly the same window.[6][7]
Significance in the interstellar medium
[edit]HNC is intricately linked to the formation and destruction of numerous other molecules of importance in the interstellar medium—aside from the obvious partners HCN, protonated hydrogen cyanide (HCNH+), and cyanide (CN), HNC is linked to the abundances of many other compounds, either directly or through a few degrees of separation. As such, an understanding of the chemistry of HNC leads to an understanding of countless other species—HNC is an integral piece in the complex puzzle representing interstellar chemistry.
Furthermore, HNC (alongside HCN) is a commonly used tracer of dense gas in molecular clouds. Aside from the potential to use HNC to investigate gravitational collapse as the means of star formation, HNC abundance (relative to the abundance of other nitrogenous molecules) can be used to determine the evolutionary stage of protostellar cores.[3]
The HCO+/HNC line ratio is used to good effect as a measure of density of gas.[8] This information provides great insight into the mechanisms of the formation of (Ultra-)Luminous Infrared Galaxies ((U)LIRGs), as it provides data on the nuclear environment, star formation, and even black hole fueling. Furthermore, the HNC/HCN line ratio is used to distinguish between photodissociation regions and X-ray-dissociation regions on the basis that [HNC]/[HCN] is roughly unity in the former, but greater than unity in the latter.
The study of HNC is relatively straightforward, which is a major motivation for its research. Its J = 1→0 transition occurs in a clear portion of the atmospheric window, and it has numerous isotopomers that are easily studied. Additionally, its large dipole moment makes observations particularly simple. Moreover, HNC is a fundamentally simple molecule in its molecular nature. This makes the study of the reaction pathways that lead to its formation and destruction a good means of obtaining insight to the workings of these reactions in space. Furthermore, the study of the tautomerization of HNC to HCN (and vice versa), which has been studied extensively, has been suggested as a model by which more complicated isomerization reactions can be studied.[5][9][10]
Chemistry in the interstellar medium
[edit]HNC is found primarily in dense molecular clouds, though it is ubiquitous in the interstellar medium. Its abundance is closely linked to the abundances of other nitrogen-containing compounds.[11] HNC is formed primarily through the dissociative recombination of HNCH+ and H2NC+, and it is destroyed primarily through ion-neutral reactions with H+
3 and C+.[12][13] Rate calculations were done at 3.16 × 105 years, which is considered early time, and at 20 K, which is a typical temperature for dense molecular clouds.[14][15]
| Formation Reactions | ||||||
|---|---|---|---|---|---|---|
| Reactant 1 | Reactant 2 | Product 1 | Product 2 | Rate constant | Rate/[H2]2 | Relative Rate |
| HCNH+ | e− | HNC | H | 9.50×10−8 | 4.76×10−25 | 3.4 |
| H2NC+ | e− | HNC | H | 1.80×10−7 | 1.39×10−25 | 1.0 |
| Destruction Reactions | ||||||
| Reactant 1 | Reactant 2 | Product 1 | Product 2 | Rate constant | Rate/[H2]2 | Relative Rate |
| H+3 | HNC | HCNH+ | H2 | 8.10×10−9 | 1.26×10−24 | 1.7 |
| C+ | HNC | C2N+ | H | 3.10×10−9 | 7.48×10−25 | 1.0 |
These four reactions are merely the four most dominant, and thus the most significant in the formation of the HNC abundances in dense molecular clouds; there are dozens more reactions for the formation and destruction of HNC. Though these reactions primarily lead to various protonated species, HNC is linked closely to the abundances of many other nitrogen containing molecules, for example, NH3 and CN.[11] The abundance HNC is also inexorably linked to the abundance of HCN, and the two tend to exist in a specific ratio based on the environment.[12] This is because the reactions that form HNC can often also form HCN, and vice versa, depending on the conditions in which the reaction occurs, and also that there exist isomerization reactions for the two species.
Astronomical detections
[edit]HCN (not HNC) was first detected in June 1970 by L. E. Snyder and D. Buhl using the 36-foot radio telescope of the National Radio Astronomy Observatory.[16] The main molecular isotope, H12C14N, was observed via its J = 1→0 transition at 88.6 GHz in six different sources: W3 (OH), Orion A, Sgr A(NH3A), W49, W51, DR 21(OH). A secondary molecular isotope, H13C14N, was observed via its J = 1→0 transition at 86.3 GHz in only two of these sources: Orion A and Sgr A(NH3A). HNC was then later detected extragalactically in 1988 using the IRAM 30-m telescope at the Pico de Veleta in Spain.[17] It was observed via its J = 1→0 transition at 90.7 GHz toward IC 342.
A number of detections have been made towards the end of confirming the temperature dependence of the abundance ratio of [HNC]/[HCN]. A strong fit between temperature and the abundance ratio would allow observers to spectroscopically detect the ratio and then extrapolate the temperature of the environment, thus gaining great insight into the environment of the species. The abundance ratio of rare isotopes of HNC and HCN along the OMC-1 varies by more than an order of magnitude in warm regions versus cold regions.[18] In 1992, the abundances of HNC, HCN, and deuterated analogs along the OMC-1 ridge and core were measured and the temperature dependence of the abundance ratio was confirmed.[6] A survey of the W 3 Giant Molecular Cloud in 1997 showed over 24 different molecular isotopes, comprising over 14 distinct chemical species, including HNC, HN13C, and H15NC. This survey further confirmed the temperature dependence of the abundance ratio, [HNC]/[HCN], this time even confirming the dependence of the isotopomers.[19]
These are not the only detections of importance of HNC in the interstellar medium. In 1997, HNC was observed along the TMC-1 ridge and its abundance relative to HCO+ was found to be constant along the ridge—this led credence to the reaction pathway that posits that HNC is derived initially from HCO+.[7] One significant astronomical detection that demonstrated the practical use of observing HNC occurred in 2006, when abundances of various nitrogenous compounds (including HN13C and H15NC) were used to determine the stage of evolution of the protostellar core Cha-MMS1 based on the relative magnitudes of the abundances.[3]
On 11 August 2014, astronomers released studies, using the Atacama Large Millimeter/Submillimeter Array (ALMA) for the first time, that detailed the distribution of HCN, HNC, H2CO, and dust inside the comae of comets C/2012 F6 (Lemmon) and C/2012 S1 (ISON).[20][21]
See also
[edit]External links
[edit]References
[edit]- ^ The suffix ylidyne refers to the loss of three hydrogen atoms from the nitrogen atom in azanium ([NH4]+) See the IUPAC Red Book 2005 Table III, "Suffixes and endings", p. 257.
- ^ Pau, Chin Fong; Hehre, Warren J. (1982-02-01). "Heat of formation of hydrogen isocyanide by ion cyclotron double resonance spectroscopy". The Journal of Physical Chemistry. 86 (3): 321–322. doi:10.1021/j100392a006. ISSN 0022-3654.
- ^ a b c Tennekes, P. P.; et al. (2006). "HCN and HNC mapping of the protostellar core Chamaeleon-MMS1". Astronomy and Astrophysics. 456 (3): 1037–1043. arXiv:astro-ph/0606547. Bibcode:2006A&A...456.1037T. doi:10.1051/0004-6361:20040294. S2CID 54492819.
- ^ Hirota, T.; et al. (1998). "Abundances of HCN and HNC in Dark Cloud Cores". Astrophysical Journal. 503 (2): 717–728. Bibcode:1998ApJ...503..717H. doi:10.1086/306032.
- ^ a b Bentley, J. A.; et al. (1993). "Highly vibrationally excited HCN/HNC: Eigenvalues, wave functions, and stimulated emission pumping spectra". J. Chem. Phys. 98 (7): 5209. Bibcode:1993JChPh..98.5207B. doi:10.1063/1.464921.
- ^ a b Schilke, P.; et al. (1992). "A study of HCN, HNC and their isotopomers in OMC-1. I. Abundances and chemistry". Astronomy and Astrophysics. 256: 595–612. Bibcode:1992A&A...256..595S.
- ^ a b Pratap, P.; et al. (1997). "A Study of the Physics and Chemistry of TMC-1". Astrophysical Journal. 486 (2): 862–885. Bibcode:1997ApJ...486..862P. doi:10.1086/304553. PMID 11540493.
- ^ Loenen, A. F.; et al. (2007). "Molecular properties of (U)LIRGs: CO, HCN, HNC and HCO+". Proceedings IAU Symposium. 242: 1–5. arXiv:0709.3423. Bibcode:2007IAUS..242..462L. doi:10.1017/S1743921307013609. S2CID 14398456.
- ^ Skurski, P.; et al. (2001). "Ab initio electronic structure of HCN− and HNC− dipole-bound anions and a description of electron loss upon tautomerization". J. Chem. Phys. 114 (17): 7446. Bibcode:2001JChPh.114.7443S. doi:10.1063/1.1358863.
- ^ Jakubetz, W.; Lan, B. L. (1997). "A simulation of ultrafast state-selective IR-laser-controlled isomerization of hydrogen cyanide based on global 3D ab initio potential and dipole surfaces". Chem. Phys. 217 (2–3): 375–388. Bibcode:1997CP....217..375J. doi:10.1016/S0301-0104(97)00056-6.
- ^ a b Turner, B. E.; et al. (1997). "The Physics and Chemistry of Small Translucent Molecular Clouds. VIII. HCN and HNC". Astrophysical Journal. 483 (1): 235–261. Bibcode:1997ApJ...483..235T. doi:10.1086/304228.
- ^ a b Hiraoka, K.; et al. (2006). "How are CH3OH, HNC/HCN, and NH3 Formed in the Interstellar Medium?". AIP Conf. Proc. 855: 86–99. Bibcode:2006AIPC..855...86H. doi:10.1063/1.2359543.
- ^ Doty, S. D.; et al. (2004). "Physical-chemical modeling of the low-mass protostar IRAS 16293-2422". Astronomy and Astrophysics. 418 (3): 1021–1034. arXiv:astro-ph/0402610. Bibcode:2004A&A...418.1021D. doi:10.1051/0004-6361:20034476. S2CID 2960790.
- ^ "The UMIST Database for Astrochemistry".
- ^ Millar, T. J.; et al. (1997). "The UMIST database for astrochemistry 1995". Astronomy and Astrophysics Supplement Series. 121: 139–185. arXiv:1212.6362. Bibcode:1997A&AS..121..139M. doi:10.1051/aas:1997118.
- ^ Snyder, L. E.; Buhl, D. (1971). "Observations of Radio Emission from Interstellar Hydrogen Cyanide". Astrophysical Journal. 163: L47–L52. Bibcode:1971ApJ...163L..47S. doi:10.1086/180664.
- ^ Henkel, C.; et al. (1988). "Molecules in external galaxies: the detection of CN, C2H, and HNC, and the tentative detection of HC3N". Astronomy and Astrophysics. 201: L23–L26. Bibcode:1988A&A...201L..23H.
- ^ Goldsmith, P. F.; et al. (1986). "Variations in the HCN/HNC Abundance Ratio in the Orion Molecular Cloud". Astrophysical Journal. 310 (1): 383–391. Bibcode:1986ApJ...310..383G. doi:10.1086/164692. PMID 11539669.
- ^ Helmich, F. P.; van Dishoeck, E. F. (1997). "Physical and chemical variations within the W3 star-forming region". Astronomy and Astrophysics. 124 (2): 205–253. Bibcode:1997A&AS..124..205H. doi:10.1051/aas:1997357. hdl:1887/2219.
- ^ Zubritsky, Elizabeth; Neal-Jones, Nancy (11 August 2014). "RELEASE 14-038 - NASA's 3-D Study of Comets Reveals Chemical Factory at Work". NASA. Retrieved 12 August 2014.
- ^ Cordiner, M.A.; et al. (11 August 2014). "Mapping the Release of Volatiles in the Inner Comae of Comets C/2012 F6 (Lemmon) and C/2012 S1 (ISON) Using the Atacama Large Millimeter/Submillimeter Array". The Astrophysical Journal. 792 (1): L2. arXiv:1408.2458. Bibcode:2014ApJ...792L...2C. doi:10.1088/2041-8205/792/1/L2. S2CID 26277035.

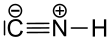

![{\textstyle \left({\frac {[HNC]}{[HCN]}}\right)_{eq}}](https://wikimedia.org/enwiki/api/rest_v1/media/math/render/svg/9946fd6d39f47bca239a36b586eb8713fca9ee74)
![{\textstyle \left({\frac {[HNC]}{[HCN]}}\right)_{observed}}](https://wikimedia.org/enwiki/api/rest_v1/media/math/render/svg/9de0d6165221ef7a08b2139f903f72341f87a06f)
