Amino acid: Difference between revisions
Undid revision 925078937 by 192.158.3.253 (talk) |
some dark mode improvements |
||
| (631 intermediate revisions by more than 100 users not shown) | |||
| Line 1: | Line 1: | ||
{{short description|Organic compounds containing amine and carboxylic groups}} |
|||
{{About|the class of chemicals|the structures and properties of the standard proteinogenic amino acids|Proteinogenic amino acid}} |
{{About|the class of chemicals|the structures and properties of the standard proteinogenic amino acids|Proteinogenic amino acid}} |
||
{{Pp-move |
{{Pp-move}} |
||
{{Use dmy dates|date=October 2020}} |
|||
{{short description|Organic compounds containing amine and carboxylic groups}} |
|||
[[File:L-amino acid structure.svg|class=skin-invert-image|thumb|upright=1.15|Structure of a typical <small>L</small>-alpha-amino acid in the "neutral" form]] |
|||
{{Use dmy dates|date=August 2017}} |
|||
{{Good article}} |
|||
[[Image:AminoAcidball.svg|thumbnail|300px|The structure of an alpha amino acid in its un-ionized form]] |
|||
'''Amino acids''' are [[organic compound]]s that contain both [[amino]] and [[carboxylic acid]] [[functional group]]s.<ref>{{Lehninger4th | name-list-style = vanc }}</ref> Although over 500 amino acids exist in nature, by far the most important are the [[Proteinogenic amino acid|22 α-amino acids]] incorporated into [[protein]]s.<ref>{{cite journal | title = Norine: update of the nonribosomal peptide resource | first1 =Areski | last1 = Flissi | first2 = Emma | last2= Ricart | first3 = Clémentine | last3= Campart | first4 = Mickael | last4= Chevalier | first5 = Yoann | last5= Dufresne | first6 = Juraj | last6= Michalik | first7 = Philippe | last7= Jacques | first8 = Christophe | last8= Flahaut | first9 = Frédérique | last9= Lisacek | first10 = Valérie | last10= Leclère | first11 = Maude | last11= Pupin | journal = Nucleic Acids Research | volume = 48 | issue = D1 | year = 2020 | pages = D465–D469 | doi = 10.1093/nar/gkz1000 | pmid =31691799 | pmc = 7145658 }}</ref><!--<ref>{{cite journal |title = New Naturally Occurring Amino Acids |vauthors = Wagner I, Musso H|doi = 10.1002/anie.198308161 |journal = [[Angewandte Chemie International Edition in English]] |volume = 22 |issue = 11 |pages = 816–828 |date = November 1983}}{{Closed access}}</ref>--> Only these 22 appear in the [[genetic code]] of life.<ref>{{Cite web | year=2009 | editor=Richard Cammack | title=Newsletter 2009 | url=http://www.chem.qmul.ac.uk/iubmb/newsletter/2009.html#item35 | url-status=dead | archive-url=https://web.archive.org/web/20170912194130/http://www.chem.qmul.ac.uk/iubmb/newsletter/2009.html#item35 | archive-date=2017-09-12 | access-date=2012-04-16 | publisher=Biochemical Nomenclature Committee of IUPAC and NC-IUBMB | at=Pyrrolysine}}</ref><ref name="pmid20847933">{{Cite journal | last1=Rother | first1=Michael | last2=Krzycki | first2=Joseph A. | date=2010-01-01 | title=Selenocysteine, Pyrrolysine, and the Unique Energy Metabolism of Methanogenic Archaea | journal=Archaea |volume=2010 | pages=1–14 | doi=10.1155/2010/453642 |issn=1472-3646 |pmc=2933860 |pmid=20847933 |doi-access=free}}</ref> |
|||
'''Amino acids''' are [[organic compound]]s that contain [[amine]] (-NH<sub>2</sub>) and [[Carboxylic acid|carboxyl]] (-COOH) [[functional group]]s, along with a [[Substituent|side chain]] (R group) specific to each amino acid.<ref>{{Lehninger4th}}</ref><ref>{{Cite encyclopedia|title = amino acid|date = 2015|encyclopedia = Cambridge Dictionaries Online|publisher = Cambridge University Press|url = https://dictionary.cambridge.org/us/dictionary/british/amino-acid|accessdate = 3 July 2015}}</ref> The key [[Chemical element|elements]] of an amino acid are [[carbon]] (C), [[hydrogen]] (H), [[oxygen]] (O), and [[nitrogen]] (N), although other elements are found in the side chains of certain amino acids. About 500 naturally occurring amino acids are known (though only 20 appear in the [[genetic code]]) and can be classified in many ways.<ref>{{cite journal |title = New Naturally Occurring Amino Acids|vauthors = Wagner I, Musso H|doi = 10.1002/anie.198308161|journal = [[Angewandte Chemie International Edition in English]]|volume = 22|issue = 11|pages = 816–28|date = November 1983}}{{Closed access}}</ref> They can be classified according to the core structural functional groups' locations as [[Alpha and beta carbon|alpha- <span style="white-space: nowrap">(α-)</span>, beta- <span style="white-space: nowrap">(β-)</span>, gamma- <span style="white-space: nowrap">(γ-)</span> or delta- <span style="white-space: nowrap">(δ-)</span>]] amino acids; other categories relate to [[Chemical polarity|polarity]], [[pH]] level, and side chain group type ([[aliphatic]], [[Open-chain compound|acyclic]], [[aromatic]], containing [[Hydroxy group|hydroxyl]] or [[sulfur]], etc.). In the form of [[protein]]s, amino acid [[Residue (chemistry)|residues]] form the second-largest component ([[water]] is the largest) of human [[muscle]]s and other [[tissue (biology)|tissues]].<ref>{{cite book|title = Human nutrition in the developing world|last = Latham|first = Michael C. | name-list-format = vanc |publisher = Food and Agriculture Organization of the United Nations|year = 1997|isbn = |location = Rome|pages = |chapter = Chapter 8. Body composition, the functions of food, metabolism and energy|chapter-url = http://www.fao.org/docrep/W0073E/w0073e04.htm#P1625_217364|series = Food and Nutrition Series – No. 29}}</ref> Beyond their role as residues in proteins, amino acids participate in a number of processes such as [[neurotransmitter]] transport and [[biosynthesis]]. |
|||
Amino acids can be classified according to the locations of the core structural functional groups ([[Alpha and beta carbon|alpha- <span style="white-space: nowrap">(α-)</span>, beta- <span style="white-space: nowrap">(β-)</span>, gamma- <span style="white-space: nowrap">(γ-)</span>]] amino acids, etc.); other categories relate to [[Chemical polarity|polarity]], [[ionization]], and side-chain group type ([[aliphatic]], [[Open-chain compound|acyclic]], [[aromatic]], [[Chemical polarity|polar]], etc.). In the form of proteins, amino-acid ''[[Residue (chemistry)#Biochemistry|residues]]'' form the second-largest component ([[water]] being the largest) of human [[muscle]]s and other [[tissue (biology)|tissues]].<ref>{{cite book |title = Human nutrition in the developing world |last = Latham |first = Michael C. |name-list-style = vanc |publisher = Food and Agriculture Organization of the United Nations |year = 1997 |location = Rome |chapter = Chapter 8. Body composition, the functions of food, metabolism and energy |chapter-url = http://www.fao.org/docrep/W0073E/w0073e04.htm#P1625_217364 |series = Food and Nutrition Series – No. 29|access-date = 9 September 2012|archive-date = 8 October 2012|archive-url = https://web.archive.org/web/20121008212843/http://www.fao.org/docrep/W0073e/w0073e04.htm#P1625_217364 |url-status = dead}}</ref> Beyond their role as residues in proteins, amino acids participate in a number of processes such as [[neurotransmitter]] transport and [[biosynthesis]]. It is thought that they played a key role in [[abiogenesis|enabling life on Earth and its emergence]].<ref> |
|||
In [[biochemistry]], amino acids having both the amine and the carboxylic acid groups attached to the [[alpha-carbon|first (alpha-) carbon]] atom have particular importance. They are known as '''2-, alpha-,''' or '''α-amino acids''' (generic [[Chemical formula|formula]] H<sub>2</sub>NCHRCOOH in most cases,<ref>[[Proline]] is an exception to this general formula. It lacks the NH<sub>2</sub> group because of the [[cyclization]] of the side chain and is known as an [[imino acid]]; it falls under the category of special structured amino acids.</ref> where R is an [[organic chemistry|organic]] [[substituent]] known as a "[[Substituent|side chain]]");<ref>{{Cite web|title = an introduction to amino acids|url = http://www.chemguide.co.uk/organicprops/aminoacids/background.html|website = chemguide|accessdate = 4 July 2015|date = August 2007|last = Clark|first = Jim}}</ref> often the term "amino acid" is used to refer specifically to these. They include the 22 [[Proteinogenic amino acid|proteinogenic]] ("protein-building") amino acids,<ref>{{cite encyclopedia|year = 2008|title = Amino acids|encyclopedia = Peptides from A to Z: A Concise Encyclopedia|url = https://books.google.com/books?id=doe9NwgJTAsC&pg=PA20|publisher = Wiley-VCH|location = Germany|isbn = 9783527621170|via = Google Books|page = 20|last = Jakubke|first = Hans-Dieter|last2 = Sewald|first2 = Norbert | name-list-format = vanc }}</ref><ref>{{cite book |editor1-first = Loredano|editor1-last = Pollegioni|editor2-first = Stefano|editor2-last = Servi | name-list-format = vanc | title = Unnatural Amino Acids: Methods and Protocols|volume = 794|year = 2012|publisher = Humana Press|isbn = 978-1-61779-331-8|page = v|oclc = 756512314|series = Methods in Molecular Biology – Volume 794|doi = 10.1007/978-1-61779-331-8}}</ref><ref>{{cite journal | vauthors = Hertweck C | title = Biosynthesis and Charging of Pyrrolysine, the 22nd Genetically Encoded Amino Acid| journal = [[Angewandte Chemie International Edition]] | volume = 50 | issue = 41 | pages = 9540–1 | date = October 2011 | pmid = 21796749 | doi = 10.1002/anie.201103769 }}{{Closed access}} |
|||
{{cite book |
|||
</ref> which combine into [[peptide]] chains ("polypeptides") to form the building-blocks of a vast array of [[protein]]s.<ref name="NIGMS">{{cite web|url = http://publications.nigms.nih.gov/structlife/chapter1.html|title = Chapter 1: Proteins are the Body's Worker Molecules|publisher = National Institute of General Medical Sciences|accessdate = 20 May 2008|date = 27 October 2011|website = The Structures of Life}}</ref> These are all <small>L</small>-[[stereoisomerism|stereoisomers]] ("[[Chirality (chemistry)|left-handed]]" [[isomer]]s), although a few <small>D</small>-amino acids ("right-handed") occur in [[bacterial envelope]]s, as a [[Neuromodulation|neuromodulator]] (<small>D</small>-[[serine]]), and in some [[antibiotic]]s.<ref>{{cite book | title = Biochemical Pathways: An Atlas of Biochemistry and Molecular Biology | publisher = Wiley-Blackwell | year = 2012 | isbn = 978-0-470-14684-2 | location = Oxford | editor-last = Michal | editor-first = Gerhard | editor-last2 = Schomburg | editor-first2 = Dietmar | name-list-format = vanc | page = 5 | edition = 2nd }}</ref> |
|||
|last1 = Luisi |
|||
|first1 = Pier Luigi |
|||
|author-link1 = Pier Luigi Luisi |
|||
|date = 13 July 2006 |
|||
|title = The Emergence of Life: From Chemical Origins to Synthetic Biology |
|||
|url = https://books.google.com/books?id=1Oxfq5VTcDkC |
|||
|publisher = Cambridge University Press |
|||
|page = 13 |
|||
|isbn = 9781139455640 |
|||
|access-date = 5 August 2024 |
|||
|quote = Of course if on Earth there had only been diketopiperazines and not amino acids; or if sugars did not have the size they have; or if lipids were three times shorter, then we would not have life. |
|||
}} |
|||
</ref> |
|||
Amino acids are formally named by the [[International Union of Pure and Applied Chemistry|IUPAC]]-[[International Union of Biochemistry and Molecular Biology|IUBMB]] [[Joint Commission]] on Biochemical Nomenclature in terms of the fictitious "neutral" structure shown in the illustration. For example, the systematic name of alanine is 2-aminopropanoic acid, based on the formula {{chem2|CH3\sCH(NH2)\sCOOH}}. The Commission justified this approach as follows:<ref name = iupaciub>{{cite web | url = http://www.chem.qmul.ac.uk/iupac/AminoAcid/AA1n2.html | title = Nomenclature and Symbolism for Amino Acids and Peptides | publisher = IUPAC-IUB Joint Commission on Biochemical Nomenclature | year = 1983 | access-date = 17 November 2008 | archive-url = https://web.archive.org/web/20081009023202/http://www.chem.qmul.ac.uk/iupac/AminoAcid/AA1n2.html | archive-date = 9 October 2008 | url-status=dead}}</ref> |
|||
Twenty of the proteinogenic amino acids are encoded directly by triplet [[codon]]s in the [[genetic code]] and are known as "standard" amino acids. The other two ("non-standard" or "non-canonical") are [[selenocysteine]] (present in many [[prokaryote]]s as well as most [[eukaryote]]s, but not coded directly by [[DNA]]), and [[pyrrolysine]] (found only in some [[archea|archaea]] and one [[bacterium]]). Pyrrolysine and selenocysteine are encoded via variant codons; for example, selenocysteine is encoded by [[stop codon]] and [[SECIS element]].<ref name="books.google.com">[https://books.google.com/books?id=BDn-AI_YBlMC&pg=PA1&lpg=PA1 Modeling Electrostatic Contributions to Protein Folding and Binding] – Tjong, p.1 footnote</ref><ref name="VoJw6fIISSkC p.299">[https://books.google.com/books?id=VoJw6fIISSkC&pg=PA299&lpg=PA299 Frontiers in Drug Design and Discovery] ed. Atta-Ur-Rahman & others, p.299</ref><ref name="url_The_Genetic_Codes_NCBI">{{cite web | url = https://www.ncbi.nlm.nih.gov/Taxonomy/Utils/wprintgc.cgi?mode=c | title = The Genetic Codes |vauthors=Elzanowski A, Ostell J | date = 7 April 2008 | website = | publisher = National Center for Biotechnology Information (NCBI) | pages = | quote = | accessdate = 10 March 2010 }}</ref> [[N-Formylmethionine|''N''-formylmethionine]] (which is often the initial amino acid of proteins in bacteria, [[Mitochondrion|mitochondria]], and [[chloroplast]]s) is generally considered as a form of [[methionine]] rather than as a separate proteinogenic amino acid. Codon–[[transfer RNA|tRNA]] combinations not found in nature can also be used to [[Expanded genetic code|"expand" the genetic code]] and form novel proteins known as [[alloprotein]]s incorporating [[non-proteinogenic amino acid]]s.<ref name="pmid16260173">{{cite journal | vauthors = Xie J, Schultz PG | title = Adding amino acids to the genetic repertoire | journal = Current Opinion in Chemical Biology | volume = 9 | issue = 6 | pages = 548–54 | date = December 2005 | pmid = 16260173 | doi = 10.1016/j.cbpa.2005.10.011 }}</ref><ref name="pmid19318213">{{cite journal | vauthors = Wang Q, Parrish AR, Wang L | title = Expanding the genetic code for biological studies | journal = Chemistry & Biology | volume = 16 | issue = 3 | pages = 323–36 | date = March 2009 | pmid = 19318213 | pmc = 2696486 | doi = 10.1016/j.chembiol.2009.03.001 }}</ref><ref name="isbn0-387-22046-1">{{cite book | vauthors = Simon M | title = Emergent computation: emphasizing bioinformatics | publisher = AIP Press/Springer Science+Business Media | location = New York | year = 2005 | pages = 105–106 | isbn = 978-0-387-22046-8 }}</ref> |
|||
<blockquote>The systematic names and formulas given refer to hypothetical forms in which amino groups are unprotonated and carboxyl groups are undissociated. This convention is useful to avoid various nomenclatural problems but should not be taken to imply that these structures represent an appreciable fraction of the amino-acid molecules. |
|||
Many important proteinogenic and non-proteinogenic amino acids have biological functions. For example, in the [[human brain]], glutamate (standard [[glutamic acid]]) and [[gamma-amino-butyric acid]] ("GABA", non-standard gamma-amino acid) are, respectively, the main [[Neurotransmitter#Excitatory and inhibitory|excitatory and inhibitory neurotransmitters]].<ref name="pmid12467378">{{cite journal | vauthors = Petroff OA | title = GABA and glutamate in the human brain | journal = The Neuroscientist | volume = 8 | issue = 6 | pages = 562–73 | date = December 2002 | pmid = 12467378 | doi = 10.1177/1073858402238515 | url = http://nro.sagepub.com/cgi/pmidlookup?view=long&pmid=12467378 }}</ref> [[Hydroxyproline]], a major component of the [[connective tissue]] [[collagen]], is synthesised from [[proline]]. [[Glycine]] is a biosynthetic precursor to porphyrins used in [[red blood cell]]s. [[Carnitine]] is used in [[lipid|lipid transport]]. |
|||
</blockquote> |
|||
==History== |
|||
Nine proteinogenic amino acids are called "[[essential amino acid|essential]]" for humans because they cannot be produced from other [[chemical compound|compounds]] by the human body and so must be taken in as food. Others may be [[Essential amino acid#Essentiality vs. conditional essentiality in humans|conditionally essential]] for certain ages or medical conditions. Essential amino acids may also differ between [[species]].<ref>For example, [[ruminant]]s such as cows obtain a number of amino acids via [[microbe]]s in the [[reticulorumen|first two stomach chambers]].</ref> |
|||
The first few amino acids were discovered in the early 1800s.<ref>{{cite journal | vauthors = Vickery HB, Schmidt CL | year = 1931 | title = The history of the discovery of the amino acids | journal = Chem. Rev. | volume = 9 | issue = 2| pages = 169–318 | doi=10.1021/cr60033a001}}</ref><ref>{{cite web |last=Hansen |first=Sabine |name-list-style=vanc |title=Die Entdeckung der proteinogenen Aminosäuren von 1805 in Paris bis 1935 in Illinois |location=Berlin |date=May 2015 |url=https://www.arginium.de/wp-content/uploads/2015/12/Entdeckung-der-Aminos%C3%A4uren.pdf |archive-url=https://web.archive.org/web/20171201232937/https://www.arginium.de/wp-content/uploads/2015/12/Entdeckung-der-Aminos%C3%A4uren.pdf |archive-date=1 December 2017 |language=de}}</ref> In 1806, French chemists [[Louis-Nicolas Vauquelin]] and [[Pierre Jean Robiquet]] isolated a compound from [[asparagus]] that was subsequently named [[asparagine]], the first amino acid to be discovered.<ref>{{Cite journal|title=The discovery of a new plant principle in Asparagus sativus |vauthors=Vauquelin LN, Robiquet PJ |journal=Annales de Chimie |year=1806 |volume=57 |pages=88–93}}</ref><ref name=Anfinsen>{{Cite book |title=Advances in Protein Chemistry |vauthors=Anfinsen CB, Edsall JT, Richards FM |year=1972 |pages=[https://archive.org/details/advancesinprotei26anfi/page/99 99, 103] |publisher=Academic Press |location=New York |isbn=978-0-12-034226-6 |url=https://archive.org/details/advancesinprotei26anfi/page/99 }}</ref> [[Cystine]] was discovered in 1810,<ref>{{Cite journal|title=On cystic oxide, a new species of urinary calculus | vauthors = Wollaston WH |s2cid=110151163 |journal=Philosophical Transactions of the Royal Society |year=1810 |volume=100|pages=223–230 |doi=10.1098/rstl.1810.0015}}</ref> although its monomer, [[cysteine]], remained undiscovered until 1884.<ref>{{Cite journal |title=Über cystin und cystein | vauthors = Baumann E |journal=Z Physiol Chem |year=1884 |volume=8 |issue=4 |pages=299–305 |url=http://vlp.mpiwg-berlin.mpg.de/library/data/lit16533 |access-date=28 March 2011 |archive-url=https://web.archive.org/web/20110314075450/http://vlp.mpiwg-berlin.mpg.de/library/data/lit16533 |archive-date=14 March 2011 |url-status=dead }}</ref><ref name=Anfinsen/>{{efn|The late discovery is explained by the fact that cysteine becomes oxidized to cystine in air.}} [[Glycine]] and [[leucine]] were discovered in 1820.<ref>{{Cite journal|title=Sur la conversion des matières animales en nouvelles substances par le moyen de l'acide sulfurique | vauthors = Braconnot HM |journal=Annales de Chimie et de Physique |series=2nd Series |year=1820 |volume=13 |pages=113–125}}</ref> The last of the 20 common amino acids to be discovered was [[threonine]] in 1935 by [[William Cumming Rose]], who also determined the [[essential amino acid]]s and established the minimum daily requirements of all amino acids for optimal growth.<ref>{{cite journal |vauthors=Simoni RD, Hill RL, Vaughan M |date=September 2002 |title=The discovery of the amino acid threonine: the work of William C. Rose [classical article] |journal=The Journal of Biological Chemistry |volume=277 |issue=37 |pages=56-58 |doi=10.1016/S0021-9258(20)74369-3 |pmid=12218068 |doi-access=free}}</ref><ref>{{cite journal|title=Feeding Experiments with Mixtures of Highly Purified Amino Acids. VIII. Isolation and Identification of a New Essential Amino Acid|vauthors = McCoy RH, Meyer CE, Rose WC|year = 1935|journal = Journal of Biological Chemistry|volume = 112|pages = 283–302|doi = 10.1016/S0021-9258(18)74986-7|doi-access = free}}</ref> |
|||
Because of their biological significance, amino acids are important in nutrition and are commonly used in [[nutritional supplement]]s, [[fertilizer]]s, [[animal feed|feed]], and [[food technology]]. Industrial uses include the production of [[Pharmaceutical drug|drugs]], [[biodegradable plastic]]s, and [[asymmetric catalysis|chiral catalysts]]. |
|||
The unity of the chemical category was recognized by [[Charles Adolphe Wurtz|Wurtz]] in 1865, but he gave no particular name to it.<ref>Menten, P. ''Dictionnaire de chimie: Une approche étymologique et historique''. De Boeck, Bruxelles. [https://books.google.com/books?id=NKTKDgAAQBAJ link] {{Webarchive|url=https://web.archive.org/web/20191228193229/https://books.google.com/books?id=NKTKDgAAQBAJ |date=28 December 2019 }}.</ref> The first use of the term "amino acid" in the English language dates from 1898,<ref>{{cite web |url=https://www.etymonline.com/word/amino- |last=Harper |first=Douglas |name-list-style=vanc |work=Online Etymology Dictionary |title=amino- |access-date=19 July 2010 |archive-date=2 December 2017 |archive-url=https://web.archive.org/web/20171202102757/https://www.etymonline.com/word/amino- |url-status=live }}</ref> while the German term, {{lang|de|Aminosäure}}, was used earlier.<ref>{{cite journal | vauthors = Paal C | year = 1894 | title = Ueber die Einwirkung von Phenyl-i-cyanat auf organische Aminosäuren | journal = Berichte der Deutschen Chemischen Gesellschaft | volume = 27 | pages = 974–979 | doi = 10.1002/cber.189402701205 | url = https://zenodo.org/record/1425732 | archive-url = https://web.archive.org/web/20200725075835/https://zenodo.org/record/1425732 | url-status = dead | archive-date = 2020-07-25 }}</ref> [[Protein]]s were found to yield amino acids after enzymatic digestion or acid [[hydrolysis]]. In 1902, [[Hermann Emil Fischer|Emil Fischer]] and [[Franz Hofmeister]] independently proposed that proteins are formed from many amino acids, whereby bonds are formed between the amino group of one amino acid with the carboxyl group of another, resulting in a linear structure that Fischer termed "[[peptide]]".<ref>{{cite book | first = Joseph S. | last = Fruton | name-list-style = vanc | title = Contrasts in Scientific Style: Research Groups in the Chemical and Biochemical Sciences |volume=191 |year=1990 |chapter=Chapter 5- Emil Fischer and Franz Hofmeister |chapter-url=https://books.google.com/books?id=tRlC9NyNNN8C&pg=PA163 |pages=163–165 |publisher=American Philosophical Society |isbn=978-0-87169-191-0 }}</ref> |
|||
==History== |
|||
The first few amino acids were discovered in the early 19th century.<ref>{{cite journal | vauthors = Vickery HB, Schmidt CL | year = 1931 | title = The history of the discovery of the amino acids | journal = Chem. Rev. | volume = 9 | issue = 2| pages = 169–318 | doi=10.1021/cr60033a001}}</ref><ref>Hansen, Sabine. ''Die Entdeckung der proteinogenen Aminosäuren von 1805 in Paris bis 1935 in Illinois''. Berlin, im Mai 2015. [https://www.arginium.de/wp-content/uploads/2015/12/Entdeckung-der-Aminos%C3%A4uren.pdf link] {{Webarchive|url=https://web.archive.org/web/20171201232937/https://www.arginium.de/wp-content/uploads/2015/12/Entdeckung-der-Aminos%C3%A4uren.pdf |date=1 December 2017 }}.</ref> In 1806, French chemists [[Louis-Nicolas Vauquelin]] and [[Pierre Jean Robiquet]] isolated a compound in [[asparagus]] that was subsequently named [[asparagine]], the first amino acid to be discovered.<ref>{{Cite journal|title=The discovery of a new plant principle in Asparagus sativus |vauthors=Vauquelin LN, Robiquet PJ |journal=Annales de Chimie |year=1806 |volume=57 |pages=88–93}}</ref><ref name=Anfinsen>{{Cite book |title=Advances in Protein Chemistry |vauthors=Anfinsen CB, Edsall JT, Richards FM |year=1972 |pages=[https://archive.org/details/advancesinprotei26anfi/page/99 99, 103] |publisher=Academic Press |location=New York |isbn=978-0-12-034226-6 |url=https://archive.org/details/advancesinprotei26anfi/page/99 }}</ref> [[Cystine]] was discovered in 1810,<ref>{{Cite journal|title=On cystic oxide, a new species of urinary calculus |author=Wollaston WH |journal=Philosophical Transactions of the Royal Society |year=1810 |volume=100|pages=223–30 |doi=10.1098/rstl.1810.0015}}</ref> although its monomer, [[cysteine]], remained undiscovered until 1884.<ref name=Anfinsen/><ref>{{Cite journal|title=Über cystin und cystein |author=Baumann E |journal=Z Physiol Chem |year=1884 |volume=8 |issue=4|pages=299–305 | url=http://vlp.mpiwg-berlin.mpg.de/library/data/lit16533 | accessdate=28 March 2011 | archiveurl= https://web.archive.org/web/20110314075450/http://vlp.mpiwg-berlin.mpg.de/library/data/lit16533| archivedate= 14 March 2011 | url-status=live}}</ref> [[Glycine]] and [[leucine]] were discovered in 1820.<ref>{{Cite journal|title=Sur la conversion des matières animales en nouvelles substances par le moyen de l'acide sulfurique |author=Braconnot HM |journal=Annales de Chimie et de Physique |series=2nd Series |year=1820 |volume=13 |pages=113–25}}</ref> The last of the 20 common amino acids to be discovered was [[threonine]] in 1935 by [[William Cumming Rose]], who also determined the [[essential amino acid]]s and established the minimum daily requirements of all amino acids for optimal growth.<ref>{{cite journal|vauthors = Simoni RD, Hill RL, Vaughan M|title = The discovery of the amino acid threonine: the work of William C. Rose [classical article]|journal = The Journal of Biological Chemistry| volume = 277|issue = 37|pages = E25|date = September 2002|pmid = 12218068|url = http://www.jbc.org/content/277/37/e25}}</ref><ref>{{cite journal|title=Feeding Experiments with Mixtures of Highly Purified Amino Acids. VIII. Isolation and Identification of a New Essential Amino Acid|vauthors = McCoy RH, Meyer CE, Rose WC|year = 1935|journal = Journal of Biological Chemistry|volume = 112|pages = 283–302}}</ref> |
|||
The unity of the chemical category was recognized by [[Charles Adolphe Wurtz|Wurtz]] in 1865, but he gave no particular name to it.<ref>Menten, P. ''Dictionnaire de chimie: Une approche étymologique et historique''. De Boeck, Bruxelles. [https://books.google.com/books?id=NKTKDgAAQBAJ link].</ref> First use of the term "amino acid" in the English language dates from 1898,<ref>{{cite web| url = https://www.etymonline.com/word/amino- | publisher = www.etymonline.com | title = etymonline.com entry for ''amino'' | accessdate = 19 July 2010}}</ref> while the German term, ''Aminosäure'', was used earlier.<ref>{{cite journal | vauthors = Paal C | year = 1894 | title = Ueber die Einwirkung von Phenyl‐i‐cyanat auf organische Aminosäuren | journal = Berichte der Deutschen Chemischen Gesellschaft | volume = 27 | issue = | pages = 974–979 | doi=10.1002/cber.189402701205}}</ref> Proteins were found to yield amino acids after enzymatic digestion or acid [[hydrolysis]]. In 1902, [[Hermann Emil Fischer|Emil Fischer]] and [[Franz Hofmeister]] independently proposed that proteins are formed from many amino acids, whereby bonds are formed between the amino group of one amino acid with the carboxyl group of another, resulting in a linear structure that Fischer termed "[[peptide]]".<ref>{{cite book | first = Joseph S. | last = Fruton | name-list-format = vanc | title = Contrasts in Scientific Style: Research Groups in the Chemical and Biochemical Sciences |volume=191 |year=1990 |chapter=Chapter 5- Emil Fischer and Franz Hofmeister |pages=163–165 |publisher=American Philosophical Society |isbn=978-0-87169-191-0 }}</ref> |
|||
==General structure== |
==General structure== |
||
[[File: |
[[File:ProteinogenicAminoAcids.svg|thumb|upright=2.75|The 21 [[Proteinogenic amino acid|proteinogenic α-amino acids]] found in [[eukaryote]]s, grouped according to their side chains' [[PKa|p''K''<sub>a</sub>]] values and charges carried at [[PH#Living systems|physiological pH (7.4)]]]] |
||
'''2-''', '''alpha-''', or '''α-amino acids'''<ref>{{cite web |url=http://www.merriam-webster.com/medical/alpha-amino%20acid |title=Alpha amino acid |work=Merriam-Webster Medical |access-date=3 January 2015|archive-date=3 January 2015|archive-url=https://web.archive.org/web/20150103191856/http://www.merriam-webster.com/medical/alpha-amino%20acid|url-status=live}}.</ref> have the generic [[Chemical formula|formula]] {{chem2|H2NCHRCOOH}} in most cases,{{efn|[[Proline]] and other cyclic amino acids are an exception to this general formula. Cyclization of the α-amino acid creates the corresponding secondary amine. These are occasionally referred to as [[imino acid]]s.}} where R is an [[organic chemistry|organic]] [[substituent]] known as a "[[Substituent|side chain]]".<ref>{{Cite web |last=Clark |first=Jim |date=August 2007 |title=An introduction to amino acids |url=http://www.chemguide.co.uk/organicprops/aminoacids/background.html |website=chemguide |access-date=4 July 2015 |url-status=live |archive-date=30 April 2015|archive-url=https://web.archive.org/web/20150430051143/http://www.chemguide.co.uk/organicprops/aminoacids/background.html}}</ref> |
|||
Of the many hundreds of described amino acids, 22 are [[Proteinogenic amino acid|proteinogenic]] ("protein-building").<ref>{{cite encyclopedia |year=2008|title=Amino acids |encyclopedia=Peptides from A to Z: A Concise Encyclopedia |url=https://books.google.com/books?id=doe9NwgJTAsC&pg=PA20 |publisher=Wiley-VCH|location=Germany|isbn=9783527621170 |via=Google Books|page=20|last1=Jakubke|first1=Hans-Dieter |last2=Sewald|first2=Norbert |name-list-style=vanc|access-date=5 January 2016|archive-date=17 May 2016|archive-url = https://web.archive.org/web/20160517144350/https://books.google.com/books?id=doe9NwgJTAsC&pg=PA20|url-status = live}}</ref><ref>{{cite book |editor1-first = Loredano|editor1-last = Pollegioni|editor2-first = Stefano|editor2-last = Servi | name-list-style = vanc | title = Unnatural Amino Acids: Methods and Protocols|year = 2012|publisher = Humana Press|isbn = 978-1-61779-331-8|page = v|oclc = 756512314|series = Methods in Molecular Biology |volume=794|doi = 10.1007/978-1-61779-331-8|s2cid = 3705304 }}</ref><ref>{{cite journal | vauthors = Hertweck C | title = Biosynthesis and Charging of Pyrrolysine, the 22nd Genetically Encoded Amino Acid| journal = [[Angewandte Chemie International Edition]] | volume = 50 | issue = 41 | pages = 9540–9541 | date = October 2011 | pmid = 21796749 | doi = 10.1002/anie.201103769 | s2cid = 5359077}}{{Closed access}} |
|||
In the structure shown at the top of the page, '''R''' represents a [[Substituent|side chain]] specific to each amino acid. The [[carbon]] atom next to the [[carboxyl group]] (which is therefore numbered 2 in the [[carbon chain]] starting from that functional group) is called the [[alpha carbon|α–carbon]]. Amino acids containing an [[amino group]] bonded directly to the alpha carbon are referred to as ''alpha amino acids''.<ref>{{cite web|url=http://www.merriam-webster.com/medical/alpha-amino%20acid|title=Alpha amino acid – Medical definition|publisher=Merriam-Webster dictionary}}</ref> These include amino acids such as [[proline]] which contain [[secondary amine]]s, which used to be often referred to as "imino acids".<ref>{{MeshName|Proline}}</ref><ref>{{cite web|url=http://opbs.okstate.edu/5753/Amino%20Acids.html |title=Archived copy |accessdate=3 January 2015 |url-status=dead |archiveurl=https://web.archive.org/web/20080118064634/http://opbs.okstate.edu/5753/Amino%20Acids.html |archivedate=18 January 2008 }}</ref><ref name=goldbook>{{GoldBookRef|file=I02959|title=Imino acids|accessdate=2 April 2012}}</ref> |
|||
</ref> It is these 22 compounds that combine to give a vast array of peptides and proteins assembled by [[ribosome]]s.<ref name="NIGMS">{{cite web |title=Chapter 1: Proteins are the Body's Worker Molecules |date=27 October 2011 |website=The Structures of Life |publisher=National Institute of General Medical Sciences |url=https://publications.nigms.nih.gov/structlife/chapter1.html |access-date=20 May 2008 |archive-date=7 June 2014 |archive-url=https://web.archive.org/web/20140607084902/https://publications.nigms.nih.gov/structlife/chapter1.html}}</ref> Non-proteinogenic or modified amino acids may arise from [[post-translational modification]] or during [[nonribosomal peptide]] synthesis. |
|||
=== |
===Chirality=== |
||
The [[carbon]] atom next to the [[carboxyl group]] is called the [[alpha carbon|α–carbon]]. In proteinogenic amino acids, it bears the amine and the R group or [[Substituent|side chain]] specific to each amino acid, as well as a hydrogen atom. With the exception of glycine, for which the side chain is also a hydrogen atom, the α–carbon is [[stereogenic]]. All [[chiral]] proteogenic amino acids have the <small>L</small> configuration. They are "left-handed" [[enantiomer]]s, which refers to the [[stereoisomers]] of the alpha carbon. |
|||
A few <small>D</small>-amino acids ("right-handed") have been found in nature, e.g., in [[bacterial envelope]]s, as a [[Neuromodulation|neuromodulator]] (<small>D</small>-[[serine]]), and in some [[antibiotic]]s.<ref>{{cite book | title = Biochemical Pathways: An Atlas of Biochemistry and Molecular Biology | publisher = Wiley-Blackwell | year = 2012 | isbn = 978-0-470-14684-2 | location = Oxford | editor-last = Michal | editor-first = Gerhard | editor2-last = Schomburg | editor2-first = Dietmar | name-list-style = vanc | page = 5 | edition = 2nd }}</ref><ref name="Creighton">{{Cite book |last=Creighton |first=Thomas H. |name-list-style=vanc |title=Proteins: structures and molecular properties |publisher=W. H. Freeman |location=San Francisco |year=1993 |chapter=Chapter 1 |isbn=978-0-7167-7030-5 |chapter-url-access=registration |chapter-url=https://archive.org/details/proteinsstructur0000crei }}</ref> Rarely, [[D-Amino acid|<small>D</small>-amino acid residues]] are found in proteins, and are converted from the <small>L</small>-amino acid as a [[post-translational modification]].<ref>{{Cite journal |last=Genchi |first=Giuseppe |date=2017-09-01 |title=An overview on d-amino acids |url=https://doi.org/10.1007/s00726-017-2459-5 |journal=Amino Acids |language=en |volume=49 |issue=9 |pages=1521–1533 |doi=10.1007/s00726-017-2459-5 |pmid=28681245 |s2cid=254088816 |issn=1438-2199}}</ref>{{efn|The <small>L</small> and <small>D</small> convention for amino acid configuration refers not to the optical activity of the amino acid itself but rather to the optical activity of the isomer of [[glyceraldehyde]] from which that amino acid can, in theory, be synthesized (<small>D</small>-glyceraldehyde is dextrorotatory; <small>L</small>-glyceraldehyde is levorotatory). |
|||
In alternative fashion, the [[Cahn–Ingold–Prelog priority rules|''(S)'' and ''(R)'' designators]] are used to indicate the ''absolute configuration''. Almost all of the amino acids in proteins are ''(S)'' at the α carbon, with cysteine being ''(R)'' and glycine non-[[Chirality|chiral]].<ref>{{cite web| last = Hatem | first = Salama Mohamed Ali | year = 2006 | url = http://geb.uni-giessen.de/geb/volltexte/2006/3038/index.html | title = Gas chromatographic determination of Amino Acid Enantiomers in tobacco and bottled wines | publisher = University of Giessen | accessdate = 17 November 2008}}</ref> Cysteine has its side chain in the same geometric position as the other amino acids, but the ''R/S'' terminology is reversed because of the higher atomic number of [[sulfur]] compared to the carboxyl oxygen gives the side chain a higher priority by the Cahn–Ingold–Prelog rules, whereas the atoms in other side chains give them lower priority compared to the carboxyl group. |
|||
An alternative convention is to use the [[Cahn–Ingold–Prelog priority rules|(''S'') and (''R'') designators]] to specify the ''absolute configuration''.<ref name=Cahn>{{Cite journal | author = Cahn, R.S. | author-link = Robert Sidney Cahn | author2 = Ingold, C.K. | author2-link = Christopher Kelk Ingold | author3 = Prelog, V. | author3-link = Vladimir Prelog | title = Specification of Molecular Chirality | journal = [[Angewandte Chemie International Edition]] | volume = 5 | issue = 4 | pages = 385–415 | year = 1966 | doi = 10.1002/anie.196603851}}</ref> Almost all of the amino acids in proteins are (''S'') at the α carbon, with [[cysteine]] being (''R'') and glycine non-[[Chirality (chemistry)|chiral]].<ref>{{cite web | last = Hatem | first = Salama Mohamed Ali |name-list-style=vanc | year = 2006 | url = http://geb.uni-giessen.de/geb/volltexte/2006/3038/index.html | title = Gas chromatographic determination of Amino Acid Enantiomers in tobacco and bottled wines | publisher = University of Giessen | access-date = 17 November 2008 | archive-url = https://web.archive.org/web/20090122104055/http://geb.uni-giessen.de/geb/volltexte/2006/3038/index.html | archive-date = 22 January 2009 | url-status = dead }}</ref> Cysteine has its side chain in the same geometric location as the other amino acids, but the ''R''/''S'' terminology is reversed because [[sulfur]] has higher atomic number compared to the carboxyl oxygen which gives the side chain a higher priority by the [[Cahn–Ingold–Prelog priority rules|Cahn-Ingold-Prelog sequence rules]].}} |
|||
===Side chains=== |
===Side chains=== |
||
[[File:Lysine fisher structure and 3d ball.svg|thumb|right|[[Lysine]] with carbon atoms labeled by position{{clarify|reason=The structural formula of the zwitterion on the left does not matched the space-filling model of the un-ionized form on the right.|date=January 2019}}]] |
|||
==== Polar charged side chains ==== |
|||
In amino acids that have a carbon chain attached to the α–carbon (such as [[lysine]], shown to the right) the carbons are labeled in order as α, β, γ, δ, and so on.<ref>{{cite web | url = http://www.chem.qmul.ac.uk/iupac/AminoAcid/AA1n2.html | title = Nomenclature and Symbolism for Amino Acids and Peptides | publisher = IUPAC-IUB Joint Commission on Biochemical Nomenclature | year = 1983 | accessdate = 17 November 2008 | archiveurl = https://web.archive.org/web/20081009023202/http://www.chem.qmul.ac.uk/iupac/AminoAcid/AA1n2.html | archivedate = 9 October 2008 | url-status=dead | df = dmy-all }}</ref> In some amino acids, the amine group is attached to the β or γ-carbon, and these are therefore referred to as ''beta'' or ''gamma amino acids''. |
|||
Five amino acids possess a charge at neutral pH. Often these side chains appear at the surfaces on proteins to enable their solubility in water, and side chains with opposite charges form important electrostatic contacts called [[Salt bridge (protein and supramolecular)|salt bridges]] that maintain structures within a single protein or between interfacing proteins.<ref name="Garrett-2010">{{Cite book |last1=Garrett |first1=Reginald H. |title=Biochemistry |last2=Grisham |first2=Charles M. |date=2010 |publisher=Brooks/Cole, Cengage Learning |isbn=978-0-495-10935-8 |edition=4th |location=Belmont, CA |pages=74,134–176,430–442 |oclc=297392560}}</ref> Many proteins bind metal into their structures specifically, and these interactions are commonly mediated by charged side chains such as [[aspartate]], [[glutamate]] and [[histidine]]. Under certain conditions, each ion-forming group can be charged, forming double salts.<ref>{{Cite journal |last1=Novikov |first1=Anton P. |last2=Safonov |first2=Alexey V. |last3=German |first3=Konstantin E. |last4=Grigoriev |first4=Mikhail S. |date=2023-12-01 |title=What kind of interactions we may get moving from zwitter to "dritter" ions: C–O⋯Re(O<sub>4</sub>) and Re–O⋯Re(O<sub>4</sub>) anion⋯anion interactions make structural difference between <small>L</small>-histidinium perrhenate and pertechnetate |journal=CrystEngComm |volume=26 |pages=61–69 |language=en |doi=10.1039/D3CE01164J |s2cid=265572280 |issn=1466-8033}}</ref> |
|||
Amino acids are usually classified by the [[chemical property|properties]] of their side chain into four groups. The side chain can make an amino acid a [[weak acid]] or a [[weak base]], and a [[hydrophile]] if the side chain is [[polar molecule|polar]] or a [[hydrophobe]] if it is [[nonpolar]].<ref name="Creighton" /> The [[chemical structure]]s of the 22 standard amino acids, along with their chemical properties, are described more fully in the article on these [[proteinogenic amino acid]]s. |
|||
The two negatively charged amino acids at neutral pH are [[Aspartic acid|aspartate]] (Asp, D) and [[Glutamic acid|glutamate]] (Glu, E). The anionic carboxylate groups behave as [[Brønsted–Lowry acid–base theory|Brønsted bases]] in most circumstances.<ref name="Garrett-2010" /> Enzymes in very low pH environments, like the aspartic protease [[pepsin]] in mammalian stomachs, may have catalytic aspartate or glutamate residues that act as Brønsted acids. |
|||
The phrase "[[branched-chain amino acid]]s" or BCAA refers to the amino acids having [[aliphatic]] side chains that are non-linear; these are [[leucine]], [[isoleucine]], and [[valine]]. [[Proline]] is the only [[proteinogenic]] amino acid whose side-group links to the α-amino group and, thus, is also the only proteinogenic amino acid containing a secondary amine at this position.<ref name="Creighton" /> In chemical terms, proline is, therefore, an [[imino acid]], since it lacks a [[amine|primary amino group]],<ref>{{Cite journal | doi = 10.1021/ja01414a033 | volume = 48 | issue = 3 | pages = 751–753 | last = Jodidi | first = S. L. | title = The Formol Titration of Certain Amino Acids | journal = Journal of the American Chemical Society | date = 1 March 1926 }}</ref> although it is still classed as an amino acid in the current biochemical nomenclature,<ref>{{Cite book|editor1-first=Claude |editor1-last=Liebecq | name-list-format = vanc |title=Biochemical Nomenclature and Related Documents |edition=2nd |publisher=Portland Press |year=1992 |pages=39–69 |isbn=978-1-85578-005-7}}</ref> and may also be called an "N-alkylated alpha-amino acid".<ref>{{cite book|first=Anthony D. |last=Smith | name-list-format = vanc |title=Oxford dictionary of biochemistry and molecular biology |publisher=Oxford University Press |location=Oxford |year=1997 |page=535 |isbn=978-0-19-854768-6 |oclc=37616711}}</ref> |
|||
[[File:Histidine lysine arginine sidechains.png|class=skin-invert-image|thumb|upright=2.05 |Functional groups found in histidine (left), lysine (middle) and arginine (right)]] |
|||
===Zwitterions=== |
|||
[[File:Amino acid zwitterions.svg |thumb|right|An amino acid in its (1) un-ionized and (2) zwitterionic forms]] |
|||
{{main|Zwitterion}} |
|||
The α-[[carboxylic acid]] group of amino acids is a [[weak acid]], meaning that it releases a [[Hydron (chemistry)|hydron]] (such as a [[proton]]) at moderate pH values. In other words, carboxylic acid groups (−CO<sub>2</sub>H) can be [[deprotonated]] to become negatively charged [[carboxylate]]s (−CO<sub>2</sub><sup>−</sup> ). The negatively charged carboxylate ion predominates at pH values greater than the [[pKa]] of the carboxylic acid group (typically around 2.2 for the 20 common amino acids; see the table of amino acid structures above). In a complementary fashion, the α-[[amine]] of amino acids is a [[weak base]], meaning that it accepts a proton at moderate pH values. In other words, α-amino groups (NH<sub>2</sub>−) can be [[protonated]] to become positively charged α-ammonium groups (<sup>+</sup>H<sub>3</sub>N−). The positively charged α-ammonium group predominates at pH values less than the pKa of the α-ammonium group (mean for the 20 common α-amino acids is about 9.4). |
|||
There are three amino acids with side chains that are cations at neutral pH: [[arginine]] (Arg, R), [[lysine]] (Lys, K) and [[histidine]] (His, H). Arginine has a charged [[Guanidine|guanidino]] group and lysine a charged alkyl amino group, and are fully protonated at pH 7. Histidine's imidazole group has a pK<sub>a</sub> of 6.0, and is only around 10% protonated at neutral pH. Because histidine is easily found in its basic and conjugate acid forms it often participates in catalytic proton transfers in enzyme reactions.<ref name="Garrett-2010" /> |
|||
Because all amino acids contain amine and carboxylic acid functional groups, they share [[Amphoterism|amphiprotic]] properties.<ref name="Creighton" /> Below pH 2.2, the predominant form will have a neutral carboxylic acid group and a positive α-ammonium ion (net charge +1), and above pH 9.4, a negative carboxylate and neutral α-amino group (net charge −1). At a pH between 2.2 and 9.4, an amino acid typically contains both a negative carboxylate and a positive α-ammonium group, as shown in structure (2) on the right and exhibits a net zero charge. This molecular state is known as a [[zwitterion]], from the German ''{{lang|de|Zwitter}}'' meaning "hermaphrodite" or "hybrid".<ref>{{cite book | last1 = Simmons | first1 = William J. | first2 = Gerhard | last2 = Meisenberg | name-list-format = vanc | title = Principles of medical biochemistry |publisher=Mosby Elsevier |location= |year=2006 |isbn=978-0-323-02942-1 |oclc= |page=19}}</ref> The fully neutral form (structure (1) on the left) is a very minor species in aqueous solution throughout the pH range (less than 1 part in 10<sup>7</sup>). Altogether, amino acids exist as zwitterions in the solid phase and crystallize with salt-like properties unlike typical organic acids or amines. |
|||
==== Polar uncharged side chains ==== |
|||
===Isoelectric point=== |
|||
[[File:Amino Acid Titration Curves By Side Chain.png|thumb|right|360px|Composite of [[titration curve]]s of twenty proteinogenic amino acids grouped by side chain category]] |
|||
The polar, uncharged amino acids [[serine]] (Ser, S), [[threonine]] (Thr, T), [[asparagine]] (Asn, N) and [[glutamine]] (Gln, Q) readily form hydrogen bonds with water and other amino acids.<ref name="Garrett-2010" /> They do not ionize in normal conditions, a prominent exception being the catalytic serine in [[Serine protease#Catalytic mechanism|serine proteases]]. This is an example of severe perturbation, and is not characteristic of serine residues in general. Threonine has two chiral centers, not only the <small>L</small> (2''S'') chiral center at the α-carbon shared by all amino acids apart from achiral glycine, but also (3''R'') at the β-carbon. The full [[stereochemical]] specification is (2''S'',3''R'')-<small>L</small>-[[threonine]]. |
|||
The variation in titration curves when the amino acids can be grouped by category.{{clarify|issue=By eye, polar-uncharged, hydrophobic, and special-case curve-sets all look the same, but one AA in each of them looks different.|date=August 2017}} With the exception of tyrosine, using titration to distinguish among hydrophobic amino acids is problematic. |
|||
==== Hydrophobic side chains ==== |
|||
At pH values between the two pKa values, the zwitterion predominates, but coexists in [[dynamic equilibrium]] with small amounts of net negative and net positive ions. At the exact midpoint between the two pKa values, the trace amount of net negative and trace of net positive ions exactly balance, so that average net charge of all forms present is zero.<ref>{{cite book | vauthors = Fennema OR |title=Food Chemistry 3rd Ed |publisher=CRC Press |location= |pages=327–8 |isbn=978-0-8247-9691-4 |oclc= |date=1996-06-19 }}</ref> This pH is known as the [[isoelectric point]] pI, so pI = ½(pKa<sub>1</sub> + pKa<sub>2</sub>). The individual amino acids all have slightly different pKa values and therefore have different isoelectric points. For amino acids with charged side chains, the pKa of the side chain is involved. Thus for Asp or Glu with negative side chains, pI = ½(pKa<sub>1</sub> + pKa<sub>R</sub>), where pKa<sub>R</sub> is the side chain pKa. Cysteine also has potentially negative side chain with pKa<sub>R</sub> = 8.14, so pI should be calculated as for Asp and Glu, even though the side chain is not significantly charged at physiological pH. For His, Lys, and Arg with positive side chains, pI = ½(pKa<sub>R</sub> + pKa<sub>2</sub>). Amino acids have zero mobility in electrophoresis at their isoelectric point, although this behaviour is more usually exploited for peptides and proteins than single amino acids. Zwitterions have minimum solubility at their isoelectric point, and some amino acids (in particular, with non-polar side chains) can be isolated by precipitation from water by adjusting the pH to the required isoelectric point. |
|||
Nonpolar amino acid interactions are the primary driving force behind the processes that [[Protein folding|fold proteins]] into their functional three dimensional structures.<ref name="Garrett-2010" /> None of these amino acids' side chains ionize easily, and therefore do not have pK<sub>a</sub>s, with the exception of [[tyrosine]] (Tyr, Y). The hydroxyl of tyrosine can deprotonate at high pH forming the negatively charged phenolate. Because of this one could place tyrosine into the polar, uncharged amino acid category, but its very low solubility in water matches the characteristics of hydrophobic amino acids well. |
|||
==Occurrence and functions in biochemistry== |
|||
{{multiple image |
|||
<!-- Layout parameters --> |
|||
| align = right |
|||
| direction = vertical |
|||
| total_width = 380 |
|||
<!-- Header --> |
|||
| header_align = <!-- center (default), left, right --> |
|||
| header = |
|||
==== Special case side chains ==== |
|||
<!--image 5--> |
|||
| image5 = Protein primary structure.svg |
|||
| alt5 = A protein depicted as a long unbranched string of linked circles each representing amino acids |
|||
| width5 = |
|||
| height5 = |
|||
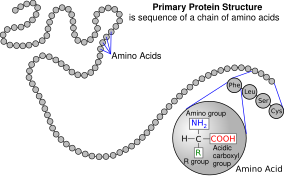
| caption5 = A [[polypeptide]] is an unbranched chain of amino acids |
|||
Several side chains are not described well by the charged, polar and hydrophobic categories. [[Glycine]] (Gly, G) could be considered a polar amino acid since its small size means that its solubility is largely determined by the amino and carboxylate groups. However, the lack of any side chain provides glycine with a unique flexibility among amino acids with large ramifications to protein folding.<ref name="Garrett-2010" /> [[Cysteine]] (Cys, C) can also form hydrogen bonds readily, which would place it in the polar amino acid category, though it can often be found in protein structures forming covalent bonds, called [[disulphide bonds]], with other cysteines. These bonds influence the folding and stability of proteins, and are essential in the formation of [[Antibody#Structure|antibodies]]. [[Proline]] (Pro, P) has an alkyl side chain and could be considered hydrophobic, but because the side chain joins back onto the alpha amino group it becomes particularly inflexible when incorporated into proteins. Similar to glycine this influences protein structure in a way unique among amino acids. [[Selenocysteine]] (Sec, U) is a rare amino acid not directly encoded by DNA, but is incorporated into proteins via the ribosome. Selenocysteine has a lower redox potential compared to the similar cysteine, and participates in several unique enzymatic reactions.<ref>{{Cite journal |last1=Papp |first1=Laura Vanda |last2=Lu |first2=Jun |last3=Holmgren |first3=Arne |last4=Khanna |first4=Kum Kum |date=2007-07-01 |title=From Selenium to Selenoproteins: Synthesis, Identity, and Their Role in Human Health |journal=Antioxidants & Redox Signaling |volume=9 |issue=7 |pages=775–806 |doi=10.1089/ars.2007.1528 |pmid=17508906 |issn=1523-0864}}</ref> [[Pyrrolysine]] (Pyl, O) is another amino acid not encoded in DNA, but synthesized into protein by ribosomes.<ref>{{Cite journal |last1=Hao |first1=Bing |last2=Gong |first2=Weimin |last3=Ferguson |first3=Tsuneo K. |last4=James |first4=Carey M. |last5=Krzycki |first5=Joseph A. |last6=Chan |first6=Michael K. |date=2002-05-24 |title=A New UAG-Encoded Residue in the Structure of a Methanogen Methyltransferase |journal=Science |language=en |volume=296 |issue=5572 |pages=1462–1466 |doi=10.1126/science.1069556 |pmid=12029132 |bibcode=2002Sci...296.1462H |s2cid=35519996 |issn=0036-8075}}</ref> It is found in archaeal species where it participates in the catalytic activity of several methyltransferases. |
|||
<!--image 6--> |
|||
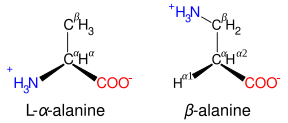
| image6 = Beta alanine comparison.svg |
|||
| alt6 = Diagrammatic comparison of the structures of β-alanine and α-alanine |
|||
| width6 = |
|||
| height6 = |
|||
| caption6 = β-alanine and its α-alanine isomer |
|||
==== β- and γ-amino acids ==== |
|||
<!--image 7--> |
|||
Amino acids with the structure {{chem2|NH3+\sCXY\sCXY\sCO2-}}, such as [[β-alanine]], a component of [[carnosine]] and a few other peptides, are β-amino acids. Ones with the structure {{chem2|NH3+\sCXY\sCXY\sCXY\sCO2-}} are γ-amino acids, and so on, where X and Y are two substituents (one of which is normally H).<ref name = iupaciub /> |
|||
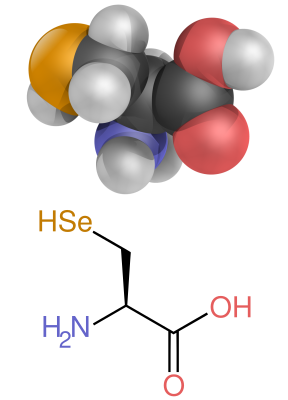
| image7 = Selenocysteine skeletal 3D.svg |
|||
| alt7 = A diagram showing the structure of selenocysteine |
|||
| width7 = |
|||
| height7 = |
|||
| caption7 = The amino acid [[selenocysteine]] |
|||
}} |
|||
===Zwitterions=== |
|||
===Proteinogenic amino acids=== |
|||
<!--[[File:Amino acid zwitterions.svg|thumb|right|An amino acid in its (1) molecular and (2) zwitterionic forms]]--> |
|||
{{main|Proteinogenic amino acids}} {{See also|Protein primary structure|Posttranslational modification}} |
|||
{{main|Zwitterion}} |
|||
[[File:Bronsted_character_of_ionizing_groups_in_proteins.png|class=skin-invert-image|thumb|upright=1.5|Ionization and Brønsted character of N-terminal amino, C-terminal carboxylate, and side chains of amino acid residues]] |
|||
Amino acids are the structural units (monomers) that make up proteins. They join together to form short [[polymer]] chains called [[peptide]]s or longer chains called either [[polypeptides]] or [[protein]]s. These polymers are linear and unbranched, with each amino acid within the chain attached to two neighboring amino acids. The process of making proteins encoded by DNA/RNA genetic material is called ''[[translation (biology)|translation]]'' and involves the step-by-step addition of amino acids to a growing protein chain by a [[ribozyme]] that is called a [[ribosome]].<ref>{{cite journal | vauthors = Rodnina MV, Beringer M, Wintermeyer W | title = How ribosomes make peptide bonds | journal = Trends in Biochemical Sciences | volume = 32 | issue = 1 | pages = 20–6 | date = January 2007 | pmid = 17157507 | doi = 10.1016/j.tibs.2006.11.007 }}</ref> The order in which the amino acids are added is read through the [[genetic code]] from an [[Messenger RNA|mRNA]] template, which is an [[RNA]] copy of one of the organism's [[gene]]s. |
|||
The common natural forms of amino acids have a [[zwitterionic]] structure, with {{chem2|\sNH3+}} ({{chem2|\sNH2+\s}} in the case of proline) and {{chem2|\sCO2-}} functional groups attached to the same C atom, and are thus α-amino acids, and are the only ones found in proteins during translation in the ribosome. |
|||
In aqueous solution at pH close to neutrality, amino acids exist as [[zwitterion]]s, i.e. as dipolar ions with both {{chem2|NH3+}} and {{chem2|CO2-}} in charged states, so the overall structure is {{chem2|NH3+\sCHR\sCO2-}}. At [[Acid–base homeostasis|physiological pH]] the so-called "neutral forms" {{chem2|\sNH2\sCHR\sCO2H}} are not present to any measurable degree.<ref>{{cite book |last1=Steinhardt |first1=J. |first2=J. A. |last2=Reynolds |title=Multiple equilibria in proteins |publisher=Academic Press |place=New York |isbn=978-0126654509| pages=176–21 |date=1969}}</ref> Although the two charges in the zwitterion structure add up to zero it is misleading to call a species with a net charge of zero "uncharged". |
|||
In strongly acidic conditions (pH below 3), the carboxylate group becomes protonated and the structure becomes an ammonio carboxylic acid, {{chem2|NH3+\sCHR\sCO2H}}. This is relevant for enzymes like pepsin that are active in acidic environments such as the mammalian stomach and [[lysosomes]], but does not significantly apply to intracellular enzymes. In highly basic conditions (pH greater than 10, not normally seen in physiological conditions), the ammonio group is deprotonated to give {{chem2|NH2\sCHR\sCO2-}}. |
|||
Twenty-two amino acids are naturally incorporated into polypeptides and are called [[proteinogenic]] or natural amino acids.<ref name="Creighton" /> Of these, 20 are encoded by the universal [[genetic code]]. The remaining 2, [[selenocysteine]] and [[pyrrolysine]], are incorporated into proteins by unique synthetic mechanisms. [[Selenocysteine]] is incorporated when the mRNA being translated includes a [[SECIS element]], which causes the UGA codon to encode selenocysteine instead of a [[stop codon]].<ref>{{cite journal | vauthors = Driscoll DM, Copeland PR | title = Mechanism and regulation of selenoprotein synthesis | journal = Annual Review of Nutrition | volume = 23 | issue = 1 | pages = 17–40 | year = 2003 | pmid = 12524431 | doi = 10.1146/annurev.nutr.23.011702.073318 }}</ref> [[Pyrrolysine]] is used by some [[methanogen]]ic [[archaea]] in enzymes that they use to produce [[methane]]. It is coded for with the codon UAG, which is normally a stop codon in other organisms.<ref>{{cite journal | vauthors = Krzycki JA | title = The direct genetic encoding of pyrrolysine | journal = Current Opinion in Microbiology | volume = 8 | issue = 6 | pages = 706–12 | date = December 2005 | pmid = 16256420 | doi = 10.1016/j.mib.2005.10.009 }}</ref> This UAG codon is followed by a [[PYLIS downstream sequence]].<ref name="pmid16164991">{{cite journal | vauthors = Théobald-Dietrich A, Giegé R, Rudinger-Thirion J | title = Evidence for the existence in mRNAs of a hairpin element responsible for ribosome dependent pyrrolysine insertion into proteins | journal = Biochimie | volume = 87 | issue = 9–10 | pages = 813–7 | year = 2005 | pmid = 16164991 | doi = 10.1016/j.biochi.2005.03.006 }}</ref> |
|||
Although various definitions of acids and bases are used in chemistry, the only one that is useful for chemistry in aqueous solution is [[Brønsted–Lowry acid–base theory|that of Brønsted]]:<ref>{{cite journal | last1 = Brønsted | first1= J. N. | journal = Recueil des Travaux Chimiques des Pays-Bas | volume = 42 | pages = 718–728 |year= 1923| title = Einige Bemerkungen über den Begriff der Säuren und Basen| issue= 8 | doi= 10.1002/recl.19230420815 |trans-title = Remarks on the concept of acids and bases}}</ref><ref name="Vollhardt-2007" /> an acid is a species that can donate a proton to another species, and a base is one that can accept a proton. This criterion is used to label the groups in the above illustration. The carboxylate side chains of aspartate and glutamate residues are the principal Brønsted bases in proteins. Likewise, lysine, tyrosine and cysteine will typically act as a Brønsted acid. Histidine under these conditions can act both as a Brønsted acid and a base. |
|||
===Non-proteinogenic amino acids=== |
|||
{{main|Non-proteinogenic amino acids}} |
|||
===Isoelectric point=== |
|||
Aside from the 22 [[proteinogenic amino acid]]s, many ''non-proteinogenic'' amino acids are known. Those either are not found in proteins (for example [[carnitine]], [[Gamma-aminobutyric acid|GABA]], [[levothyroxine]]) or are not produced directly and in isolation by standard cellular machinery (for example, [[hydroxyproline]] and [[selenomethionine]]). |
|||
[[File:Titration Curves of 20 Amino Acids Organized by Side Chain.png|class=skin-invert-image|thumb|right|upright=1.5|Composite of [[titration curve]]s of twenty proteinogenic amino acids grouped by side chain category]] |
|||
For amino acids with uncharged side-chains the zwitterion predominates at pH values between the two p''K''<sub>a</sub> values, but coexists in [[Chemical equilibrium|equilibrium]] with small amounts of net negative and net positive ions. At the midpoint between the two p''K''<sub>a</sub> values, the trace amount of net negative and trace of net positive ions balance, so that average net charge of all forms present is zero.<ref>{{cite book | vauthors = Fennema OR |title=Food Chemistry 3rd Ed |publisher=CRC Press |pages=327–328 |isbn=978-0-8247-9691-4 |date=1996-06-19 }}</ref> This pH is known as the [[isoelectric point]] p''I'', so p''I'' = {{sfrac|1|2}}(p''K''<sub>a1</sub> + p''K''<sub>a2</sub>). |
|||
Non-proteinogenic amino acids that are found in proteins are formed by [[post-translational modification]], which is modification after translation during protein synthesis. These modifications are often essential for the function or regulation of a protein. For example, the [[carboxylation]] of [[glutamate]] allows for better binding of [[calcium in biology|calcium cations]],<ref>{{cite journal | vauthors = Vermeer C | title = Gamma-carboxyglutamate-containing proteins and the vitamin K-dependent carboxylase | journal = The Biochemical Journal | volume = 266 | issue = 3 | pages = 625–36 | date = March 1990 | pmid = 2183788 | pmc = 1131186 | doi = 10.1042/bj2660625 }}</ref> and [[collagen]] contains hydroxyproline, generated by [[hydroxylation]] of [[proline]].<ref>{{cite journal | vauthors = Bhattacharjee A, Bansal M | title = Collagen structure: the Madras triple helix and the current scenario | journal = IUBMB Life | volume = 57 | issue = 3 | pages = 161–72 | date = March 2005 | pmid = 16036578 | doi = 10.1080/15216540500090710 }}</ref> Another example is the formation of [[hypusine]] in the [[Eukaryotic initiation factor|translation initiation factor]] [[EIF5A]], through modification of a lysine residue.<ref>{{cite journal | vauthors = Park MH | title = The post-translational synthesis of a polyamine-derived amino acid, hypusine, in the eukaryotic translation initiation factor 5A (eIF5A) | journal = Journal of Biochemistry | volume = 139 | issue = 2 | pages = 161–9 | date = February 2006 | pmid = 16452303 | pmc = 2494880 | doi = 10.1093/jb/mvj034 }}</ref> Such modifications can also determine the localization of the protein, e.g., the addition of long hydrophobic groups can cause a protein to bind to a [[phospholipid]] membrane.<ref>{{cite journal | vauthors = Blenis J, Resh MD | title = Subcellular localization specified by protein acylation and phosphorylation | journal = Current Opinion in Cell Biology | volume = 5 | issue = 6 | pages = 984–9 | date = December 1993 | pmid = 8129952 | doi = 10.1016/0955-0674(93)90081-Z }}</ref> |
|||
For amino acids with charged side chains, the p''K''<sub>a</sub> of the side chain is involved. Thus for aspartate or glutamate with negative side chains, the terminal amino group is essentially entirely in the charged form {{chem2|\sNH3+}}, but this positive charge needs to be balanced by the state with just one C-terminal carboxylate group is negatively charged. This occurs halfway between the two carboxylate p''K''<sub>a</sub> values: p''I'' = {{sfrac|1|2}}(p''K''<sub>a1</sub> + p''K''<sub>a(R)</sub>), where p''K''<sub>a(R)</sub> is the side chain p''K''<sub>a</sub>.<ref name="Vollhardt-2007">{{Cite book |last=Vollhardt |first=K. Peter C. |title=Organic chemistry : structure and function |date=2007 |publisher=W.H. Freeman |others=Neil Eric Schore |isbn=978-0-7167-9949-8 |edition=5th |location=New York |pages=58–66 |oclc=61448218}}</ref> |
|||
Some non-proteinogenic amino acids are not found in proteins. Examples include [[2-aminoisobutyric acid]] and the neurotransmitter [[gamma-aminobutyric acid]]. Non-proteinogenic amino acids often occur as intermediates in the [[metabolic pathway]]s for standard amino acids – for example, [[ornithine]] and [[citrulline]] occur in the [[urea cycle]], part of amino acid [[catabolism]] (see below).<ref>{{cite journal | vauthors = Curis E, Nicolis I, Moinard C, Osowska S, Zerrouk N, Bénazeth S, Cynober L | title = Almost all about citrulline in mammals | journal = Amino Acids | volume = 29 | issue = 3 | pages = 177–205 | date = November 2005 | pmid = 16082501 | doi = 10.1007/s00726-005-0235-4 }}</ref> A rare exception to the dominance of α-amino acids in biology is the β-amino acid [[beta alanine]] (3-aminopropanoic acid), which is used in plants and microorganisms in the synthesis of [[pantothenic acid]] (vitamin B<sub>5</sub>), a component of [[coenzyme A]].<ref>{{cite journal | vauthors = Coxon KM, Chakauya E, Ottenhof HH, Whitney HM, Blundell TL, Abell C, Smith AG | title = Pantothenate biosynthesis in higher plants | journal = Biochemical Society Transactions | volume = 33 | issue = Pt 4 | pages = 743–6 | date = August 2005 | pmid = 16042590 | doi = 10.1042/BST0330743 }}</ref> |
|||
Similar considerations apply to other amino acids with ionizable side-chains, including not only glutamate (similar to aspartate), but also cysteine, histidine, lysine, tyrosine and arginine with positive side chains. |
|||
===<small>D</small>-amino acid natural abundance=== |
|||
{{update section|date=July 2019}}<!-- see talkpage note, recommending [[doi:10.1007/s00726-017-2459-5]] --> |
|||
<small>Although D</small>-isomers are uncommon in live organisms, [[gramicidin]] is a polypeptide made up from mixture of <small>D</small>- and <small>L</small>-amino acids.<ref>{{cite journal | vauthors = Ketchem RR, Hu W, Cross TA | title = High-resolution conformation of gramicidin A in a lipid bilayer by solid-state NMR | journal = Science | volume = 261 | issue = 5127 | pages = 1457–60 | date = September 1993 | pmid = 7690158 | doi = 10.1126/science.7690158 | bibcode = 1993Sci...261.1457K }}</ref> Other compounds containing <small>D</small>-amino acids are [[tyrocidine]] and [[valinomycin]]. These compounds disrupt bacterial cell walls, particularly in [[Gram-positive]] bacteria. {{As of|2011}}, only 837 <small>D</small>-amino acids were found in the [[Swiss-Prot]] database out of a total of 187 million amino acids analysed.<ref>{{cite journal | vauthors = Khoury GA, Baliban RC, Floudas CA | title = Proteome-wide post-translational modification statistics: frequency analysis and curation of the swiss-prot database | journal = Scientific Reports | volume = 1 | issue = 90 | pages = 90 | date = September 2011 | pmid = 22034591 | pmc = 3201773 | doi = 10.1038/srep00090 | bibcode = 2011NatSR...1E..90K }}</ref> |
|||
Amino acids have zero mobility in [[electrophoresis]] at their isoelectric point, although this behaviour is more usually exploited for peptides and proteins than single amino acids. Zwitterions have minimum solubility at their isoelectric point, and some amino acids (in particular, with nonpolar side chains) can be isolated by precipitation from water by adjusting the pH to the required isoelectric point. |
|||
===Non-standard amino acids=== |
|||
The 20 amino acids that are encoded directly by the codons of the universal [[genetic code]] are called ''standard'' or ''canonical'' amino acids. A modified form of methionine ([[N-Formylmethionine|''N''-formylmethionine]]) is often incorporated in place of methionine as the initial amino acid of proteins in bacteria, mitochondria and chloroplasts. Other amino acids are called ''non-standard'' or ''non-canonical''. Most of the non-standard amino acids are also non-proteinogenic (i.e. they cannot be incorporated into proteins during translation), but two of them are proteinogenic, as they can be incorporated translationally into proteins by exploiting information not encoded in the universal genetic code. |
|||
==Physicochemical properties== |
|||
The two non-standard proteinogenic amino acids are [[selenocysteine]] (present in many non-eukaryotes as well as most eukaryotes, but not coded directly by DNA) and [[pyrrolysine]] (found only in some [[archaea]] and one [[bacterium]]). The incorporation of these non-standard amino acids is rare. For example, 25 human proteins include selenocysteine (Sec) in their primary structure,<ref>{{cite journal | vauthors = Kryukov GV, Castellano S, Novoselov SV, Lobanov AV, Zehtab O, Guigó R, Gladyshev VN | title = Characterization of mammalian selenoproteomes | journal = Science | volume = 300 | issue = 5624 | pages = 1439–43 | date = May 2003 | pmid = 12775843 | doi = 10.1126/science.1083516 | bibcode = 2003Sci...300.1439K | url = http://digitalcommons.unl.edu/cgi/viewcontent.cgi?article=1072&context=biochemgladyshev }}</ref> and the structurally characterized enzymes (selenoenzymes) employ Sec as the catalytic [[moiety (chemistry)|moiety]] in their active sites.<ref>{{cite journal | vauthors = Gromer S, Urig S, Becker K | title = The thioredoxin system—from science to clinic | journal = Medicinal Research Reviews | volume = 24 | issue = 1 | pages = 40–89 | date = January 2004 | pmid = 14595672 | doi = 10.1002/med.10051 }}</ref> Pyrrolysine and selenocysteine are encoded via variant codons. For example, selenocysteine is encoded by [[stop codon]] and [[SECIS element]].<ref name="books.google.com"/><ref name="VoJw6fIISSkC p.299"/><ref name="url_The_Genetic_Codes_NCBI" /> |
|||
The 20 canonical amino acids can be classified according to their properties. Important factors are charge, [[hydrophilicity]] or [[hydrophobicity]], size, and functional groups.<ref name="Creighton" /> These properties influence [[protein structure]] and [[protein–protein interaction]]s. The water-soluble proteins tend to have their hydrophobic residues ([[leucine|Leu]], [[isoleucine|Ile]], [[valine|Val]], [[phenylalanine|Phe]], and [[tryptophan|Trp]]) buried in the middle of the protein, whereas hydrophilic side chains are exposed to the aqueous solvent. (In [[biochemistry]], a residue refers to a specific [[monomer]] ''within'' the [[polymer]]ic chain of a [[polysaccharide]], protein or [[nucleic acid]].) The [[integral membrane protein]]s tend to have outer rings of exposed [[hydrophobic]] amino acids that anchor them in the [[lipid bilayer]]. Some [[peripheral membrane protein]]s have a patch of hydrophobic amino acids on their surface that sticks to the membrane. In a similar fashion, proteins that have to bind to positively charged molecules have surfaces rich in negatively charged amino acids such as [[glutamate]] and [[aspartate]], while proteins binding to negatively charged molecules have surfaces rich in positively charged amino acids like [[lysine]] and [[arginine]]. For example, lysine and arginine are present in large amounts in the [[Low complexity regions in proteins|low-complexity regions]] of nucleic-acid binding proteins.<ref name="Ntountoumi-2019">{{cite journal | vauthors = Ntountoumi C, Vlastaridis P, Mossialos D, Stathopoulos C, Iliopoulos I, Promponas V, Oliver SG, Amoutzias GD | display-authors = 6 | title = Low complexity regions in the proteins of prokaryotes perform important functional roles and are highly conserved | journal = Nucleic Acids Research | volume = 47 | issue = 19 | pages = 9998–10009 | date = November 2019 | pmid = 31504783 | pmc = 6821194 | doi = 10.1093/nar/gkz730 }}</ref> There are various [[hydrophobicity scale]]s of amino acid residues.<ref>{{cite journal| vauthors = Urry DW | title = The change in Gibbs free energy for hydrophobic association: Derivation and evaluation by means of inverse temperature transitions | journal = Chemical Physics Letters | volume = 399 | issue = 1–3 | pages = 177–183 | year = 2004 | doi = 10.1016/S0009-2614(04)01565-9 | bibcode = 2004CPL...399..177U }}</ref> |
|||
Some amino acids have special properties. Cysteine can form covalent [[disulfide bond]]s to other cysteine residues. [[Proline]] forms [[cyclic compound|a cycle]] to the polypeptide backbone, and glycine is more flexible than other amino acids. |
|||
===In human nutrition=== |
|||
[[File:Amino acids in food and blood.png|thumb|right|380px|Share of amino acid in different human diets and the resulting mix of amino acids in human blood serum. Glutamate and glutamine are the most frequent in food at over 10%, while alanine, glutamine, and glycine are the most common in blood.|alt=Diagram showing the relative occurrence of different amino acids in blood serum as obtained from different diets]] |
|||
{{Main|Essential amino acids}} |
|||
{{further|Protein (nutrient)|Amino acid synthesis}} |
|||
Glycine and proline are strongly present within low complexity regions of both eukaryotic and prokaryotic proteins, whereas the opposite is the case with cysteine, phenylalanine, tryptophan, methionine, valine, leucine, isoleucine, which are highly reactive, or complex, or hydrophobic.<ref name="Ntountoumi-2019" /><ref>{{cite journal | vauthors = Marcotte EM, Pellegrini M, Yeates TO, Eisenberg D | title = A census of protein repeats | journal = Journal of Molecular Biology | volume = 293 | issue = 1 | pages = 151–60 | date = October 1999 | pmid = 10512723 | doi = 10.1006/jmbi.1999.3136 }}</ref><ref>{{cite journal | vauthors = Haerty W, Golding GB | title = Low-complexity sequences and single amino acid repeats: not just "junk" peptide sequences | journal = Genome | volume = 53 | issue = 10 | pages = 753–62 | date = October 2010 | pmid = 20962881 | doi = 10.1139/G10-063 | veditors = Bonen L }}</ref> |
|||
When taken up into the human body from the diet, the 20 standard amino acids either are used to synthesize proteins, other biomolecules, or are oxidized to [[urea]] and [[carbon dioxide]] as a source of energy.<ref>{{cite journal | vauthors = Sakami W, Harrington H | title = Amino acid metabolism | journal = Annual Review of Biochemistry | volume = 32 | issue = 1 | pages = 355–98 | year = 1963 | pmid = 14144484 | doi = 10.1146/annurev.bi.32.070163.002035 }}</ref> The oxidation pathway starts with the removal of the amino group by a [[transaminase]]; the amino group is then fed into the [[urea cycle]]. The other product of transamidation is a [[keto acid]] that enters the [[citric acid cycle]].<ref>{{cite journal | vauthors = Brosnan JT | title = Glutamate, at the interface between amino acid and carbohydrate metabolism | journal = The Journal of Nutrition | volume = 130 | issue = 4S Suppl | pages = 988S–90S | date = April 2000 | pmid = 10736367 | url = http://jn.nutrition.org/content/130/4/988S.full | doi = 10.1093/jn/130.4.988S }}</ref> [[Glucogenic amino acid]]s can also be converted into glucose, through [[gluconeogenesis]].<ref>{{cite journal | vauthors = Young VR, Ajami AM | title = Glutamine: the emperor or his clothes? | journal = The Journal of Nutrition | volume = 131 | issue = 9 Suppl | pages = 2449S–59S; discussion 2486S–7S | date = September 2001 | pmid = 11533293 | url = http://jn.nutrition.org/cgi/pmidlookup?view=long&pmid=11533293 | doi = 10.1093/jn/131.9.2449S }}</ref> Of the 20 standard amino acids, nine ([[Histidine|His]], [[Isoleucine|Ile]], [[Leucine|Leu]], [[Lysine|Lys]], [[Methionine|Met]], [[Phenylalanine|Phe]], [[Threonine|Thr]], [[Tryptophan|Trp]] and [[Valine|Val]]) are called [[essential amino acid]]s because the [[human body]] cannot [[biosynthesis|synthesize]] them from other [[chemical compound|compounds]] at the level needed for normal growth, so they must be obtained from food.<ref>{{cite journal | vauthors = Young VR | title = Adult amino acid requirements: the case for a major revision in current recommendations | journal = The Journal of Nutrition | volume = 124 | issue = 8 Suppl | pages = 1517S–1523S | date = August 1994 | pmid = 8064412 | url = http://jn.nutrition.org/cgi/pmidlookup?view=long&pmid=8064412 | doi = 10.1093/jn/124.suppl_8.1517S }}</ref><ref>{{cite journal | vauthors = Fürst P, Stehle P | title = What are the essential elements needed for the determination of amino acid requirements in humans? | journal = The Journal of Nutrition | volume = 134 | issue = 6 Suppl | pages = 1558S–1565S | date = June 2004 | pmid = 15173430 | url = http://jn.nutrition.org/cgi/pmidlookup?view=long&pmid=15173430 | doi = 10.1093/jn/134.6.1558S }}</ref><ref>{{cite journal | vauthors = Reeds PJ | title = Dispensable and indispensable amino acids for humans | journal = The Journal of Nutrition | volume = 130 | issue = 7 | pages = 1835S–40S | date = July 2000 | pmid = 10867060 | url = http://jn.nutrition.org/cgi/pmidlookup?view=long&pmid=10867060 | doi = 10.1093/jn/130.7.1835S }}</ref> In addition, [[cysteine]], [[taurine]], [[tyrosine]], and [[arginine]] are considered semiessential amino-acids in children (though taurine is not technically an amino acid), because the metabolic pathways that synthesize these amino acids are not fully developed.<ref>{{cite journal | vauthors = Imura K, Okada A | title = Amino acid metabolism in pediatric patients | journal = Nutrition | volume = 14 | issue = 1 | pages = 143–8 | date = January 1998 | pmid = 9437700 | doi = 10.1016/S0899-9007(97)00230-X }}</ref><ref>{{cite journal | vauthors = Lourenço R, Camilo ME | title = Taurine: a conditionally essential amino acid in humans? An overview in health and disease | journal = Nutricion Hospitalaria | volume = 17 | issue = 6 | pages = 262–70 | year = 2002 | pmid = 12514918 }}</ref> The amounts required also depend on the age and health of the individual, so it is hard to make general statements about the dietary requirement for some amino acids. Dietary exposure to the non-standard amino acid [[beta-Methylamino-L-alanine|BMAA]] has been linked to human neurodegenerative diseases, including [[ALS]].<ref name="Holtcamp">{{cite journal | vauthors = Holtcamp W | title = The emerging science of BMAA: do cyanobacteria contribute to neurodegenerative disease? | journal = Environmental Health Perspectives | volume = 120 | issue = 3 | pages = A110–6 | date = March 2012 | pmid = 22382274 | pmc = 3295368 | doi = 10.1289/ehp.120-a110 }}</ref><ref name="Cox and Davis">{{cite journal | vauthors = Cox PA, Davis DA, Mash DC, Metcalf JS, Banack SA | title = Dietary exposure to an environmental toxin triggers neurofibrillary tangles and amyloid deposits in the brain | journal = Proceedings: Biological Sciences | volume = 283 | issue = 1823 | pages = 20152397 | date = January 2016 | pmid = 26791617 | pmc = 4795023 | doi = 10.1098/rspb.2015.2397 }}</ref> |
|||
{{multiple image |
|||
<!-- Layout parameters --> |
|||
| align = center |
|||
| direction = horizontal |
|||
| total_width = 800 |
|||
<!-- Header --> |
|||
| header_align = <!-- center (default), left, right --> |
|||
| header = |
|||
<!--image 1--> |
|||
| image1 = Muscle protein synthesis signaling cascades.jpg |
|||
| alt1 = Signaling cascade diagram |
|||
| width1 = 619 |
|||
| height1 = 347 |
|||
| caption1 = Diagram of the molecular [[signaling cascade]]s that are involved in [[myofibrillar]] muscle protein synthesis and [[mitochondrial biogenesis]] in response to physical exercise and specific amino acids or their derivatives (primarily [[leucine|{{nowrap|<small>L</small>-leucine}}]] and [[Beta-Hydroxy beta-methylbutyric acid|HMB]]).<ref name="Skeletal muscle homeostasis 2016 review">{{cite journal | vauthors = Brook MS, Wilkinson DJ, Phillips BE, Perez-Schindler J, Philp A, Smith K, Atherton PJ | title = Skeletal muscle homeostasis and plasticity in youth and ageing: impact of nutrition and exercise | journal = Acta Physiologica | volume = 216 | issue = 1 | pages = 15–41 | date = January 2016 | pmid = 26010896 | pmc = 4843955 | doi = 10.1111/apha.12532 }}</ref> Many amino acids derived from food protein promote the activation of [[mTORC1]] and increase [[protein translation|protein synthesis]] by [[signal transduction|signaling]] through [[Rag GTPase]]s.<ref name="Skeletal muscle homeostasis 2016 review" /><ref name="The neurology of mTOR">{{cite journal | vauthors = Lipton JO, Sahin M | title = The neurology of mTOR | journal = Neuron | volume = 84 | issue = 2 | pages = 275–91 | date = October 2014 | pmid = 25374355 | pmc = 4223653 | doi = 10.1016/j.neuron.2014.09.034 }}<br />[https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4223653/figure/F2/ Figure 2: The mTOR Signaling Pathway]</ref><br />{{hidden|Abbreviations and representations:|{{bull}}PLD: [[phospholipase D]]<br />{{bull}}PA: [[phosphatidic acid]]<br />{{bull}}mTOR: [[mechanistic target of rapamycin]]<br />{{bull}}AMP: [[adenosine monophosphate]]<br />{{bull}}ATP: [[adenosine triphosphate]]<br />{{bull}}AMPK: [[AMP-activated protein kinase]]<br />{{bull}}PGC‐1α: [[PGC-1α|peroxisome proliferator-activated receptor gamma coactivator-1α]]<br />{{bull}}S6K1: [[p70S6 kinase]]<br />{{bull}}4EBP1: [[EIF4EBP1|eukaryotic translation initiation factor 4E-binding protein 1]]<br />{{bull}}eIF4E: [[eukaryotic translation initiation factor 4E]]<br />{{bull}}RPS6: [[ribosomal protein S6]]<br />{{bull}}eEF2: [[eukaryotic elongation factor 2]]<br />{{bull}}RE: resistance exercise; EE: endurance exercise<br />{{bull}}Myo: [[myofibrillar]]; Mito: [[Mitochondrion|mitochondria]]l<br />{{bull}}AA: amino acids<br />{{bull}}HMB: [[β-hydroxy β-methylbutyric acid]]<br />{{bull}}↑ represents activation<br />{{bull}}Τ represents inhibition | headerstyle=background:#ccccff | style=text-align:center; }} |
|||
<!--image 2--> |
|||
| image2 = Resistance exercise-induced muscle protein synthesis.jpg |
|||
| alt2 = Graph of muscle protein synthesis vs time |
|||
| width2 = 387 |
|||
| height2 = 221 |
|||
| caption2 = Resistance training stimulates muscle protein synthesis (MPS) for a period of up to 48 hours following exercise (shown by lighter dotted line).<ref name="Muscle hypertrophy review" /> Ingestion of a protein-rich meal at any point during this period will augment the exercise-induced increase in muscle protein synthesis (shown by solid lines).<ref name="Muscle hypertrophy review">{{cite journal | vauthors = Phillips SM | title = A brief review of critical processes in exercise-induced muscular hypertrophy | journal = Sports Medicine | volume = 44 Suppl 1 | issue = | pages = S71–7 | date = May 2014 | pmid = 24791918 | pmc = 4008813 | doi = 10.1007/s40279-014-0152-3 }}</ref> |
|||
}} |
|||
Many proteins undergo a range of [[posttranslational modification]]s, whereby additional chemical groups are attached to the amino acid residue side chains sometimes producing [[lipoprotein]]s (that are hydrophobic),<ref>{{cite journal | vauthors = Magee T, Seabra MC | title = Fatty acylation and prenylation of proteins: what's hot in fat | journal = Current Opinion in Cell Biology | volume = 17 | issue = 2 | pages = 190–196 | date = April 2005 | pmid = 15780596 | doi = 10.1016/j.ceb.2005.02.003 }}</ref> or [[glycoprotein]]s (that are hydrophilic)<ref>{{cite journal | vauthors = Pilobello KT, Mahal LK | title = Deciphering the glycocode: the complexity and analytical challenge of glycomics | journal = Current Opinion in Chemical Biology | volume = 11 | issue = 3 | pages = 300–305 | date = June 2007 | pmid = 17500024 | doi = 10.1016/j.cbpa.2007.05.002 }}</ref> allowing the protein to attach temporarily to a membrane. For example, a signaling protein can attach and then detach from a cell membrane, because it contains cysteine residues that can have the fatty acid [[palmitic acid]] added to them and subsequently removed.<ref>{{cite journal | vauthors = Smotrys JE, Linder ME | title = Palmitoylation of intracellular signaling proteins: regulation and function | journal = Annual Review of Biochemistry | volume = 73 | issue = 1 | pages = 559–587 | year = 2004 | pmid = 15189153 | doi = 10.1146/annurev.biochem.73.011303.073954 }}</ref> |
|||
===Non-protein functions=== |
|||
{{Catecholamine and trace amine biosynthesis|align=right|caption=[[Catecholamine]]s and [[trace amine]]s are synthesized from phenylalanine and tyrosine in humans.}} |
|||
{{Further|Amino acid neurotransmitter}} |
|||
===Table of standard amino acid abbreviations and properties=== |
|||
In humans, non-protein amino acids also have important roles as [[metabolic intermediate]]s, such as in the biosynthesis of the [[neurotransmitter]] [[gamma-amino-butyric acid]] (GABA). Many amino acids are used to synthesize other molecules, for example: |
|||
{{Redirect|Amino acid code|base-pair encoding of amino acids|Genetic code#Codons}} |
|||
* [[Tryptophan]] is a precursor of the neurotransmitter [[serotonin]].<ref>{{cite journal | vauthors = Savelieva KV, Zhao S, Pogorelov VM, Rajan I, Yang Q, Cullinan E, Lanthorn TH | title = Genetic disruption of both tryptophan hydroxylase genes dramatically reduces serotonin and affects behavior in models sensitive to antidepressants | journal = PLOS ONE | volume = 3 | issue = 10 | pages = e3301 | year = 2008 | pmid = 18923670 | pmc = 2565062 | doi = 10.1371/journal.pone.0003301 | editor1-last = Bartolomucci | bibcode = 2008PLoSO...3.3301S | editor1-first = Alessandro }}</ref> |
|||
{{Main|Proteinogenic amino acid}} |
|||
* [[Tyrosine]] (and its precursor phenylalanine) are precursors of the [[catecholamine]] [[neurotransmitter]]s [[dopamine]], [[epinephrine]] and [[norepinephrine]] and various [[trace amine]]s. |
|||
* [[Phenylalanine]] is a precursor of [[phenethylamine]] and tyrosine in humans. In plants, it is a precursor of various [[phenylpropanoid]]s, which are important in plant metabolism. |
|||
* [[Glycine]] is a precursor of [[porphyrin]]s such as [[heme]].<ref>{{cite journal | vauthors = Shemin D, Rittenberg D | title = The biological utilization of glycine for the synthesis of the protoporphyrin of hemoglobin | journal = The Journal of Biological Chemistry | volume = 166 | issue = 2 | pages = 621–5 | date = December 1946 | pmid = 20276176 | url = http://www.jbc.org/cgi/reprint/166/2/621 }}</ref> |
|||
* [[Arginine]] is a precursor of [[nitric oxide]].<ref>{{cite journal | vauthors = Tejero J, Biswas A, Wang ZQ, Page RC, Haque MM, Hemann C, Zweier JL, Misra S, Stuehr DJ | title = Stabilization and characterization of a heme-oxy reaction intermediate in inducible nitric-oxide synthase | journal = The Journal of Biological Chemistry | volume = 283 | issue = 48 | pages = 33498–507 | date = November 2008 | pmid = 18815130 | pmc = 2586280 | doi = 10.1074/jbc.M806122200 }}</ref> |
|||
* [[Ornithine]] and [[S-Adenosyl methionine|S-adenosylmethionine]] are precursors of [[polyamine]]s.<ref>{{cite journal | vauthors = Rodríguez-Caso C, Montañez R, Cascante M, Sánchez-Jiménez F, Medina MA | title = Mathematical modeling of polyamine metabolism in mammals | journal = The Journal of Biological Chemistry | volume = 281 | issue = 31 | pages = 21799–812 | date = August 2006 | pmid = 16709566 | doi = 10.1074/jbc.M602756200 }}</ref> |
|||
* [[Aspartate]], [[glycine]], and [[glutamine]] are precursors of [[nucleotide]]s.<ref name = "Stryer_2002">{{cite book | last1 = Stryer | first1 = Lubert | last2 = Berg | first2 = Jeremy M. | last3 = Tymoczko | first3 = John L. | name-list-format = vanc | title = Biochemistry | date = 2002 | publisher = W.H. Freeman | location = New York | isbn = 978-0-7167-4684-3 | edition = 5th | pages = 693–8 }}</ref> However, not all of the functions of other abundant non-standard amino acids are known. |
|||
Although one-letter symbols are included in the table, IUPAC–IUBMB recommend<ref name = iupaciub/> that "Use of the one-letter symbols should be restricted to the comparison of long sequences". |
|||
Some non-standard amino acids are used as [[Plant defense against herbivory|defenses against herbivores]] in plants.<ref name="Hylin1969">{{Cite journal|last=Hylin |first=John W. |year=1969 |title=Toxic peptides and amino acids in foods and feeds |journal=Journal of Agricultural and Food Chemistry |volume=17 |issue=3 |pages=492–6 |doi=10.1021/jf60163a003}}</ref> For example, [[canavanine]] is an analogue of [[arginine]] that is found in many [[legume]]s,<ref name="Turner1967">{{cite journal| vauthors = Turner BL, Harborne JB | year = 1967 | title = Distribution of canavanine in the plant kingdom | journal = Phytochemistry | volume = 6 | issue = 6 | pages = 863–66 | doi = 10.1016/S0031-9422(00)86033-1 }}</ref> and in particularly large amounts in ''[[Canavalia gladiata]]'' (sword bean).<ref>{{cite journal | vauthors = Ekanayake S, Skog K, Asp NG | title = Canavanine content in sword beans (Canavalia gladiata): analysis and effect of processing | journal = Food and Chemical Toxicology | volume = 45 | issue = 5 | pages = 797–803 | date = May 2007 | pmid = 17187914 | doi = 10.1016/j.fct.2006.10.030 }}</ref> This amino acid protects the plants from predators such as insects and can cause illness in people if some types of legumes are eaten without processing.<ref>{{cite journal | vauthors = Rosenthal GA | title = L-Canavanine: a higher plant insecticidal allelochemical | journal = Amino Acids | volume = 21 | issue = 3 | pages = 319–30 | year = 2001 | pmid = 11764412 | doi = 10.1007/s007260170017 }}</ref> The non-protein amino acid [[mimosine]] is found in other species of legume, in particular ''[[Leucaena leucocephala]]''.<ref>{{cite journal|vauthors=Hammond AC |title=Leucaena toxicosis and its control in ruminants |journal=Journal of Animal Science |volume=73 |issue=5 |pages=1487–92 |date=May 1995 |pmid=7665380 |url=http://jas.fass.org/cgi/pmidlookup?view=long&pmid=7665380 |doi=10.2527/1995.7351487x }}{{dead link|date=July 2017 |bot=InternetArchiveBot |fix-attempted=yes }}</ref> This compound is an analogue of [[tyrosine]] and can poison animals that graze on these plants. |
|||
The one-letter notation was chosen by IUPAC-IUB based on the following rules:<ref name="IUPAC-1968">{{Cite journal |date=10 July 1968 |title=IUPAC-IUB Commission on Biochemical Nomenclature A One-Letter Notation for Amino Acid Sequences |journal=Journal of Biological Chemistry |language=en |volume=243 |issue=13 |pages=3557–3559 |doi=10.1016/S0021-9258(19)34176-6|doi-access=free }}</ref> |
|||
==Uses in industry== |
|||
Amino acids are used for a variety of applications in industry, but their main use is as additives to [[Compound feed|animal feed]]. This is necessary, since many of the bulk components of these feeds, such as [[soybean]]s, either have low levels or lack some of the [[essential amino acid]]s: lysine, methionine, threonine, and tryptophan are most important in the production of these feeds.<ref name=Leuchtenberger2005>{{cite journal | vauthors = Leuchtenberger W, Huthmacher K, Drauz K | title = Biotechnological production of amino acids and derivatives: current status and prospects | journal = Applied Microbiology and Biotechnology | volume = 69 | issue = 1 | pages = 1–8 | date = November 2005 | pmid = 16195792 | doi = 10.1007/s00253-005-0155-y }}</ref> In this industry, amino acids are also used to chelate metal cations in order to improve the absorption of minerals from supplements, which may be required to improve the health or production of these animals.<ref>{{cite book|last=Ashmead|first=H. DeWayne | name-list-format = vanc |title=The Role of Amino Acid Chelates in Animal Nutrition|year=1993|publisher=Noyes Publications|location=Westwood}}</ref> |
|||
* Initial letters are used where there is no ambuiguity: C cysteine, H histidine, I isoleucine, M methionine, S serine, V valine,<ref name="IUPAC-1968" /> |
|||
The [[food industry]] is also a major consumer of amino acids, in particular, [[glutamic acid]], which is used as a [[flavor enhancer]],<ref name=Garattini>{{cite journal | vauthors = Garattini S | title = Glutamic acid, twenty years later | journal = The Journal of Nutrition | volume = 130 | issue = 4S Suppl | pages = 901S–9S | date = April 2000 | pmid = 10736350 | url = http://jn.nutrition.org/cgi/pmidlookup?view=long&pmid=10736350 | doi = 10.1093/jn/130.4.901S }}</ref> and [[aspartame]] (aspartyl-phenylalanine-1-methyl ester) as a low-calorie [[artificial sweetener]].<ref>{{cite journal | vauthors = Stegink LD | title = The aspartame story: a model for the clinical testing of a food additive | journal = The American Journal of Clinical Nutrition | volume = 46 | issue = 1 Suppl | pages = 204–15 | date = July 1987 | pmid = 3300262 | url = http://www.ajcn.org/cgi/pmidlookup?view=long&pmid=3300262 | doi = 10.1093/ajcn/46.1.204 }}</ref> Similar technology to that used for animal nutrition is employed in the human nutrition industry to alleviate symptoms of mineral deficiencies, such as anemia, by improving mineral absorption and reducing negative side effects from inorganic mineral supplementation.<ref>{{cite web|last=Albion Laboratories, Inc.|title=Albion Ferrochel Website|url=http://www.albionferrochel.com|accessdate=12 July 2011}}</ref> |
|||
* Where arbitrary assignment is needed, the structurally simpler amino acids are given precedence: A Alanine, G glycine, L leucine, P proline, T threonine,<ref name="IUPAC-1968" /> |
|||
* F ''PH''enylalanine and R a''R''ginine are assigned by being phonetically suggestive,<ref name="IUPAC-1968" /> |
|||
The chelating ability of amino acids has been used in fertilizers for agriculture to facilitate the delivery of minerals to plants in order to correct mineral deficiencies, such as iron chlorosis. These fertilizers are also used to prevent deficiencies from occurring and improving the overall health of the plants.<ref>{{cite book|last=Ashmead|first=H. DeWayne | name-list-format = vanc |title=Foliar Feeding of Plants with Amino Acid Chelates|year=1986|publisher=Noyes Publications|location=Park Ridge}}</ref> The remaining production of amino acids is used in the synthesis of [[Pharmaceutical drug|drugs]] and [[cosmetics]].<ref name=Leuchtenberger2005/> |
|||
* W tryptophan is assigned based on the double ring being visually suggestive to the bulky letter W,<ref name="IUPAC-1968" /> |
|||
* K lysine and Y tyrosine are assigned as alphabetically nearest to their initials L and T (note that U was avoided for its similarity with V, while X was reserved for undetermined or atypical amino acids); for tyrosine the mnemonic t''Y''rosine was also proposed,<ref name="Saffran-1998">{{Cite journal |last=Saffran |first=M. |date=April 1998 |title=Amino acid names and parlor games: from trivial names to a one-letter code, amino acid names have strained students' memories. Is a more rational nomenclature possible? |journal=Biochemical Education |language=en |volume=26 |issue=2 |pages=116–118 |doi=10.1016/S0307-4412(97)00167-2}}</ref> |
|||
Similarly, some amino acids derivatives are used in pharmaceutical industry. They include [[5-HTP]] (5-hydroxytryptophan) used for experimental treatment of depression,<ref>{{cite journal | vauthors = Turner EH, Loftis JM, Blackwell AD | title = Serotonin a la carte: supplementation with the serotonin precursor 5-hydroxytryptophan | journal = Pharmacology & Therapeutics | volume = 109 | issue = 3 | pages = 325–38 | date = March 2006 | pmid = 16023217 | doi = 10.1016/j.pharmthera.2005.06.004 | url = https://escholarship.org/uc/item/58h866d5 }}</ref> [[L-DOPA|<small>L</small>-DOPA]] (<small>L</small>-dihydroxyphenylalanine) for [[Parkinson's]] treatment,<ref>{{cite journal | vauthors = Kostrzewa RM, Nowak P, Kostrzewa JP, Kostrzewa RA, Brus R | title = Peculiarities of L: -DOPA treatment of Parkinson's disease | journal = Amino Acids | volume = 28 | issue = 2 | pages = 157–64 | date = March 2005 | pmid = 15750845 | doi = 10.1007/s00726-005-0162-4 }}</ref> and [[eflornithine]] drug that inhibits [[ornithine decarboxylase]] and used in the treatment of [[African trypanosomiasis|sleeping sickness]].<ref>{{cite journal | vauthors = Heby O, Persson L, Rentala M | title = Targeting the polyamine biosynthetic enzymes: a promising approach to therapy of African sleeping sickness, Chagas' disease, and leishmaniasis | journal = Amino Acids | volume = 33 | issue = 2 | pages = 359–66 | date = August 2007 | pmid = 17610127 | doi = 10.1007/s00726-007-0537-9 }}</ref> |
|||
* D aspartate was assigned arbitrarily, with the proposed mnemonic aspar''D''ic acid;<ref name="Adoga-1988">{{Cite journal |last=Adoga |first=Godwin I |last2=Nicholson |first2=Bh |date=January 1988 |title=Letters to the editor |journal=Biochemical Education |language=en |volume=16 |issue=1 |pages=49 |doi=10.1016/0307-4412(88)90026-X}}</ref> E glutamate was assigned in alphabetical sequence being larger by merely one [[Methylene group|methylene]] –CH<sub>2</sub>– group,<ref name="Saffran-1998" /> |
|||
* N asparagine was assigned arbitrarily, with the proposed mnemonic asparagi''N''e;<ref name="Adoga-1988" /> Q glutamine was assigned in alphabetical sequence of those still available (note again that O was avoided due to similarity with D), with the proposed mnemonic ''Q''lutamine.<ref name="Adoga-1988" /> |
|||
===Expanded genetic code=== |
|||
{{Main|Expanded genetic code}} |
|||
Since 2001, 40 non-natural amino acids have been added into protein by creating a unique codon (recoding) and a corresponding transfer-RNA:aminoacyl – tRNA-synthetase pair to encode it with diverse physicochemical and biological properties in order to be used as a tool to exploring [[protein structure]] and function or to create novel or enhanced proteins.<ref name="pmid16260173"/><ref name="pmid19318213"/> |
|||
===Nullomers=== |
|||
{{Main|Nullomers}} |
|||
Nullomers are codons that in theory code for an amino acid, however in nature there is a selective bias against using this codon in favor of another, for example bacteria prefer to use CGA instead of AGA to code for arginine.<ref>{{cite journal | vauthors = Cruz-Vera LR, Magos-Castro MA, Zamora-Romo E, Guarneros G | title = Ribosome stalling and peptidyl-tRNA drop-off during translational delay at AGA codons | journal = Nucleic Acids Research | volume = 32 | issue = 15 | pages = 4462–8 | year = 2004 | pmid = 15317870 | pmc = 516057 | doi = 10.1093/nar/gkh784 }}</ref> This creates some sequences that do not appear in the genome. This characteristic can be taken advantage of and used to create new selective cancer-fighting drugs<ref>{{cite web|url=https://www.newscientist.com/article/dn22424-molecules-too-dangerous-for-nature-kill-cancer-cells.html|title=Molecules 'too dangerous for nature' kill cancer cells|work=New Scientist|date= October 2012| first = Coghlan | last = Andy | name-list-format = vanc }}</ref> and to prevent cross-contamination of DNA samples from crime-scene investigations.<ref>{{cite magazine|title=Lethal DNA tags could keep innocent people out of jail|url=https://www.newscientist.com/article/mg21829155.900-lethal-dna-tags-could-keep-innocent-people-out-of-jail.html|magazine=New Scientist|date=2 May 2013}}</ref> |
|||
===Chemical building blocks=== |
|||
{{further|Asymmetric synthesis}} |
|||
Amino acids are important as low-cost [[feedstock]]s. These compounds are used in [[chiral pool synthesis]] as [[enantiomer|enantiomerically pure]] building-blocks.<ref name=Hanessian1993>{{cite journal | vauthors = Hanessian S | year =1993 | title = Reflections on the total synthesis of natural products: Art, craft, logic, and the chiron approach |journal=Pure and Applied Chemistry | volume = 65 | issue = 6 | pages = 1189–204 | doi = 10.1351/pac199365061189 }}</ref> |
|||
Amino acids have been investigated as precursors [[chiral catalyst]]s, e.g., for asymmetric [[hydrogenation]] reactions, although no commercial applications exist.<ref name=Blaser1992>{{cite journal | last = Blaser | first = Hans Ulrich | name-list-format = vanc | year = 1992 | title = The chiral pool as a source of enantioselective catalysts and auxiliaries |journal=Chemical Reviews |volume=92 |issue=5 |pages=935–52 |doi=10.1021/cr00013a009}}</ref> |
|||
===Biodegradable plastics=== |
|||
{{further|Biodegradable plastic|Biopolymer}} |
|||
{| class="wikitable sortable" style="text-align:center;" |
|||
Amino acids have been considered as components of biodegradable polymers, which have applications as [[environmentally friendly]] packaging and in medicine in [[drug delivery]] and the construction of [[prosthesis|prosthetic implants]].<ref name=Sanda1999>{{cite journal | vauthors = Sanda F, Endo T | year = 1999 | title = Syntheses and functions of polymers based on amino acids | journal = Macromolecular Chemistry and Physics | volume = 200 | issue = 12 | pages = 2651–61 | doi = 10.1002/(SICI)1521-3935(19991201)200:12<2651::AID-MACP2651>3.0.CO;2-P }}</ref> An interesting example of such materials is [[Sodium poly(aspartate)|polyaspartate]], a water-soluble biodegradable polymer that may have applications in disposable [[diaper]]s and agriculture.<ref name=Gross2002>{{cite journal | vauthors = Gross RA, Kalra B | title = Biodegradable polymers for the environment | journal = Science | volume = 297 | issue = 5582 | pages = 803–7 | date = August 2002 | pmid = 12161646 | doi = 10.1126/science.297.5582.803 | bibcode = 2002Sci...297..803G | url = https://zenodo.org/record/1231185 }}</ref> Due to its solubility and ability to [[chelation|chelate]] metal ions, polyaspartate is also being used as a biodegradeable anti-[[Fouling|scaling]] agent and a [[corrosion inhibitor]].<ref>{{Cite book|title= Commercial poly(aspartic acid) and Its Uses | vauthors = Low KC, Wheeler AP, Koskan LP |series= Advances in Chemistry Series |volume= 248 |publisher= [[American Chemical Society]] |location= Washington, D.C. |year= 1996}}</ref><ref name=Thombre2005>{{cite journal| vauthors = Thombre SM, Sarwade BD | year = 2005 | title = Synthesis and Biodegradability of Polyaspartic Acid: A Critical Review | journal = Journal of Macromolecular Science, Part A | volume = 42 | issue = 9 | pages = 1299–1315 | doi = 10.1080/10601320500189604}}</ref> In addition, the aromatic amino acid [[tyrosine]] has been considered as a possible replacement for [[phenols]] such as [[bisphenol A]] in the manufacture of [[polycarbonate]]s.<ref name=Bourke2003>{{cite journal | vauthors = Bourke SL, Kohn J | title = Polymers derived from the amino acid L-tyrosine: polycarbonates, polyarylates and copolymers with poly(ethylene glycol) | journal = Advanced Drug Delivery Reviews | volume = 55 | issue = 4 | pages = 447–66 | date = April 2003 | pmid = 12706045 | doi = 10.1016/S0169-409X(03)00038-3 }}</ref> |
|||
! rowspan=2 | Amino acid |
|||
! colspan=2 | 3- and 1-letter symbols |
|||
==Synthesis== |
|||
! colspan=3 | Side chain |
|||
{{Main|Amino acid synthesis}} |
|||
! rowspan=2 | [[Hydropathy index|Hydropathy <br/>index]]<ref>{{cite journal |vauthors=Kyte J, Doolittle RF |title=A simple method for displaying the hydropathic character of a protein |journal=Journal of Molecular Biology |volume=157 |issue=1 |pages=105–132 |date=May 1982 |pmid=7108955 |doi=10.1016/0022-2836(82)90515-0 |citeseerx=10.1.1.458.454}}</ref> |
|||
[[Image:Strecker amino acid synthesis scheme.svg|thumb|400px|right|The Strecker amino acid synthesis|alt=For the steps in the reaction, see the text.]] |
|||
! colspan=2 | [[Molar absorptivity]]<ref name="Freifelder">{{Cite book| title=Physical Biochemistry| vauthors=Freifelder D| publisher=W. H. Freeman and Company| isbn=978-0-7167-1315-9| edition=2nd| year=1983}}{{Page needed|date=September 2010}}</ref> |
|||
! rowspan=2 | [[Molecular mass]] |
|||
===Chemical synthesis=== |
|||
! rowspan=2 | Abundance in proteins (%)<ref>{{cite journal |vauthors=Kozlowski LP |title=Proteome-p''I'': proteome isoelectric point database |journal=Nucleic Acids Research |volume=45 |issue=D1 |pages=D1112–D1116 |date=January 2017 |pmid=27789699 |pmc=5210655 |doi=10.1093/nar/gkw978}}</ref> |
|||
The commercial production of amino acids usually relies on mutant bacteria that overproduce individual amino acids using glucose as a carbon source. Some amino acids are produced by enzymatic conversions of synthetic intermediates. [[2-Aminothiazoline-4-carboxylic acid]] is an intermediate in one industrial synthesis of L-[[cysteine]] for example. [[Aspartic acid]] is produced by the addition of ammonia to [[fumarate]] using a lyase.<ref name=Ullmann>{{Ullmann|author=Karlheinz Drauz, Ian Grayson, Axel Kleemann, Hans-Peter Krimmer, Wolfgang Leuchtenberger, Christoph Weckbecker|year=2006| doi=10.1002/14356007.a02_057.pub2}}</ref> |
|||
! rowspan=2 | Standard genetic coding,<br/>[[Nucleic acid notation#IUPAC notation|IUPAC notation]] |
|||
===Biosynthesis=== |
|||
In plants, nitrogen is first assimilated into organic compounds in the form of [[glutamate]], formed from alpha-ketoglutarate and ammonia in the mitochondrion. For other amino acids, plants use [[transaminase]]s to move the amino group from glutamate to another alpha-keto acids. For example, aspartate aminotransferase converts glutamate and oxaloacetate to alpha-ketoglutarate and aspartate.<ref>{{Cite book | last1 = Jones | first1 = Russell Celyn | last2 = Buchanan | first2 = Bob B. | last3 = Gruissem | first3 = Wilhelm | name-list-format = vanc | title = Biochemistry & molecular biology of plants | publisher = American Society of Plant Physiologists | location = Rockville, Md | year = 2000 | pages = [https://archive.org/details/biochemistrymole00buch/page/371 371–2] | isbn = 978-0-943088-39-6 | url = https://archive.org/details/biochemistrymole00buch/page/371 }}</ref> Other organisms use transaminases for amino acid synthesis, too. |
|||
Nonstandard amino acids are usually formed through modifications to standard amino acids. For example, [[homocysteine]] is formed through the [[transsulfuration pathway]] or by the demethylation of methionine via the intermediate metabolite [[S-adenosyl methionine]],<ref name="Brosnan">{{cite journal | vauthors = Brosnan JT, Brosnan ME | title = The sulfur-containing amino acids: an overview | journal = The Journal of Nutrition | volume = 136 | issue = 6 Suppl | pages = 1636S–1640S | date = June 2006 | pmid = 16702333 | url = http://jn.nutrition.org/cgi/pmidlookup?view=long&pmid=16702333 | doi = 10.1093/jn/136.6.1636S }}</ref> while [[hydroxyproline]] is made by a [[posttranslational modification]] of [[proline]].<ref>{{cite book | vauthors = Kivirikko KI, Pihlajaniemi T | title = Collagen hydroxylases and the protein disulfide isomerase subunit of prolyl 4-hydroxylases | journal = Advances in Enzymology and Related Areas of Molecular Biology | volume = 72 | issue = | pages = 325–98 | year = 1998 | pmid = 9559057 | doi = 10.1002/9780470123188.ch9 | isbn = 9780470123188 | series = Advances in Enzymology – and Related Areas of Molecular Biology }}</ref> |
|||
[[Microorganism]]s and plants synthesize many uncommon amino acids. For example, some microbes make [[2-aminoisobutyric acid]] and [[lanthionine]], which is a sulfide-bridged derivative of alanine. Both of these amino acids are found in peptidic [[lantibiotics]] such as [[alamethicin]].<ref>{{cite journal | vauthors = Whitmore L, Wallace BA | title = Analysis of peptaibol sequence composition: implications for in vivo synthesis and channel formation | journal = European Biophysics Journal | volume = 33 | issue = 3 | pages = 233–7 | date = May 2004 | pmid = 14534753 | doi = 10.1007/s00249-003-0348-1 }}</ref> However, in plants, [[1-aminocyclopropane-1-carboxylic acid]] is a small disubstituted cyclic amino acid that is a key intermediate in the production of the plant hormone [[ethylene#Ethylene as a plant hormone|ethylene]].<ref>{{cite journal | vauthors = Alexander L, Grierson D | title = Ethylene biosynthesis and action in tomato: a model for climacteric fruit ripening | journal = Journal of Experimental Botany | volume = 53 | issue = 377 | pages = 2039–55 | date = October 2002 | pmid = 12324528 | doi = 10.1093/jxb/erf072 }}</ref> |
|||
==Reactions== |
|||
Amino acids undergo the reactions expected of the constituent functional groups.<ref>{{cite book | last1 = Elmore | first1 = Donald Trevor | last2 = Barrett | first2 = GC | name-list-format = vanc | title = Amino acids and peptides |publisher=Cambridge University Press |location=Cambridge, UK |year=1998 |pages=48–60 |isbn=978-0-521-46827-5}}</ref><ref>{{cite journal | vauthors = Gutteridge A, Thornton JM | title = Understanding nature's catalytic toolkit | journal = Trends in Biochemical Sciences | volume = 30 | issue = 11 | pages = 622–9 | date = November 2005 | pmid = 16214343 | doi = 10.1016/j.tibs.2005.09.006 }}</ref> The types of these reactions are determined by the groups on these side chains and are, therefore, different between the various types of amino acid. |
|||
===Peptide bond formation=== |
|||
{{see also|Peptide synthesis|Peptide bond}} |
|||
[[File:Peptidformationball.svg|thumbnail|right|400px|The condensation of two amino acids to form a ''[[dipeptide]]'' through a ''[[peptide bond]]''|alt=Two amino acids are shown next to each other. One loses a hydrogen and oxygen from its carboxyl group (COOH) and the other loses a hydrogen from its amino group (NH2). This reaction produces a molecule of water (H2O) and two amino acids joined by a peptide bond (-CO-NH-). The two joined amino acids are called a dipeptide.]] |
|||
As both the amine and carboxylic acid groups of amino acids can react to form amide bonds, one amino acid molecule can react with another and become joined through an amide linkage. This [[polymerization]] of amino acids is what creates proteins. This [[condensation reaction]] yields the newly formed [[peptide bond]] and a molecule of water. In cells, this reaction does not occur directly; instead, the amino acid is first activated by attachment to a [[transfer RNA]] molecule through an [[ester]] bond. This aminoacyl-tRNA is produced in an [[Adenosine triphosphate|ATP]]-dependent reaction carried out by an [[aminoacyl tRNA synthetase]].<ref>{{cite journal | vauthors = Ibba M, Söll D | title = The renaissance of aminoacyl-tRNA synthesis | journal = EMBO Reports | volume = 2 | issue = 5 | pages = 382–7 | date = May 2001 | pmid = 11375928 | pmc = 1083889 | doi = 10.1093/embo-reports/kve095 }}</ref> This aminoacyl-tRNA is then a substrate for the [[ribosome]], which catalyzes the attack of the amino group of the elongating protein chain on the ester bond.<ref>{{cite journal | vauthors = Lengyel P, Söll D | title = Mechanism of protein biosynthesis | journal = Bacteriological Reviews | volume = 33 | issue = 2 | pages = 264–301 | date = June 1969 | pmid = 4896351 | pmc = 378322 }}</ref> As a result of this mechanism, all proteins made by ribosomes are synthesized starting at their N-terminus and moving toward their C-terminus. |
|||
However, not all peptide bonds are formed in this way. In a few cases, peptides are synthesized by specific enzymes. For example, the tripeptide [[glutathione]] is an essential part of the defenses of cells against oxidative stress. This peptide is synthesized in two steps from free amino acids.<ref>{{cite journal | vauthors = Wu G, Fang YZ, Yang S, Lupton JR, Turner ND | title = Glutathione metabolism and its implications for health | journal = The Journal of Nutrition | volume = 134 | issue = 3 | pages = 489–92 | date = March 2004 | pmid = 14988435 | url = http://jn.nutrition.org/cgi/pmidlookup?view=long&pmid=14988435 | doi = 10.1093/jn/134.3.489 }}</ref> In the first step, [[gamma-glutamylcysteine synthetase]] condenses [[cysteine]] and [[glutamic acid]] through a peptide bond formed between the side chain carboxyl of the glutamate (the gamma carbon of this side chain) and the amino group of the cysteine. This dipeptide is then condensed with [[glycine]] by [[glutathione synthetase]] to form glutathione.<ref>{{cite journal | vauthors = Meister A | title = Glutathione metabolism and its selective modification | journal = The Journal of Biological Chemistry | volume = 263 | issue = 33 | pages = 17205–8 | date = November 1988 | pmid = 3053703 | url = http://www.jbc.org/cgi/pmidlookup?view=long&pmid=3053703 }}</ref> |
|||
In chemistry, peptides are synthesized by a variety of reactions. One of the most-used in [[peptide synthesis|solid-phase peptide synthesis]] uses the aromatic oxime derivatives of amino acids as activated units. These are added in sequence onto the growing peptide chain, which is attached to a solid resin support.<ref>{{cite journal | first1 = Louis A. | last1 = Carpino | name-list-format = vanc | year = 1992 | title = 1-Hydroxy-7-azabenzotriazole. An efficient peptide coupling additive |journal=Journal of the American Chemical Society |volume=115 |pages=4397–8 |doi=10.1021/ja00063a082 |issue=10}}</ref> The ability to easily synthesize vast numbers of different peptides by varying the types and order of amino acids (using [[combinatorial chemistry]]) has made peptide synthesis particularly important in creating libraries of peptides for use in drug discovery through [[high-throughput screening]].<ref>{{cite journal | vauthors = Marasco D, Perretta G, Sabatella M, Ruvo M | title = Past and future perspectives of synthetic peptide libraries | journal = Current Protein & Peptide Science | volume = 9 | issue = 5 | pages = 447–67 | date = October 2008 | pmid = 18855697 | doi = 10.2174/138920308785915209 }}</ref> |
|||
The combination of functional groups allow amino acids to be effective polydentate ligands for metal-amino acid chelates.<ref>{{cite journal | vauthors = Konara S, Gagnona K, Clearfield A, Thompson C, Hartle J, Ericson C, Nelson C | title = Structural determination and characterization of copper and zinc bis-glycinates with X-ray crystallography and mass spectrometry | journal = Journal of Coordination Chemistry | year = 2010 | volume = 63 | issue = 19 | doi = 10.1080/00958972.2010.514336 | pages = 3335–47 }}</ref> |
|||
The multiple side chains of amino acids can also undergo chemical reactions. |
|||
===Catabolism=== |
|||
[[File:Amino acid catabolism revised.png|thumb|300px|Catabolism of proteinogenic amino acids. Amino acids can be classified according to the properties of their main products as either of the following:<ref>Stipanuk, M. H. (2006). Biochemical, physiological, & molecular aspects of human nutrition (2 ed.): Saunders Elsevier.</ref> |
|||
<br />* ''Glucogenic'', with the products having the ability to form [[glucose]] by [[gluconeogenesis]] |
|||
<br />* ''Ketogenic'', with the products not having the ability to form glucose. These products may still be used for [[ketogenesis]] or [[lipid synthesis]]. |
|||
<br />* Amino acids catabolized into both glucogenic and ketogenic products.]] |
|||
Amino acids must first pass out of organelles and cells into blood circulation via [[amino acid transporter]]s, since the amine and carboxylic acid groups are typically ionized. Degradation of an amino acid, occurring in the liver and kidneys, often involves [[deamination]] by moving its amino group to alpha-ketoglutarate, forming [[glutamate]]. This process involves transaminases, often the same as those used in amination during synthesis. In many vertebrates, the amino group is then removed through the [[urea cycle]] and is excreted in the form of [[urea]]. However, amino acid degradation can produce [[uric acid]] or ammonia instead. For example, [[serine dehydratase]] converts serine to pyruvate and ammonia.<ref name = "Stryer_2002" /> After removal of one or more amino groups, the remainder of the molecule can sometimes be used to synthesize new amino acids, or it can be used for energy by entering [[glycolysis]] or the [[citric acid cycle]], as detailed in image at right. |
|||
'''Some Human Genetic Disorders Affecting Amino Acid Catabolism'''<ref>{{Cite book|url=https://www.worldcat.org/oclc/818809626|title=Fundamentals of biochemistry|last=Jain, J. L.|publisher=S. Chand and Co|isbn=8121903432|location=New Delhi|oclc=818809626}}</ref> |
|||
{| class="wikitable" |
|||
|+ |
|||
!Sr.No |
|||
!Medical condition |
|||
!Approximate incidence |
|||
(per 100,000 births) |
|||
!Defective process |
|||
!Defective enzyme |
|||
!Symptoms and effects |
|||
|- |
|- |
||
! 3 |
|||
|1 |
|||
! 1 |
|||
|Albinism |
|||
! Class |
|||
|3 |
|||
! [[Chemical polarity]]<ref name="Hausman">{{cite book | last1 = Hausman | first1 = Robert E. | last2 = Cooper | first2 = Geoffrey M. | name-list-style = vanc |title=The cell: a molecular approach |publisher=ASM Press |location=Washington, D.C. |year=2004 |page=51 |isbn=978-0-87893-214-6}}</ref> |
|||
|Melanin synthesis from tyrosine |
|||
! Net charge<br/>at pH 7.4<ref name="Hausman" /> |
|||
|Tyrosine 3-monooxygenase |
|||
! Wavelength,<br/>''λ''<sub>max</sub> (nm) |
|||
|Lack of pigmentation, White hair, Pink skin |
|||
! Coefficient ''ε''<br/>(mM<sup>−1</sup>·cm<sup>−1</sup>) |
|||
|- |
|- |
||
|2 |
|||
|Alkaptonuria |
|||
|0.4 |
|||
|Tyrosine degradation |
|||
|Homogentisate |
|||
|Dark pigment in urine |
|||
|- |
|||
|3 |
|||
|Argininemia |
|||
|0.5 |
|||
|Urea synthesis |
|||
|Arginase |
|||
|Mental retardation |
|||
|- |
|||
|4 |
|||
|Arginosuccinic acidemia |
|||
|1.5 |
|||
|Urea synthesis |
|||
|Arginosuccinate lyase |
|||
|Vomiting, convulsions |
|||
|- |
|||
|5 |
|||
|Carbamoyl phosphate |
|||
synthetase I deficiency |
|||
|0.5 |
|||
|Urea synthesis |
|||
|Carbamoyl phosphate synthetase I |
|||
|Lethargy, convulsions and early death |
|||
|- |
|||
|6 |
|||
|Homocystinuria |
|||
|0.5 |
|||
|Methionine degradation |
|||
|Cystathione beta-synthase |
|||
|Faulty bone development, mental retadation |
|||
|- |
|||
|7 |
|||
|Maple syrup urine disease |
|||
|0.4 |
|||
|Isoleucine, leucine and valine degradation |
|||
|Branched chain alpha-keto acid dehydrogenase complex |
|||
|Vomiting, mental retardation and Early death |
|||
|- |
|||
|8 |
|||
|methylmalonic acidemia |
|||
|0.5 |
|||
|Conversion of propionyl-CoA into succinyl-CoA |
|||
|Methylmalonyl-Co A mutase |
|||
|Vomiting, mental retardation and Early death |
|||
|- |
|||
|9 |
|||
|Phenylketonuria |
|||
|8 |
|||
|Conversion of phenyalanine to tyrosine |
|||
|Phenylalanine hydroxylase |
|||
|Neonatal vomiting, Mental retardation |
|||
|} |
|||
===Complexation=== |
|||
Amino acids are bidentate ligands, forming [[transition metal amino acid complexes]].<ref>{{cite journal|journal=Angew. Chem. Int. Ed. |authors=Severin, K.; Bergs, R.; Beck, W.|title=Bioorganometallic Chemistry-Transition Metal Complexes with α-Amino Acids and Peptides|year=1998|volume=37|issue=12|pages=1635–1654|doi=10.1002/(SICI)1521-3773(19980703)37:12<1634::AID-ANIE1634>3.0.CO;2-C}}</ref> |
|||
:[[File:AAcomplexation.png|420 px]] |
|||
==Physicochemical properties of amino acids== |
|||
The ca. 20 canonical amino acids can be classified according to their properties. Important factors are charge, [[hydrophile|hydrophilicity]] or [[hydrophobe|hydrophobicity]], size, and functional groups.<ref name="Creighton" /> These properties are important for [[protein structure]] and [[protein–protein interaction]]s. The water-soluble proteins tend to have their hydrophobic residues (Leu, Ile, Val, Phe, and Trp) buried in the middle of the protein, whereas hydrophilic side chains are exposed to the aqueous solvent. (Note that in [[biochemistry]], a residue refers to a specific [[monomer]] within the [[polymer|polymeric chain]] of a [[polysaccharide]], [[protein]] or [[nucleic acid]].) The [[integral membrane protein]]s tend to have outer rings of exposed [[hydrophobic]] amino acids that anchor them into the [[lipid bilayer]]. In the case part-way between these two extremes, some [[peripheral membrane protein]]s have a patch of hydrophobic amino acids on their surface that locks onto the membrane. In similar fashion, proteins that have to bind to positively charged molecules have surfaces rich with negatively charged amino acids like [[glutamate]] and [[aspartate]], while proteins binding to negatively charged molecules have surfaces rich with positively charged chains like [[lysine]] and [[arginine]]. There are different [[hydrophobicity scale]]s of amino acid residues.<ref>{{cite journal| vauthors = Urry DW | title = The change in Gibbs free energy for hydrophobic association: Derivation and evaluation by means of inverse temperature transitions | journal = Chemical Physics Letters | volume = 399 | issue = 1–3 | pages = 177–83 | year = 2004 | doi = 10.1016/S0009-2614(04)01565-9 }}</ref> |
|||
Some amino acids have special properties such as [[cysteine]], that can form covalent [[disulfide bond]]s to other cysteine residues, [[proline]] that forms [[cyclic compound|a cycle]] to the polypeptide backbone, and [[glycine]] that is more flexible than other amino acids. |
|||
Many proteins undergo a range of [[posttranslational modification]]s, when additional chemical groups are attached to the amino acids in proteins. Some modifications can produce hydrophobic [[lipoprotein]]s,<ref>{{cite journal | vauthors = Magee T, Seabra MC | title = Fatty acylation and prenylation of proteins: what's hot in fat | journal = Current Opinion in Cell Biology | volume = 17 | issue = 2 | pages = 190–6 | date = April 2005 | pmid = 15780596 | doi = 10.1016/j.ceb.2005.02.003 }}</ref> or hydrophilic [[glycoprotein]]s.<ref>{{cite journal | vauthors = Pilobello KT, Mahal LK | title = Deciphering the glycocode: the complexity and analytical challenge of glycomics | journal = Current Opinion in Chemical Biology | volume = 11 | issue = 3 | pages = 300–5 | date = June 2007 | pmid = 17500024 | doi = 10.1016/j.cbpa.2007.05.002 }}</ref> These type of modification allow the reversible targeting of a protein to a membrane. For example, the addition and removal of the fatty acid [[palmitic acid]] to cysteine residues in some signaling proteins causes the proteins to attach and then detach from cell membranes.<ref>{{cite journal | vauthors = Smotrys JE, Linder ME | title = Palmitoylation of intracellular signaling proteins: regulation and function | journal = Annual Review of Biochemistry | volume = 73 | issue = 1 | pages = 559–87 | year = 2004 | pmid = 15189153 | doi = 10.1146/annurev.biochem.73.011303.073954 }}</ref> |
|||
===Table of standard amino acid abbreviations and properties=== |
|||
{{Main|Proteinogenic amino acid}} |
|||
{| class="wikitable sortable" |
|||
|- |
|||
! Amino acid |
|||
! 3-letter<ref name="Hausman" /> |
|||
! 1-letter<ref name="Hausman" /> |
|||
! Side chain |
|||
class |
|||
! Side chain |
|||
polarity<ref name="Hausman">{{cite book | last1 = Hausman | first1 = Robert E. | last2 = Cooper | first2 = Geoffrey M. | name-list-format = vanc |title=The cell: a molecular approach |publisher=ASM Press |location=Washington, D.C |year=2004 |page=51 |isbn=978-0-87893-214-6}}</ref> |
|||
! Side chain |
|||
charge (pH 7.4)<ref name="Hausman" /> |
|||
! [[Hydropathy index|Hydropathy<br />index]]<ref>{{cite journal | vauthors = Kyte J, Doolittle RF | title = A simple method for displaying the hydropathic character of a protein | journal = Journal of Molecular Biology | volume = 157 | issue = 1 | pages = 105–32 | date = May 1982 | pmid = 7108955 | doi = 10.1016/0022-2836(82)90515-0 | citeseerx = 10.1.1.458.454 }}</ref> |
|||
! [[Absorbance]] |
|||
λ<sub>max</sub>(nm)<ref name="Freifelder" /> |
|||
! [[Molar absorptivity|ε]] at |
|||
λ<sub>max</sub> (mM<sup>−1</sup> cm<sup>−1</sup>)<ref name="Freifelder">{{Cite book| title=Physical Biochemistry| edition=2nd|vauthors = Freifelder D | publisher=W. H. Freeman and Company| isbn=978-0-7167-1315-9 | year=1983}}{{Page needed|date=September 2010}}</ref> |
|||
! [[Molecular mass|MW]] |
|||
(weight) |
|||
!Occurrence |
|||
in proteins |
|||
(%)<ref>{{cite journal | vauthors = Kozlowski LP | title = Proteome-pI: proteome isoelectric point database | journal = Nucleic Acids Research | volume = 45 | issue = D1 | pages = D1112–D1116 | date = January 2017 | pmid = 27789699 | pmc = 5210655 | doi = 10.1093/nar/gkw978 }}</ref> |
|||
!Coding in the Standard Genetic Code (using [[Nucleic acid notation#IUPAC notation|IUPAC notation]]) |
|||
|- style="text-align:center;" |
|||
| [[Alanine]] |
| [[Alanine]] |
||
| Ala |
| Ala |
||
| A |
| A |
||
| Aliphatic |
|||
| aliphatic |
|||
| Nonpolar |
|||
| nonpolar |
|||
| |
| Neutral |
||
| 1.8 |
| 1.8 |
||
| |
| |
||
| |
| |
||
| 89.094 |
| 89.094 |
||
|8.76 |
| 8.76 |
||
|GCN |
| GCN |
||
|- |
|||
|- style="text-align:center;" |
|||
| [[Arginine]] |
| [[Arginine]] |
||
| Arg |
| Arg |
||
| R |
| R |
||
| Fixed cation |
|||
| basic |
|||
| |
| Basic polar |
||
| Positive |
|||
| positive |
|||
| −4.5 |
| −4.5 |
||
| |
| |
||
| |
| |
||
| 174.203 |
| 174.203 |
||
|5.78 |
| 5.78 |
||
| |
| MGR, CGY{{efn|Codons can also be expressed by: CGN, AGR.}} |
||
|- |
|||
|- style="text-align:center;" |
|||
| [[Asparagine]] |
| [[Asparagine]] |
||
| Asn |
| Asn |
||
| N |
| N |
||
| |
| Amide |
||
| |
| Polar |
||
| |
| Neutral |
||
| −3.5 |
| −3.5 |
||
| |
| |
||
| |
| |
||
| 132.119 |
| 132.119 |
||
|3.93 |
| 3.93 |
||
|AAY |
| AAY |
||
|- |
|||
|- style="text-align:center;" |
|||
| [[ |
| [[Aspartate]] |
||
| Asp |
| Asp |
||
| D |
| D |
||
| |
| Anion |
||
| [[Brønsted base]] |
|||
| acidic polar |
|||
| Negative |
|||
| negative |
|||
| −3.5 |
| −3.5 |
||
| |
| |
||
| |
| |
||
| 133.104 |
| 133.104 |
||
|5.49 |
| 5.49 |
||
|GAY |
| GAY |
||
|- |
|||
|- style="text-align:center;" |
|||
| [[Cysteine]] |
| [[Cysteine]] |
||
| Cys |
| Cys |
||
| C |
| C |
||
| Thiol |
|||
| sulfur-containing |
|||
| Brønsted acid |
|||
| nonpolar |
|||
| |
| Neutral |
||
| 2.5 |
| 2.5 |
||
| 250 |
| 250 |
||
| 0.3 |
| 0.3 |
||
| 121.154 |
| 121.154 |
||
|1.38 |
| 1.38 |
||
|UGY |
| UGY |
||
|- |
|||
|- style="text-align:center;" |
|||
| [[Glutamine]] |
| [[Glutamine]] |
||
| Gln |
| Gln |
||
| Q |
| Q |
||
| |
| Amide |
||
| |
| Polar |
||
| |
| Neutral |
||
| −3.5 |
| −3.5 |
||
| |
| |
||
| |
| |
||
| 146.146 |
| 146.146 |
||
|3.9 |
| 3.9 |
||
|CAR |
| CAR |
||
|- |
|||
|- style="text-align:center;" |
|||
| [[ |
| [[Glutamate]] |
||
| Glu |
| Glu |
||
| E |
| E |
||
| |
| Anion |
||
| Brønsted base |
|||
| acidic polar |
|||
| Negative |
|||
| negative |
|||
| −3.5 |
| −3.5 |
||
| |
| |
||
| |
| |
||
| 147.131 |
| 147.131 |
||
|6.32 |
| 6.32 |
||
|GAR |
| GAR |
||
|- |
|||
|- style="text-align:center;" |
|||
| [[Glycine]] |
| [[Glycine]] |
||
| Gly |
| Gly |
||
| G |
| G |
||
| Aliphatic |
|||
| aliphatic |
|||
| Nonpolar |
|||
| nonpolar |
|||
| |
| Neutral |
||
| −0.4 |
| −0.4 |
||
| |
| |
||
| |
| |
||
| 75.067 |
| 75.067 |
||
|7.03 |
| 7.03 |
||
|GGN |
| GGN |
||
|- |
|||
|- style="text-align:center;" |
|||
| [[Histidine]] |
| [[Histidine]] |
||
| His |
| His |
||
| H |
| H |
||
| Cationic |
|||
| basic aromatic |
|||
| Brønsted acid and base |
|||
| basic polar |
|||
| |
| Positive, 10%<br/>Neutral, 90% |
||
| −3.2 |
| −3.2 |
||
| 211 |
| 211 |
||
| 5.9 |
| 5.9 |
||
| 155.156 |
| 155.156 |
||
|2.26 |
| 2.26 |
||
|CAY |
| CAY |
||
|- |
|||
|- style="text-align:center;" |
|||
| [[Isoleucine]] |
| [[Isoleucine]] |
||
| Ile |
| Ile |
||
| I |
| I |
||
| Aliphatic |
|||
| aliphatic |
|||
| Nonpolar |
|||
| nonpolar |
|||
| |
| Neutral |
||
| 4.5 |
| 4.5 |
||
| |
| |
||
| |
| |
||
| 131.175 |
| 131.175 |
||
|5.49 |
| 5.49 |
||
| AUH |
| AUH |
||
|- |
|||
|- style="text-align:center;" |
|||
| [[Leucine]] |
| [[Leucine]] |
||
| Leu |
| Leu |
||
| L |
| L |
||
| Aliphatic |
|||
| aliphatic |
|||
| Nonpolar |
|||
| nonpolar |
|||
| |
| Neutral |
||
| 3.8 |
| 3.8 |
||
| |
| |
||
| |
| |
||
| 131.175 |
| 131.175 |
||
|9.68 |
| 9.68 |
||
|YUR, CUY |
| YUR, CUY{{efn|Codons can also be expressed by: CUN, UUR.}} |
||
|- |
|||
|- style="text-align:center;" |
|||
| [[Lysine]] |
| [[Lysine]] |
||
| Lys |
| Lys |
||
| K |
| K |
||
| |
| Cation |
||
| Brønsted acid |
|||
| basic polar |
|||
| Positive |
|||
| positive |
|||
| −3.9 |
| −3.9 |
||
| |
| |
||
| |
| |
||
| 146.189 |
| 146.189 |
||
|5.19 |
| 5.19 |
||
|AAR |
| AAR |
||
|- |
|||
|- style="text-align:center;" |
|||
| [[Methionine]] |
| [[Methionine]] |
||
| Met |
| Met |
||
| M |
| M |
||
| Thioether |
|||
| sulfur-containing |
|||
| Nonpolar |
|||
| nonpolar |
|||
| |
| Neutral |
||
| 1.9 |
| 1.9 |
||
| |
| |
||
| |
| |
||
| 149.208 |
| 149.208 |
||
|2.32 |
| 2.32 |
||
|AUG |
| AUG |
||
|- |
|||
|- style="text-align:center;" |
|||
| [[Phenylalanine]] |
| [[Phenylalanine]] |
||
| Phe |
| Phe |
||
| F |
| F |
||
| Aromatic |
|||
| aromatic |
|||
| Nonpolar |
|||
| nonpolar |
|||
| |
| Neutral |
||
| 2.8 |
| 2.8 |
||
| 257, 206, 188 |
| 257, 206, 188 |
||
| 0.2, 9.3, 60.0 |
| 0.2, 9.3, 60.0 |
||
| 165.192 |
| 165.192 |
||
|3.87 |
| 3.87 |
||
|UUY |
| UUY |
||
|- |
|||
|- style="text-align:center;" |
|||
| [[Proline]] |
| [[Proline]] |
||
| Pro |
| Pro |
||
| P |
| P |
||
| |
| Cyclic |
||
| Nonpolar |
|||
| nonpolar |
|||
| |
| Neutral |
||
| −1.6 |
| −1.6 |
||
| |
| |
||
| |
| |
||
| 115.132 |
| 115.132 |
||
|5.02 |
| 5.02 |
||
|CCN |
| CCN |
||
|- |
|||
|- style="text-align:center;" |
|||
| [[Serine]] |
| [[Serine]] |
||
| Ser |
| Ser |
||
| S |
| S |
||
| Hydroxylic |
|||
| hydroxyl-containing |
|||
| |
| Polar |
||
| |
| Neutral |
||
| −0.8 |
| −0.8 |
||
| |
| |
||
| |
| |
||
| 105.093 |
| 105.093 |
||
|7.14 |
| 7.14 |
||
|UCN, AGY |
| UCN, AGY |
||
|- |
|||
|- style="text-align:center;" |
|||
| [[Threonine]] |
| [[Threonine]] |
||
| Thr |
| Thr |
||
| T |
| T |
||
| Hydroxylic |
|||
| hydroxyl-containing |
|||
| |
| Polar |
||
| |
| Neutral |
||
| −0.7 |
| −0.7 |
||
| |
| |
||
| |
| |
||
| 119.119 |
| 119.119 |
||
|5.53 |
| 5.53 |
||
|ACN |
| ACN |
||
|- |
|||
|- style="text-align:center;" |
|||
| [[Tryptophan]] |
| [[Tryptophan]] |
||
| Trp |
| Trp |
||
| W |
| W |
||
| Aromatic |
|||
| aromatic |
|||
| Nonpolar |
|||
| nonpolar |
|||
| |
| Neutral |
||
| −0.9 |
| −0.9 |
||
| 280, 219 |
| 280, 219 |
||
| 5.6, 47.0 |
| 5.6, 47.0 |
||
| 204.228 |
| 204.228 |
||
|1.25 |
| 1.25 |
||
|UGG |
| UGG |
||
|- |
|||
|- style="text-align:center;" |
|||
| [[Tyrosine]] |
| [[Tyrosine]] |
||
| Tyr |
| Tyr |
||
| Y |
| Y |
||
| Aromatic |
|||
| aromatic |
|||
| Brønsted acid |
|||
| polar |
|||
| |
| Neutral |
||
| −1.3 |
| −1.3 |
||
| 274, 222, 193 |
| 274, 222, 193 |
||
| 1.4, 8.0, 48.0 |
| 1.4, 8.0, 48.0 |
||
| 181.191 |
| 181.191 |
||
|2.91 |
| 2.91 |
||
|UAY |
| UAY |
||
|- |
|||
|- style="text-align:center;" |
|||
| [[Valine]] |
| [[Valine]] |
||
| Val |
| Val |
||
| V |
| V |
||
| Aliphatic |
|||
| aliphatic |
|||
| Nonpolar |
|||
| nonpolar |
|||
| |
| Neutral |
||
| 4.2 |
| 4.2 |
||
| |
| |
||
| |
| |
||
| 117.148 |
| 117.148 |
||
|6.73 |
| 6.73 |
||
|GUN |
| GUN |
||
|} |
|} |
||
Two additional amino acids are in some species coded for by [[ |
Two additional amino acids are in some species coded for by [[codons]] that are usually interpreted as [[stop codon]]s: |
||
{| class="wikitable" |
{| class="wikitable" style="text-align:center;" |
||
|- |
|- |
||
! 21st and 22nd amino acids |
! 21st and 22nd amino acids |
||
! 3-letter |
! 3-letter |
||
! 1-letter |
! 1-letter |
||
! [[Molecular mass |
! [[Molecular mass]] |
||
|- |
|||
|- style="text-align:center;" |
|||
| [[Selenocysteine]] |
| [[Selenocysteine]] |
||
| Sec |
| Sec |
||
| U |
| U |
||
| 168.064 |
| 168.064 |
||
|- |
|||
|- style="text-align:center;" |
|||
| [[Pyrrolysine]] |
| [[Pyrrolysine]] |
||
| Pyl |
| Pyl |
||
| Line 616: | Line 422: | ||
|} |
|} |
||
In addition to the specific amino acid codes, placeholders are used in cases where [[Protein sequencing|chemical]] or [[X-ray crystallography|crystallographic]] analysis of a peptide or protein cannot conclusively determine the identity of a residue. They are also used to |
In addition to the specific amino acid codes, placeholders are used in cases where [[Protein sequencing|chemical]] or [[X-ray crystallography|crystallographic]] analysis of a peptide or protein cannot conclusively determine the identity of a residue. They are also used to summarize [[Conserved sequence|conserved protein sequence]] motifs. The use of single letters to indicate sets of similar residues is similar to the use of [[Nucleic acid notation|abbreviation codes for degenerate bases]].<ref>{{cite journal | vauthors = Aasland R, Abrams C, Ampe C, Ball LJ, Bedford MT, Cesareni G, Gimona M, Hurley JH, Jarchau T, Lehto VP, Lemmon MA, Linding R, Mayer BJ, Nagai M, Sudol M, Walter U, Winder SJ | title = Normalization of nomenclature for peptide motifs as ligands of modular protein domains | journal = FEBS Letters | volume = 513 | issue = 1 | pages = 141–144 | date = February 2002 | pmid = 11911894 | doi = 10.1111/j.1432-1033.1968.tb00350.x }}</ref><ref>{{cite journal | author = IUPAC–IUB Commission on Biochemical Nomenclature | title = A one-letter notation for amino acid sequences | journal = Pure and Applied Chemistry | volume = 31 | issue = 4 | pages = 641–645 | pmid = 5080161 | year = 1972 | doi = 10.1351/pac197231040639| doi-access = free }}</ref> |
||
{| class="wikitable" |
{| class="wikitable" style="text-align:center;" |
||
|- |
|- |
||
! Ambiguous amino acids |
! Ambiguous amino acids |
||
! 3-letter |
! 3-letter |
||
! 1-letter |
! 1-letter |
||
! Amino |
! Amino acids included |
||
! Codons |
! Codons included |
||
|- |
|||
|- style="text-align:center;" |
|||
|Any / unknown |
| Any / unknown |
||
|Xaa |
| Xaa |
||
|X |
| X |
||
|All |
| All |
||
|NNN |
| NNN |
||
|- |
|||
|- style="text-align:center;" |
|||
| [[Asparagine]] or |
| [[Asparagine]] or [[aspartate]] |
||
| Asx |
| Asx |
||
| B |
| B |
||
|D, N |
| D, N |
||
|RAY |
| RAY |
||
|- |
|||
|- style="text-align:center;" |
|||
| [[Glutamine]] or |
| [[Glutamine]] or [[glutamate]] |
||
| Glx |
| Glx |
||
| Z |
| Z |
||
|E, Q |
| E, Q |
||
|SAR |
| SAR |
||
|- |
|||
|- style="text-align:center;" |
|||
| [[Leucine]] or |
| [[Leucine]] or isoleucine |
||
| Xle |
| Xle |
||
| J |
| J |
||
|I, L |
| I, L |
||
|YTR, ATH, CTY |
| YTR, ATH, CTY{{efn|Codons can also be expressed by: CTN, ATH, TTR; MTY, YTR, ATA; MTY, HTA, YTG.}} |
||
|- |
|||
|- style="text-align:center;" |
|||
|[[Hydrophobic]] |
| [[Hydrophobic]] |
||
| |
| |
||
|Φ |
| Φ |
||
|V, I, L, F, W, Y, M |
| V, I, L, F, W, Y, M |
||
|NTN, TAY, TGG |
| NTN, TAY, TGG |
||
|- |
|||
|- style="text-align:center;" |
|||
|[[Aromatic]] |
| [[Aromatic]] |
||
| |
| |
||
|Ω |
| Ω |
||
|F, W, Y, H |
| F, W, Y, H |
||
|YWY, TTY, TGG |
| YWY, TTY, TGG{{efn|Codons can also be expressed by: TWY, CAY, TGG.}} |
||
|- |
|||
|- style="text-align:center;" |
|||
|[[Aliphatic]] (non-aromatic) |
| [[Aliphatic]] (non-aromatic) |
||
| |
| |
||
|Ψ |
| Ψ |
||
|V, I, L, M |
| V, I, L, M |
||
|VTN, TTR |
| VTN, TTR{{efn|Codons can also be expressed by: NTR, VTY.}} |
||
|- |
|||
|- style="text-align:center;" |
|||
|Small |
| Small |
||
| |
| |
||
|π |
| π |
||
|P, G, A, S |
| P, G, A, S |
||
|BCN, RGY, GGR |
| BCN, RGY, GGR |
||
|- |
|||
|- style="text-align:center;" |
|||
|[[Hydrophilic]] |
| [[Hydrophilic]] |
||
| |
| |
||
|ζ |
| ζ |
||
|S, T, H, N, Q, E, D, K, R |
| S, T, H, N, Q, E, D, K, R |
||
|VAN, WCN, CGN, AGY |
| VAN, WCN, CGN, AGY{{efn|Codons can also be expressed by: VAN, WCN, MGY, CGP.}} |
||
|- |
|||
|- style="text-align:center;" |
|||
|[[Cation|Positively |
| [[Cation|Positively-charged]] |
||
| |
| |
||
| + |
| + |
||
|K, R, H |
| K, R, H |
||
|ARR, CRY, CGR |
| ARR, CRY, CGR |
||
|- |
|||
|- style="text-align:center;" |
|||
|[[Anion|Negatively |
| [[Anion|Negatively-charged]] |
||
| |
| |
||
| − |
| − |
||
|D, E |
| D, E |
||
|GAN |
| GAN |
||
|} |
|} |
||
'''Unk''' is sometimes used instead of '''Xaa''', but is less standard. |
'''Unk''' is sometimes used instead of '''Xaa''', but is less standard. |
||
'''Ter''' or '''*''' (from termination) is used in notation for mutations in proteins when a stop codon occurs. It corresponds to no amino acid at all.<ref name="HGSV_recommendations">{{Cite web |url=http://varnomen.hgvs.org/recommendations/protein/variant/substitution/ |title=HGVS: Sequence Variant Nomenclature, Protein Recommendations |access-date=23 September 2021 |url-status=live |archive-date=24 September 2021 |archive-url=https://web.archive.org/web/20210924091505/http://varnomen.hgvs.org/recommendations/protein/variant/substitution/}}</ref> |
|||
In addition, many [[Non-proteinogenic amino acids|non-standard amino acids]] have a specific code. For example, several peptide drugs, such as [[Bortezomib]] and [[MG132]], are [[peptide synthesis|artificially synthesized]] and retain their [[protecting group]]s, which have specific codes. Bortezomib is [[pyrazinoic acid|Pyz]]-Phe-boroLeu, and MG132 is [[Carboxybenzyl|Z]]-Leu-Leu-Leu-al. To aid in the analysis of protein structure, [[photo-reactive amino acid analog]]s are available. These include [[photoleucine]] ('''pLeu''') and [[photomethionine]] ('''pMet''').<ref>{{cite journal | vauthors = Suchanek M, Radzikowska A, Thiele C | title = Photo-leucine and photo-methionine allow identification of protein-protein interactions in living cells | journal = Nature Methods | volume = 2 | issue = 4 | pages = 261–7 | date = April 2005 | pmid = 15782218 | doi = 10.1038/nmeth752 }}</ref> |
|||
In addition, many [[Non-proteinogenic amino acids|nonstandard amino acids]] have a specific code. For example, several peptide drugs, such as [[Bortezomib]] and [[MG132]], are [[peptide synthesis|artificially synthesized]] and retain their [[protecting group]]s, which have specific codes. Bortezomib is [[pyrazinoic acid|Pyz]]–Phe–boroLeu, and MG132 is [[Carboxybenzyl|Z]]–Leu–Leu–Leu–al. To aid in the analysis of protein structure, [[photo-reactive amino acid analog]]s are available. These include [[photoleucine]] ('''pLeu''') and [[photomethionine]] ('''pMet''').<ref>{{cite journal |vauthors=Suchanek M, Radzikowska A, Thiele C |title=Photo-leucine and photo-methionine allow identification of protein–protein interactions in living cells |journal=Nature Methods |volume=2 |issue=4 |pages=261–267 |date=April 2005 |pmid=15782218 |doi=10.1038/nmeth752 |doi-access=free}}</ref> |
|||
==Occurrence and functions in biochemistry== |
|||
{{multiple image |
|||
<!-- Layout parameters --> |
|||
| align = right |
|||
| direction = vertical |
|||
| total_width = 300 |
|||
<!-- Header --> |
|||
| header_align = <!-- center (default), left, right --> |
|||
| header = |
|||
<!--image 5--> |
|||
| image5 = Protein primary structure.svg |
|||
| alt5 = A protein depicted as a long unbranched string of linked circles each representing amino acids |
|||
| width5 = |
|||
| height5 = |
|||
| caption5 = A [[polypeptide]] is an unbranched chain of amino acids. |
|||
<!--image 6--> |
|||
| image6 = Beta alanine comparison.svg |
|||
| alt6 = Diagrammatic comparison of the structures of β-alanine and α-alanine |
|||
| width6 = |
|||
| height6 = |
|||
| caption6 = β-Alanine and its α-alanine isomer |
|||
<!--image 7--> |
|||
| image7 = Selenocysteine skeletal 3D.svg |
|||
| alt7 = A diagram showing the structure of selenocysteine |
|||
| width7 = |
|||
| height7 = |
|||
| caption7 = The amino acid [[selenocysteine]] |
|||
}} |
|||
===Proteinogenic amino acids=== |
|||
{{main|Proteinogenic amino acid}} {{See also|Protein primary structure|Posttranslational modification}} |
|||
Amino acids are the precursors to proteins.<ref name="NIGMS"/> They join by condensation reactions to form short polymer chains called peptides or longer chains called either polypeptides or proteins. These chains are linear and unbranched, with each amino acid residue within the chain attached to two neighboring amino acids. In nature, the process of making proteins encoded by RNA genetic material is called ''[[translation (biology)|translation]]'' and involves the step-by-step addition of amino acids to a growing protein chain by a [[ribozyme]] that is called a [[ribosome]].<ref>{{cite journal | vauthors = Rodnina MV, Beringer M, Wintermeyer W | title = How ribosomes make peptide bonds | journal = Trends in Biochemical Sciences | volume = 32 | issue = 1 | pages = 20–26 | date = January 2007 | pmid = 17157507 | doi = 10.1016/j.tibs.2006.11.007 }}</ref> The order in which the amino acids are added is read through the [[genetic code]] from an [[Messenger RNA|mRNA]] template, which is an [[RNA]] derived from one of the organism's [[gene]]s. |
|||
Twenty-two amino acids are naturally incorporated into polypeptides and are called [[proteinogenic]] or natural amino acids.<ref name="Creighton" /> Of these, 20 are encoded by the universal genetic code. The remaining 2, [[selenocysteine]] and [[pyrrolysine]], are incorporated into proteins by unique synthetic mechanisms. Selenocysteine is incorporated when the mRNA being translated includes a [[SECIS element]], which causes the UGA codon to encode selenocysteine instead of a stop codon.<ref>{{cite journal | vauthors = Driscoll DM, Copeland PR | title = Mechanism and regulation of selenoprotein synthesis | journal = Annual Review of Nutrition | volume = 23 | issue = 1 | pages = 17–40 | year = 2003 | pmid = 12524431 | doi = 10.1146/annurev.nutr.23.011702.073318 }}</ref> [[Pyrrolysine]] is used by some [[methanogen]]ic [[archaea]] in enzymes that they use to produce [[methane]]. It is coded for with the codon UAG, which is normally a stop codon in other organisms.<ref>{{cite journal | vauthors = Krzycki JA | title = The direct genetic encoding of pyrrolysine | journal = Current Opinion in Microbiology | volume = 8 | issue = 6 | pages = 706–712 | date = December 2005 | pmid = 16256420 | doi = 10.1016/j.mib.2005.10.009 }}</ref> |
|||
Several independent evolutionary studies have suggested that Gly, Ala, Asp, Val, Ser, Pro, Glu, Leu, Thr may belong to a group of amino acids that constituted the early genetic code, whereas Cys, Met, Tyr, Trp, His, Phe may belong to a group of amino acids that constituted later additions of the genetic code.<ref>{{cite journal | |
|||
doi=10.1073/pnas.72.5.1909| |
|||
title=A Co-Evolution Theory of the Genetic Code| |
|||
year=1975| |
|||
last1=Wong| |
|||
first1=J. T.-F.| |
|||
journal=Proceedings of the National Academy of Sciences| |
|||
volume=72| |
|||
issue=5| |
|||
pages=1909–1912| |
|||
pmid=1057181| |
|||
pmc=432657| |
|||
bibcode=1975PNAS...72.1909T| |
|||
doi-access=free}}</ref><ref>{{cite journal| vauthors = Trifonov EN |date=December 2000|title=Consensus temporal order of amino acids and evolution of the triplet code |journal=Gene |volume=261|issue=1|pages=139–151|doi=10.1016/S0378-1119(00)00476-5|pmid=11164045}}</ref><ref>{{cite journal | vauthors = Higgs PG, Pudritz RE | title = A thermodynamic basis for prebiotic amino acid synthesis and the nature of the first genetic code | journal = Astrobiology | volume = 9 | issue = 5 | pages = 483–90 | date = June 2009 | pmid = 19566427 | doi = 10.1089/ast.2008.0280 | arxiv = 0904.0402 | bibcode = 2009AsBio...9..483H | s2cid = 9039622 }}</ref> |
|||
===Standard vs nonstandard amino acids=== |
|||
The 20 amino acids that are encoded directly by the codons of the universal genetic code are called ''standard'' or ''canonical'' amino acids. A modified form of methionine ([[N-Formylmethionine|''N''-formylmethionine]]) is often incorporated in place of methionine as the initial amino acid of proteins in bacteria, mitochondria and [[plastid]]s (including chloroplasts). Other amino acids are called ''nonstandard'' or ''non-canonical''. Most of the nonstandard amino acids are also non-proteinogenic (i.e. they cannot be incorporated into proteins during translation), but two of them are proteinogenic, as they can be incorporated translationally into proteins by exploiting information not encoded in the universal genetic code. |
|||
The two nonstandard proteinogenic amino acids are selenocysteine (present in many non-eukaryotes as well as most eukaryotes, but not coded directly by DNA) and [[pyrrolysine]] (found only in some [[archaea]] and at least one [[bacterium]]). The incorporation of these nonstandard amino acids is rare. For example, 25 human proteins include selenocysteine in their primary structure,<ref>{{cite journal | vauthors = Kryukov GV, Castellano S, Novoselov SV, Lobanov AV, Zehtab O, Guigó R, Gladyshev VN | title = Characterization of mammalian selenoproteomes | journal = Science | volume = 300 | issue = 5624 | pages = 1439–1443 | date = May 2003 | pmid = 12775843 | doi = 10.1126/science.1083516 | bibcode = 2003Sci...300.1439K | s2cid = 10363908 }}</ref> and the structurally characterized enzymes (selenoenzymes) employ selenocysteine as the catalytic [[moiety (chemistry)|moiety]] in their active sites.<ref>{{cite journal | vauthors = Gromer S, Urig S, Becker K | title = The thioredoxin system—from science to clinic | journal = Medicinal Research Reviews | volume = 24 | issue = 1 | pages = 40–89 | date = January 2004 | pmid = 14595672 | doi = 10.1002/med.10051 | s2cid = 1944741 }}</ref> Pyrrolysine and selenocysteine are encoded via variant codons. For example, selenocysteine is encoded by stop codon and [[SECIS element]].<ref name="Tjong">{{cite thesis|url=https://diginole.lib.fsu.edu/islandora/object/fsu%3A175939|last=Tjong|first=Harianto|name-list-style=vanc|title=Modeling Electrostatic Contributions to Protein Folding and Binding|date=2008|publisher=Florida State University|type=PhD thesis|page=1 footnote|access-date=28 January 2020|archive-date=28 January 2020|archive-url=https://web.archive.org/web/20200128234717/https://diginole.lib.fsu.edu/islandora/object/fsu:175939|url-status=live}}</ref><ref name="VoJw6fIISSkC p.299">{{cite journal|last1=Stewart|first1=L.|last2=Burgin|first2=A. B.|journal=Frontiers in Drug Design & Discovery |name-list-style=vanc|date=2005|title=Whole Gene Synthesis: A Gene-O-Matic Future|url=https://books.google.com/books?id=VoJw6fIISSkC&pg=PA299|publisher=[[Bentham Science Publishers]]|volume=1|page=299|doi=10.2174/1574088054583318|isbn=978-1-60805-199-1|issn=1574-0889|access-date=5 January 2016|archive-date=14 April 2021|archive-url=https://web.archive.org/web/20210414224011/https://books.google.com/books?id=VoJw6fIISSkC&pg=PA299|url-status=live}}</ref><ref name="url_The_Genetic_Codes_NCBI">{{cite web|date=7 April 2008|title=The Genetic Codes|url=https://www.ncbi.nlm.nih.gov/Taxonomy/Utils/wprintgc.cgi?mode=c|access-date=10 March 2010|publisher=National Center for Biotechnology Information (NCBI)|vauthors=Elzanowski A, Ostell J|archive-date=20 August 2016|archive-url=https://web.archive.org/web/20160820125755/http://130.14.29.110/Taxonomy/Utils/wprintgc.cgi?mode=c|url-status=live}}</ref> |
|||
[[N-Formylmethionine|''N''-formylmethionine]] (which is often the initial amino acid of proteins in bacteria, [[Mitochondrion|mitochondria]], and [[chloroplast]]s) is generally considered as a form of [[methionine]] rather than as a separate proteinogenic amino acid. Codon–[[transfer RNA|tRNA]] combinations not found in nature can also be used to [[Expanded genetic code|"expand" the genetic code]] and form novel proteins known as [[alloprotein]]s incorporating [[non-proteinogenic amino acid]]s.<ref name="pmid16260173">{{cite journal | vauthors = Xie J, Schultz PG | title = Adding amino acids to the genetic repertoire | journal = Current Opinion in Chemical Biology | volume = 9 | issue = 6 | pages = 548–554 | date = December 2005 | pmid = 16260173 | doi = 10.1016/j.cbpa.2005.10.011 }}</ref><ref name="pmid19318213">{{cite journal | vauthors = Wang Q, Parrish AR, Wang L | title = Expanding the genetic code for biological studies | journal = Chemistry & Biology | volume = 16 | issue = 3 | pages = 323–336 | date = March 2009 | pmid = 19318213 | pmc = 2696486 | doi = 10.1016/j.chembiol.2009.03.001 }}</ref><ref name="isbn0-387-22046-1">{{cite book | vauthors = Simon M | title = Emergent computation: emphasizing bioinformatics | url = https://archive.org/details/emergentcomputat00simo_754 | url-access = limited | publisher = AIP Press/Springer Science+Business Media | location = New York | year = 2005 | pages = [https://archive.org/details/emergentcomputat00simo_754/page/n116 105–106] | isbn = 978-0-387-22046-8 }}</ref> |
|||
===Non-proteinogenic amino acids=== |
|||
{{main|Non-proteinogenic amino acids}} |
|||
Aside from the 22 [[proteinogenic amino acid]]s, many ''non-proteinogenic'' amino acids are known. Those either are not found in proteins (for example [[carnitine]], [[Gamma-aminobutyric acid|GABA]], [[levothyroxine]]) or are not produced directly and in isolation by standard cellular machinery. For example, [[hydroxyproline]], is synthesised from [[proline]]. Another example is [[selenomethionine]]). |
|||
Non-proteinogenic amino acids that are found in proteins are formed by [[post-translational modification]]. Such modifications can also determine the localization of the protein, e.g., the addition of long hydrophobic groups can cause a protein to bind to a [[phospholipid]] membrane.<ref>{{cite journal | vauthors = Blenis J, Resh MD | title = Subcellular localization specified by protein acylation and phosphorylation | journal = Current Opinion in Cell Biology | volume = 5 | issue = 6 | pages = 984–989 | date = December 1993 | pmid = 8129952 | doi = 10.1016/0955-0674(93)90081-Z }}</ref> Examples: |
|||
*the [[carboxylation]] of [[glutamate]] allows for better binding of [[calcium in biology|calcium cations]],<ref>{{cite journal | vauthors = Vermeer C | title = Gamma-carboxyglutamate-containing proteins and the vitamin K-dependent carboxylase | journal = The Biochemical Journal | volume = 266 | issue = 3 | pages = 625–636 | date = March 1990 | pmid = 2183788 | pmc = 1131186 | doi = 10.1042/bj2660625 }}</ref> |
|||
*[[Hydroxyproline]], generated by [[hydroxylation]] of [[proline]], is a major component of the [[connective tissue]] [[collagen]].<ref>{{cite journal | vauthors = Bhattacharjee A, Bansal M | title = Collagen Structure: the Madras triple helix and the current scenario | journal = IUBMB Life | volume = 57 | issue = 3 | pages = 161–172 | date = March 2005 | pmid = 16036578 | doi = 10.1080/15216540500090710 | s2cid = 7211864 }}</ref> |
|||
* [[Hypusine]] in the [[Eukaryotic initiation factor|translation initiation factor]] [[EIF5A]], contains a modification of lysine.<ref>{{cite journal | vauthors = Park MH | title = The post-translational synthesis of a polyamine-derived amino acid, hypusine, in the eukaryotic translation initiation factor 5A (eIF5A) | journal = Journal of Biochemistry | volume = 139 | issue = 2 | pages = 161–169 | date = February 2006 | pmid = 16452303 | pmc = 2494880 | doi = 10.1093/jb/mvj034 }}</ref> |
|||
Some non-proteinogenic amino acids are not found in proteins. Examples include [[2-aminoisobutyric acid]] and the neurotransmitter [[gamma-aminobutyric acid]]. Non-proteinogenic amino acids often occur as intermediates in the [[metabolic pathway]]s for standard amino acids – for example, [[ornithine]] and [[citrulline]] occur in the [[urea cycle]], part of amino acid [[catabolism]] (see below).<ref>{{cite journal | vauthors = Curis E, Nicolis I, Moinard C, Osowska S, Zerrouk N, Bénazeth S, Cynober L | s2cid = 23877884 | title = Almost all about citrulline in mammals | journal = Amino Acids | volume = 29 | issue = 3 | pages = 177–205 | date = November 2005 | pmid = 16082501 | doi = 10.1007/s00726-005-0235-4 }}</ref> A rare exception to the dominance of α-amino acids in biology is the β-amino acid [[beta alanine]] (3-aminopropanoic acid), which is used in plants and microorganisms in the synthesis of [[pantothenic acid]] (vitamin B<sub>5</sub>), a component of [[coenzyme A]].<ref>{{cite journal | vauthors = Coxon KM, Chakauya E, Ottenhof HH, Whitney HM, Blundell TL, Abell C, Smith AG | title = Pantothenate biosynthesis in higher plants | journal = Biochemical Society Transactions | volume = 33 | issue = Pt 4 | pages = 743–746 | date = August 2005 | pmid = 16042590 | doi = 10.1042/BST0330743 }}</ref> |
|||
===In mammalian nutrition=== |
|||
[[File:Amino acids in food and blood.png|class=skin-invert-image|thumb|right|upright=1.75 |Share of amino acid in various human diets and the resulting mix of amino acids in human blood serum. Glutamate and glutamine are the most frequent in food at over 10%, while alanine, glutamine, and glycine are the most common in blood.|alt=Diagram showing the relative occurrence of amino acids in blood serum as obtained from diverse diets.]] |
|||
{{Main|Essential amino acid}} |
|||
{{further|Protein (nutrient)|Amino acid synthesis}} |
|||
Amino acids are not typical component of food: animals eat proteins. The protein is broken down into amino acids in the process of digestion. They are then used to synthesize new proteins, other biomolecules, or are oxidized to [[urea]] and [[carbon dioxide]] as a source of energy.<ref>{{cite journal | vauthors = Sakami W, Harrington H | title = Amino acid metabolism | journal = Annual Review of Biochemistry | volume = 32 | issue = 1 | pages = 355–398 | year = 1963 | pmid = 14144484 | doi = 10.1146/annurev.bi.32.070163.002035 }}</ref> The oxidation pathway starts with the removal of the amino group by a [[transaminase]]; the amino group is then fed into the [[urea cycle]]. The other product of transamidation is a [[keto acid]] that enters the [[citric acid cycle]].<ref>{{cite journal | vauthors = Brosnan JT | title = Glutamate, at the interface between amino acid and carbohydrate metabolism | journal = The Journal of Nutrition | volume = 130 | issue = 4S Suppl | pages = 988S–990S | date = April 2000 | pmid = 10736367 | doi = 10.1093/jn/130.4.988S | doi-access = free }}</ref> [[Glucogenic amino acid]]s can also be converted into glucose, through [[gluconeogenesis]].<ref>{{cite journal | vauthors = Young VR, Ajami AM | title = Glutamine: the emperor or his clothes? | journal = The Journal of Nutrition | volume = 131 | issue = 9 Suppl | pages = 2449S–2459S, 2486S–2487S | date = September 2001 | pmid = 11533293 | doi = 10.1093/jn/131.9.2449S | doi-access = free }}</ref> |
|||
Of the 20 standard amino acids, nine ([[Histidine|His]], [[Isoleucine|Ile]], [[Leucine|Leu]], [[Lysine|Lys]], [[Methionine|Met]], [[Phenylalanine|Phe]], [[Threonine|Thr]], [[Tryptophan|Trp]] and [[Valine|Val]]) are called [[essential amino acid]]s because the [[human body]] cannot [[biosynthesis|synthesize]] them from other compounds at the level needed for normal growth, so they must be obtained from food.<ref>{{cite journal | vauthors = Young VR | title = Adult amino acid requirements: the case for a major revision in current recommendations | journal = The Journal of Nutrition | volume = 124 | issue = 8 Suppl | pages = 1517S–1523S | date = August 1994 | pmid = 8064412 | doi = 10.1093/jn/124.suppl_8.1517S | doi-access = free }}</ref><ref>{{cite journal | vauthors = Fürst P, Stehle P | title = What are the essential elements needed for the determination of amino acid requirements in humans? | journal = The Journal of Nutrition | volume = 134 | issue = 6 Suppl | pages = 1558S–1565S | date = June 2004 | pmid = 15173430 | doi = 10.1093/jn/134.6.1558S | doi-access = free }}</ref><ref>{{cite journal | vauthors = Reeds PJ | title = Dispensable and indispensable amino acids for humans | journal = The Journal of Nutrition | volume = 130 | issue = 7 | pages = 1835S–1840S | date = July 2000 | pmid = 10867060 | doi = 10.1093/jn/130.7.1835S | doi-access = free }}</ref> |
|||
====Semi-essential and conditionally essential amino acids, and juvenile requirements==== |
|||
In addition, cysteine, [[tyrosine]], and [[arginine]] are considered semiessential amino acids, and [[taurine]] a semi-essential aminosulfonic acid in children. Some amino acids are [[Essential amino acid#Essentiality in humans|conditionally essential]] for certain ages or medical conditions. Essential amino acids may also vary from [[species]] to species.{{efn|For example, [[ruminant]]s such as cows obtain a number of amino acids via [[microbe]]s in the [[reticulorumen|first two stomach chambers]].}} The metabolic pathways that synthesize these monomers are not fully developed.<ref>{{cite journal | vauthors = Imura K, Okada A | title = Amino acid metabolism in pediatric patients | journal = Nutrition | volume = 14 | issue = 1 | pages = 143–148 | date = January 1998 | pmid = 9437700 | doi = 10.1016/S0899-9007(97)00230-X }}</ref><ref>{{cite journal | vauthors = Lourenço R, Camilo ME | title = Taurine: a conditionally essential amino acid in humans? An overview in health and disease | journal = Nutricion Hospitalaria | volume = 17 | issue = 6 | pages = 262–270 | year = 2002 | pmid = 12514918 }}</ref> |
|||
===Non-protein functions=== |
|||
{{Catecholamine and trace amine biosynthesis|align=right|caption=[[Catecholamine]]s and [[trace amine]]s are synthesized from phenylalanine and tyrosine in humans.}} |
|||
{{Further|Amino acid neurotransmitter}} |
|||
Many proteinogenic and non-proteinogenic amino acids have biological functions beyond being precursors to proteins and peptides.In humans, amino acids also have important roles in diverse biosynthetic pathways. [[Plant defense against herbivory|Defenses against herbivores]] in plants sometimes employ amino acids.<ref name="Hylin1969">{{Cite journal|last=Hylin |first=John W. | name-list-style = vanc |year=1969 |title=Toxic peptides and amino acids in foods and feeds |journal=Journal of Agricultural and Food Chemistry |volume=17 |issue=3 |pages=492–496 |doi=10.1021/jf60163a003}}</ref> Examples: |
|||
====Standard amino acids==== |
|||
* [[Tryptophan]] is a precursor of the neurotransmitter [[serotonin]].<ref>{{cite journal | vauthors = Savelieva KV, Zhao S, Pogorelov VM, Rajan I, Yang Q, Cullinan E, Lanthorn TH | title = Genetic disruption of both tryptophan hydroxylase genes dramatically reduces serotonin and affects behavior in models sensitive to antidepressants | journal = PLOS ONE| volume = 3 | issue = 10 | pages = e3301 | year = 2008 | pmid = 18923670 | pmc = 2565062 | doi = 10.1371/journal.pone.0003301 | veditors = Bartolomucci A | bibcode = 2008PLoSO...3.3301S | doi-access = free }}</ref> |
|||
* [[Tyrosine]] (and its precursor phenylalanine) are precursors of the [[catecholamine]] [[neurotransmitter]]s [[dopamine]], [[epinephrine]] and [[norepinephrine]] and various [[trace amine]]s. |
|||
* [[Phenylalanine]] is a precursor of [[phenethylamine]] and tyrosine in humans. In plants, it is a precursor of various [[phenylpropanoid]]s, which are important in plant metabolism. |
|||
* [[Glycine]] is a precursor of [[porphyrin]]s such as [[heme]].<ref>{{cite journal |vauthors=Shemin D, Rittenberg D |title=The biological utilization of glycine for the synthesis of the protoporphyrin of hemoglobin |journal=The Journal of Biological Chemistry |volume=166 |issue=2 |pages=621–625 |date=December 1946 |doi=10.1016/S0021-9258(17)35200-6 |pmid=20276176 |doi-access=free}}</ref> |
|||
* [[Arginine]] is a precursor of [[nitric oxide]].<ref>{{cite journal |vauthors=Tejero J, Biswas A, Wang ZQ, Page RC, Haque MM, Hemann C, Zweier JL, Misra S, Stuehr DJ |title=Stabilization and characterization of a heme-oxy reaction intermediate in inducible nitric-oxide synthase |journal=The Journal of Biological Chemistry |volume=283 |issue=48 |pages=33498–33507 |date=November 2008 |pmid=18815130 |pmc=2586280 |doi=10.1074/jbc.M806122200 |doi-access=free}}</ref> |
|||
* [[Ornithine]] and [[S-Adenosyl methionine|''S''-adenosylmethionine]] are precursors of [[polyamine]]s.<ref>{{cite journal |vauthors=Rodríguez-Caso C, Montañez R, Cascante M, Sánchez-Jiménez F, Medina MA |title=Mathematical modeling of polyamine metabolism in mammals |journal=The Journal of Biological Chemistry |volume=281 |issue=31 |pages=21799–21812 |date=August 2006 |pmid=16709566 |doi=10.1074/jbc.M602756200 |doi-access=free |hdl=10630/32289 |hdl-access=free}}</ref> |
|||
* [[Aspartate]], [[glycine]], and [[glutamine]] are precursors of [[nucleotide]]s.<ref name="Stryer_2002">{{cite book |last1=Stryer |first1=Lubert |last2=Berg |first2=Jeremy M. |last3=Tymoczko |first3=John L. |name-list-style=vanc |title=Biochemistry |url=https://archive.org/details/biochemistry200100jere |url-access=registration |date=2002 |publisher=W.H. Freeman |location=New York |isbn=978-0-7167-4684-3 |edition=5th |pages=[https://archive.org/details/biochemistry200100jere/page/693 693–698]}}</ref> |
|||
====Roles for nonstandard amino acids==== |
|||
*[[Carnitine]] is used in [[lipid|lipid transport]]. |
|||
*[[gamma-aminobutyric acid]] is a neurotransmitter.<ref>{{cite journal |vauthors=Petroff OA |date=December 2002 |title=GABA and glutamate in the human brain |journal=The Neuroscientist |volume=8 |issue=6 |pages=562–573 |pmid=12467378 |s2cid=84891972 |doi=10.1177/1073858402238515}}</ref> |
|||
*[[5-HTP]] (5-hydroxytryptophan) is used for experimental treatment of depression.<ref>{{cite journal |vauthors=Turner EH, Loftis JM, Blackwell AD |title=Serotonin a la carte: supplementation with the serotonin precursor 5-hydroxytryptophan |journal=Pharmacology & Therapeutics |volume=109 |issue=3 |pages=325–338 |date=March 2006 |pmid=16023217 |doi=10.1016/j.pharmthera.2005.06.004 |s2cid=2563606}}</ref> |
|||
*[[L-DOPA|<small>L</small>-DOPA]] (<small>L</small>-dihydroxyphenylalanine) for [[Parkinson's]] treatment,<ref>{{cite journal |vauthors=Kostrzewa RM, Nowak P, Kostrzewa JP, Kostrzewa RA, Brus R |s2cid=33603501 |title=Peculiarities of L-DOPA treatment of Parkinson's disease |journal=Amino Acids |volume=28 |issue=2 |pages=157–164 |date=March 2005 |pmid=15750845 |doi=10.1007/s00726-005-0162-4}}</ref> |
|||
*[[Eflornithine]] inhibits [[ornithine decarboxylase]] and used in the treatment of [[African trypanosomiasis|sleeping sickness]].<ref>{{cite journal |vauthors=Heby O, Persson L, Rentala M |s2cid=26273053 |title=Targeting the polyamine biosynthetic enzymes: a promising approach to therapy of African sleeping sickness, Chagas' disease, and leishmaniasis |journal=Amino Acids |volume=33 |issue=2 |pages=359–366 |date=August 2007 |pmid=17610127 |doi=10.1007/s00726-007-0537-9}}</ref> |
|||
*[[Canavanine]], an analogue of [[arginine]] found in many [[legume]]s is an [[antifeedant]], protecting the plant from predators.<ref>{{cite journal |vauthors=Rosenthal GA |s2cid=3144019 |title=L-Canavanine: a higher plant insecticidal allelochemical |journal=Amino Acids |volume=21 |issue=3 |pages=319–330 |year=2001 |pmid=11764412 |doi=10.1007/s007260170017}}</ref> |
|||
*[[Mimosine]] found in some legumes, is another possible [[antifeedant]].<ref>{{cite journal |last=Hammond |first=Andrew C. |date=1 May 1995 |title=Leucaena toxicosis and its control in ruminants |journal=[[Journal of Animal Science]] |volume=73 |issue=5 |pages=1487–1492 |pmid=7665380 |doi=10.2527/1995.7351487x}}</ref> This compound is an analogue of [[tyrosine]] and can poison animals that graze on these plants. |
|||
However, not all of the functions of other abundant nonstandard amino acids are known. |
|||
==Uses in industry== |
|||
===Animal feed=== |
|||
Amino acids are sometimes added to [[Compound feed|animal feed]] because some of the components of these feeds, such as [[soybean]]s, have low levels of some of the [[essential amino acid]]s, especially of lysine, methionine, threonine, and tryptophan.<ref name=Leuchtenberger2005>{{cite journal | vauthors = Leuchtenberger W, Huthmacher K, Drauz K | s2cid = 24161808 | title = Biotechnological production of amino acids and derivatives: current status and prospects | journal = Applied Microbiology and Biotechnology | volume = 69 | issue = 1 | pages = 1–8 | date = November 2005 | pmid = 16195792 | doi = 10.1007/s00253-005-0155-y }}</ref> Likewise amino acids are used to chelate metal cations in order to improve the absorption of minerals from feed supplements.<ref>{{cite book|last=Ashmead|first=H. DeWayne | name-list-style = vanc |title=The Role of Amino Acid Chelates in Animal Nutrition|year=1993|publisher=Noyes Publications|location=Westwood}}</ref> |
|||
===Food=== |
|||
The [[food industry]] is a major consumer of amino acids, especially [[glutamic acid]], which is used as a [[flavor enhancer]],<ref name=Garattini>{{cite journal | vauthors = Garattini S | title = Glutamic acid, twenty years later | journal = The Journal of Nutrition | volume = 130 | issue = 4S Suppl | pages = 901S–909S | date = April 2000 | pmid = 10736350 | doi = 10.1093/jn/130.4.901S | doi-access = free }}</ref> and [[aspartame]] (aspartylphenylalanine 1-methyl ester), which is used as an [[artificial sweetener]].<ref>{{cite journal | vauthors = Stegink LD | title = The aspartame story: a model for the clinical testing of a food additive | journal = The American Journal of Clinical Nutrition | volume = 46 | issue = 1 Suppl | pages = 204–215 | date = July 1987 | pmid = 3300262 | doi = 10.1093/ajcn/46.1.204 }}</ref> Amino acids are sometimes added to food by manufacturers to alleviate symptoms of mineral deficiencies, such as anemia, by improving mineral absorption and reducing negative side effects from inorganic mineral supplementation.<ref name=Ullmann/> |
|||
===Chemical building blocks=== |
|||
{{further|Asymmetric synthesis}} |
|||
Amino acids are low-cost [[feedstock]]s used in [[chiral pool synthesis]] as [[enantiomer|enantiomerically pure]] building blocks.<ref name=Hanessian1993>{{cite journal | vauthors = Hanessian S | year =1993 | title = Reflections on the total synthesis of natural products: Art, craft, logic, and the chiron approach |journal=Pure and Applied Chemistry | volume = 65 | issue = 6 | pages = 1189–1204 | doi = 10.1351/pac199365061189 | s2cid =43992655 | doi-access = free }}</ref><ref name=Blaser1992>{{cite journal | last = Blaser | first = Hans Ulrich | name-list-style = vanc | year = 1992 | title = The chiral pool as a source of enantioselective catalysts and auxiliaries |journal=Chemical Reviews |volume=92 |issue=5 |pages=935–952 |doi=10.1021/cr00013a009}}</ref> |
|||
Amino acids are used in the synthesis of some [[cosmetics]].<ref name=Leuchtenberger2005/> |
|||
==Aspirational uses== |
|||
===Fertilizer=== |
|||
The [[Chelation|chelating]] ability of amino acids is sometimes used in fertilizers to facilitate the delivery of minerals to plants in order to correct mineral deficiencies, such as iron chlorosis. These fertilizers are also used to prevent deficiencies from occurring and to improve the overall health of the plants.<ref>{{cite book|last=Ashmead|first=H. DeWayne | name-list-style = vanc |title=Foliar Feeding of Plants with Amino Acid Chelates|year=1986|publisher=Noyes Publications|location=Park Ridge}}</ref> |
|||
===Biodegradable plastics=== |
|||
{{further|Biodegradable plastic|Biopolymer}} |
|||
Amino acids have been considered as components of biodegradable polymers, which have applications as [[environmentally friendly]] packaging and in medicine in [[drug delivery]] and the construction of [[prosthetic implant]]s.<ref name=Sanda1999>{{cite journal | vauthors = Sanda F, Endo T | year = 1999 | title = Syntheses and functions of polymers based on amino acids | journal = Macromolecular Chemistry and Physics | volume = 200 | issue = 12 | pages = 2651–2661 | doi = 10.1002/(SICI)1521-3935(19991201)200:12<2651::AID-MACP2651>3.0.CO;2-P | doi-access = free }}</ref> An interesting example of such materials is [[polyaspartate]], a water-soluble biodegradable polymer that may have applications in disposable [[diaper]]s and agriculture.<ref name=Gross2002>{{cite journal | vauthors = Gross RA, Kalra B | title = Biodegradable polymers for the environment | journal = Science | volume = 297 | issue = 5582 | pages = 803–807 | date = August 2002 | pmid = 12161646 | doi = 10.1126/science.297.5582.803 | bibcode = 2002Sci...297..803G | url = https://zenodo.org/record/1231185 | access-date = 12 June 2019 | archive-date = 25 July 2020 | archive-url = https://web.archive.org/web/20200725075829/https://zenodo.org/record/1231185 | url-status = live }}</ref> Due to its solubility and ability to [[chelate]] metal ions, polyaspartate is also being used as a biodegradable anti[[Fouling|scaling]] agent and a [[corrosion inhibitor]].<ref>{{Cite book|title= Commercial poly(aspartic acid) and Its Uses | vauthors = Low KC, Wheeler AP, Koskan LP |series= Advances in Chemistry Series |volume= 248 |publisher= [[American Chemical Society]] |location= Washington, D.C. |year= 1996}}</ref><ref name=Thombre2005>{{cite journal| vauthors = Thombre SM, Sarwade BD | year = 2005 | title = Synthesis and Biodegradability of Polyaspartic Acid: A Critical Review | journal = Journal of Macromolecular Science, Part A | volume = 42 | issue = 9 | pages = 1299–1315 | doi = 10.1080/10601320500189604| s2cid = 94818855 }}</ref> |
|||
==Synthesis== |
|||
{{Main|Amino acid synthesis}} |
|||
===Chemical synthesis=== |
|||
The commercial production of amino acids usually relies on mutant bacteria that overproduce individual amino acids using glucose as a carbon source. Some amino acids are produced by enzymatic conversions of synthetic intermediates. [[2-Aminothiazoline-4-carboxylic acid]] is an intermediate in one industrial synthesis of [[cysteine|<small>L</small>-cysteine]] for example. [[Aspartic acid]] is produced by the addition of ammonia to [[fumarate]] using a lyase.<ref name=Ullmann>{{Ullmann|first1=Karlheinz |last1=Drauz |first2=Ian |last2=Grayson |first3=Axel |last3=Kleemann |first4=Hans-Peter |last4=Krimmer |first5=Wolfgang |last5=Leuchtenberger |first6=Christoph |last6=Weckbecker |name-list-style=vanc |year=2007| doi=10.1002/14356007.a02_057.pub2|title=Amino Acids}}</ref> |
|||
===Biosynthesis=== |
|||
In plants, nitrogen is first assimilated into organic compounds in the form of [[glutamate]], formed from alpha-ketoglutarate and ammonia in the mitochondrion. For other amino acids, plants use [[transaminase]]s to move the amino group from glutamate to another alpha-keto acid. For example, aspartate aminotransferase converts glutamate and oxaloacetate to alpha-ketoglutarate and aspartate.<ref>{{Cite book | last1 = Jones | first1 = Russell Celyn | last2 = Buchanan | first2 = Bob B. | last3 = Gruissem | first3 = Wilhelm | name-list-style = vanc | title = Biochemistry & molecular biology of plants | publisher = American Society of Plant Physiologists | location = Rockville, Md | year = 2000 | pages = [https://archive.org/details/biochemistrymole00buch/page/371 371–372] | isbn = 978-0-943088-39-6 | url = https://archive.org/details/biochemistrymole00buch/page/371 }}</ref> Other organisms use transaminases for amino acid synthesis, too. |
|||
Nonstandard amino acids are usually formed through modifications to standard amino acids. For example, [[homocysteine]] is formed through the [[transsulfuration pathway]] or by the demethylation of methionine via the intermediate metabolite [[S-adenosylmethionine|''S''-adenosylmethionine]],<ref name="Brosnan">{{cite journal | vauthors = Brosnan JT, Brosnan ME | title = The sulfur-containing amino acids: an overview | journal = The Journal of Nutrition | volume = 136 | issue = 6 Suppl | pages = 1636S–1640S | date = June 2006 | pmid = 16702333 | doi = 10.1093/jn/136.6.1636S | doi-access = free }}</ref> while [[hydroxyproline]] is made by a [[post translational modification]] of [[proline]].<ref>{{cite book | vauthors = Kivirikko KI, Pihlajaniemi T | chapter = Collagen Hydroxylases and the Protein Disulfide Isomerase Subunit of Prolyl 4-Hydroxylases | title = Advances in Enzymology and Related Areas of Molecular Biology | volume = 72 | pages = 325–398 | year = 1998 | pmid = 9559057 | doi = 10.1002/9780470123188.ch9 | isbn = 9780470123188 | series = Advances in Enzymology – and Related Areas of Molecular Biology }}</ref> |
|||
[[Microorganism]]s and plants synthesize many uncommon amino acids. For example, some microbes make [[2-aminoisobutyric acid]] and [[lanthionine]], which is a sulfide-bridged derivative of alanine. Both of these amino acids are found in peptidic [[lantibiotics]] such as [[alamethicin]].<ref>{{cite journal | vauthors = Whitmore L, Wallace BA | s2cid = 24638475 | title = Analysis of peptaibol sequence composition: implications for in vivo synthesis and channel formation | journal = European Biophysics Journal | volume = 33 | issue = 3 | pages = 233–237 | date = May 2004 | pmid = 14534753 | doi = 10.1007/s00249-003-0348-1 }}</ref> However, in plants, [[1-aminocyclopropane-1-carboxylic acid]] is a small disubstituted cyclic amino acid that is an intermediate in the production of the plant hormone [[ethylene#Ethylene as a plant hormone|ethylene]].<ref>{{cite journal | vauthors = Alexander L, Grierson D | title = Ethylene biosynthesis and action in tomato: a model for climacteric fruit ripening | journal = Journal of Experimental Botany | volume = 53 | issue = 377 | pages = 2039–2055 | date = October 2002 | pmid = 12324528 | doi = 10.1093/jxb/erf072 | doi-access = free }}</ref> |
|||
===Primordial synthesis=== |
|||
The formation of amino acids and peptides is assumed to have preceded and perhaps induced the [[abiogenesis|emergence of life on earth]]. Amino acids can form from simple precursors under various conditions.<ref name="10.1016/j.gsf.2017.07.007"/> Surface-based chemical metabolism of amino acids and very small compounds may have led to the build-up of amino acids, coenzymes and phosphate-based small carbon molecules.<ref>{{cite journal |last1=Danchin |first1=Antoine |title=From chemical metabolism to life: the origin of the genetic coding process |journal=Beilstein Journal of Organic Chemistry |date=12 June 2017 |volume=13 |issue=1 |pages=1119–1135 |doi=10.3762/bjoc.13.111 |pmid=28684991 |pmc=5480338 |language=en |issn=1860-5397}}</ref>{{additional citation needed|date=September 2022}} Amino acids and similar building blocks could have been elaborated into proto-[[peptide]]s, with peptides being considered key players in the origin of life.<ref name="10.1021/acs.chemrev.9b00664">{{cite journal |last1=Frenkel-Pinter |first1=Moran |last2=Samanta |first2=Mousumi |last3=Ashkenasy |first3=Gonen |last4=Leman |first4=Luke J. |title=Prebiotic Peptides: Molecular Hubs in the Origin of Life |journal=Chemical Reviews |date=10 June 2020 |volume=120 |issue=11 |pages=4707–4765 |doi=10.1021/acs.chemrev.9b00664 |pmid=32101414 |s2cid=211536416 |language=en |issn=0009-2665}}</ref> |
|||
[[File:Strecker amino acid synthesis scheme.svg|class=skin-invert-image|thumb|upright=1.75 |right|The Strecker amino acid synthesis|alt=For the steps in the reaction, see the text.]] |
|||
In the famous [[Urey-Miller experiment]], the passage of an electric arc through a mixture of methane, hydrogen, and ammonia produces a large number of amino acids. Since then, scientists have discovered a range of ways and components by which the potentially prebiotic formation and chemical evolution of peptides may have occurred, such as condensing agents, the design of self-replicating peptides and a number of non-enzymatic mechanisms by which amino acids could have emerged and elaborated into peptides.<ref name="10.1021/acs.chemrev.9b00664"/> Several hypotheses invoke the [[Strecker synthesis]] whereby hydrogen cyanide, simple aldehydes, ammonia, and water produce amino acids.<ref name="10.1016/j.gsf.2017.07.007">{{cite journal |doi=10.1016/j.gsf.2017.07.007|title=Origins of building blocks of life: A review |year=2018 |last1=Kitadai |first1=Norio |last2=Maruyama |first2=Shigenori |journal=Geoscience Frontiers |volume=9 |issue=4 |pages=1117–1153 |bibcode=2018GeoFr...9.1117K |s2cid=102659869 |doi-access=free }}</ref> |
|||
According to a review, amino acids, and even peptides, "turn up fairly regularly in the [[primordial soup|various experimental broths]] that have been allowed to be cooked from simple chemicals. This is because [[nucleotide]]s are far more difficult to synthesize chemically than amino acids." For a chronological order, it suggests that there must have been a 'protein world' or at least a 'polypeptide world', possibly later followed by the '[[RNA world]]' and the '[[DNA world]]'.<ref>{{cite journal |last1=Milner-White |first1=E. James |title=Protein three-dimensional structures at the origin of life |journal=Interface Focus |date=6 December 2019 |volume=9 |issue=6 |pages=20190057 |doi=10.1098/rsfs.2019.0057|pmid=31641431 |pmc=6802138 }}</ref> [[Codon]]–amino acids mappings may be the [[biology|biological]] information system at the primordial origin of life on Earth.<ref>{{cite journal |last1=Chatterjee |first1=Sankar |last2=Yadav |first2=Surya |title=The Coevolution of Biomolecules and Prebiotic Information Systems in the Origin of Life: A Visualization Model for Assembling the First Gene |journal=Life |date=June 2022 |volume=12 |issue=6 |pages=834 |doi=10.3390/life12060834 |pmid=35743865 |pmc=9225589 |bibcode=2022Life...12..834C |language=en |issn=2075-1729|doi-access=free }}</ref> While amino acids and consequently simple peptides must have formed under different experimentally probed geochemical scenarios, the transition from an abiotic world to the first life forms is to a large extent still unresolved.<ref>{{cite journal |last1=Kirschning |first1=Andreas |title=The coenzyme/protein pair and the molecular evolution of life |journal=Natural Product Reports |date=26 May 2021 |volume=38 |issue=5 |pages=993–1010 |doi=10.1039/D0NP00037J |pmid=33206101 |s2cid=227037164 |language=en |issn=1460-4752|doi-access=free }}</ref> |
|||
==Reactions== |
|||
Amino acids undergo the reactions expected of the constituent functional groups.<ref>{{cite book | vauthors = Elmore DT, Barrett GC | title = Amino acids and peptides | url = https://archive.org/details/aminoacidspeptid00barr_040 | url-access = limited |publisher=Cambridge University Press |location=Cambridge, UK |year=1998 |pages=[https://archive.org/details/aminoacidspeptid00barr_040/page/n64 48]–60 |isbn=978-0-521-46827-5}}</ref><ref>{{cite journal | vauthors = Gutteridge A, Thornton JM | title = Understanding nature's catalytic toolkit | journal = Trends in Biochemical Sciences | volume = 30 | issue = 11 | pages = 622–629 | date = November 2005 | pmid = 16214343 | doi = 10.1016/j.tibs.2005.09.006 }}</ref> |
|||
===Peptide bond formation=== |
|||
{{see also|Peptide synthesis|Peptide bond}} |
|||
[[File:Peptidformationball.svg|thumbnail|right|upright=1.75 |The condensation of two amino acids to form a [[dipeptide]]. The two amino acid ''residues'' are linked through a ''[[peptide bond]]''.|alt=Two amino acids are shown next to each other. One loses a hydrogen and oxygen from its carboxyl group (COOH) and the other loses a hydrogen from its amino group (NH2). This reaction produces a molecule of water (H2O) and two amino acids joined by a peptide bond (–CO–NH–). The two joined amino acids are called a dipeptide.]] |
|||
As both the amine and carboxylic acid groups of amino acids can react to form amide bonds, one amino acid molecule can react with another and become joined through an amide linkage. This [[polymerization]] of amino acids is what creates proteins. This [[condensation reaction]] yields the newly formed peptide bond and a molecule of water. In cells, this reaction does not occur directly; instead, the amino acid is first activated by attachment to a [[transfer RNA]] molecule through an [[ester]] bond. This aminoacyl-tRNA is produced in an [[Adenosine triphosphate|ATP]]-dependent reaction carried out by an [[aminoacyl tRNA synthetase]].<ref>{{cite journal |vauthors=Ibba M, Söll D |title=The renaissance of aminoacyl-tRNA synthesis |journal=EMBO Reports |volume=2 |issue=5 |pages=382–387 |date=May 2001 |pmid=11375928 |pmc=1083889 |doi=10.1093/embo-reports/kve095}}</ref> This aminoacyl-tRNA is then a substrate for the ribosome, which catalyzes the attack of the amino group of the elongating protein chain on the ester bond.<ref>{{cite journal |vauthors=Lengyel P, Söll D |title=Mechanism of protein biosynthesis |journal=Bacteriological Reviews |volume=33 |issue=2 |pages=264–301 |date=June 1969 |pmid=4896351 |pmc=378322 |doi=10.1128/MMBR.33.2.264-301.1969}}</ref> As a result of this mechanism, all proteins made by ribosomes are synthesized starting at their ''N''-terminus and moving toward their ''C''-terminus. |
|||
However, not all peptide bonds are formed in this way. In a few cases, peptides are synthesized by specific enzymes. For example, the tripeptide [[glutathione]] is an essential part of the defenses of cells against oxidative stress. This peptide is synthesized in two steps from free amino acids.<ref>{{cite journal |vauthors=Wu G, Fang YZ, Yang S, Lupton JR, Turner ND |title=Glutathione metabolism and its implications for health |journal=The Journal of Nutrition |volume=134 |issue=3 |pages=489–492 |date=March 2004 |pmid=14988435 |doi=10.1093/jn/134.3.489 |doi-access=free}}</ref> In the first step, [[gamma-glutamylcysteine synthetase]] condenses cysteine and [[glutamate]] through a peptide bond formed between the side chain carboxyl of the glutamate (the gamma carbon of this side chain) and the amino group of the cysteine. This dipeptide is then condensed with glycine by [[glutathione synthetase]] to form glutathione.<ref>{{cite journal |vauthors=Meister A |title=Glutathione metabolism and its selective modification |journal=The Journal of Biological Chemistry |volume=263 |issue=33 |pages=17205–17208 |date=November 1988 |doi=10.1016/S0021-9258(19)77815-6 |pmid=3053703 |doi-access=free}}</ref> |
|||
In chemistry, peptides are synthesized by a variety of reactions. One of the most-used in [[peptide synthesis|solid-phase peptide synthesis]] uses the aromatic oxime derivatives of amino acids as activated units. These are added in sequence onto the growing peptide chain, which is attached to a solid resin support.<ref>{{cite journal |first1=Louis A. |last1=Carpino |name-list-style=vanc |year=1992 |title=1-Hydroxy-7-azabenzotriazole. An efficient peptide coupling additive |journal=Journal of the American Chemical Society |volume=115 |issue=10 |pages=4397–4398 |doi=10.1021/ja00063a082}}</ref> Libraries of peptides are used in drug discovery through [[high-throughput screening]].<ref>{{cite journal |vauthors=Marasco D, Perretta G, Sabatella M, Ruvo M |title=Past and future perspectives of synthetic peptide libraries |journal=Current Protein & Peptide Science |volume=9 |issue=5 |pages=447–467 |date=October 2008 |pmid=18855697 |doi=10.2174/138920308785915209}}</ref> |
|||
The combination of functional groups allow amino acids to be effective polydentate ligands for metal–amino acid chelates.<ref>{{cite journal |vauthors=Konara S, Gagnona K, Clearfield A, Thompson C, Hartle J, Ericson C, Nelson C |title=Structural determination and characterization of copper and zinc bis-glycinates with X-ray crystallography and mass spectrometry |journal=Journal of Coordination Chemistry |year=2010 |volume=63 |issue=19 |doi=10.1080/00958972.2010.514336 |pages=3335–3347 |s2cid=94822047}}</ref> |
|||
The multiple side chains of amino acids can also undergo chemical reactions. |
|||
===Catabolism=== |
|||
[[File:Amino acid catabolism revised.png|class=skin-invert-image|thumb|upright=1.75 |Catabolism of proteinogenic amino acids. Amino acids can be classified according to the properties of their main degradation products:<ref>{{cite book |vauthors=Stipanuk MH |date=2006 |title=Biochemical, physiological, & molecular aspects of human nutrition |edition=2nd |publisher=Saunders Elsevier}}</ref> |
|||
<br/>* ''Glucogenic'', with the products having the ability to form [[glucose]] by [[gluconeogenesis]] |
|||
<br/>* ''Ketogenic'', with the products not having the ability to form glucose. These products may still be used for [[ketogenesis]] or [[lipid synthesis]]. |
|||
<br/>* Amino acids catabolized into both glucogenic and ketogenic products.]] |
|||
Degradation of an amino acid often involves [[deamination]] by moving its amino group to α-ketoglutarate, forming [[glutamate]]. This process involves transaminases, often the same as those used in amination during synthesis. In many vertebrates, the amino group is then removed through the [[urea cycle]] and is excreted in the form of [[urea]]. However, amino acid degradation can produce [[uric acid]] or ammonia instead. For example, [[serine dehydratase]] converts serine to pyruvate and ammonia.<ref name = "Stryer_2002" /> After removal of one or more amino groups, the remainder of the molecule can sometimes be used to synthesize new amino acids, or it can be used for energy by entering [[glycolysis]] or the [[citric acid cycle]], as detailed in image at right. |
|||
===Complexation=== |
|||
Amino acids are bidentate ligands, forming [[transition metal amino acid complexes]].<ref>{{cite journal |vauthors=Dghaym RD, Dhawan R, Arndtsen BA |title=The Use of Carbon Monoxide and Imines as Peptide Derivative Synthons: A Facile Palladium-Catalyzed Synthesis of α-Amino Acid Derived Imidazolines |journal=Angewandte Chemie |volume=40 |issue=17 |pages=3228–3230 |date=September 2001 |pmid=29712039 |doi=10.1002/(SICI)1521-3773(19980703)37:12<1634::AID-ANIE1634>3.0.CO;2-C}}</ref> |
|||
[[File:AAcomplexation.png|class=skin-invert-image|420px]] |
|||
==Chemical analysis== |
|||
The total nitrogen content of organic matter is mainly formed by the amino groups in proteins. The Total Kjeldahl Nitrogen ([[TKN]]) is a measure of nitrogen widely used in the analysis of (waste) water, soil, food, feed and organic matter in general. As the name suggests, the [[Kjeldahl method]] is applied. More sensitive methods are available.<ref name="pmid23959242">{{cite journal | vauthors = Muñoz-Huerta RF, Guevara-Gonzalez RG, Contreras-Medina LM, Torres-Pacheco I, Prado-Olivarez J, Ocampo-Velazquez RV | title = A review of methods for sensing the nitrogen status in plants: advantages, disadvantages and recent advances | journal = Sensors | location = Basel, Switzerland | volume = 13 | issue = 8 | pages = 10823–43 | date = August 2013 | pmid = 23959242 | pmc = 3812630 | doi = 10.3390/s130810823 | bibcode = 2013Senso..1310823M | doi-access = free }}</ref><ref>{{cite journal | vauthors = Martin PD, Malley DF, Manning G, Fuller L |date=2002 |title=Determination of soil organic carbon and nitrogen at thefield level using near-infrared spectroscopy |journal=Canadian Journal of Soil Science |volume=82 |issue=4 |pages=413–422 |doi=10.4141/S01-054 }}</ref> |
|||
== See also == |
== See also == |
||
| Line 709: | Line 706: | ||
* [[Miller–Urey experiment]] |
* [[Miller–Urey experiment]] |
||
* [[Nucleic acid sequence]] |
* [[Nucleic acid sequence]] |
||
* [[ |
* [[RNA codon table]] |
||
* [[RNA codon table|Table of codons]], 3-nucleotide sequences that encode each amino acid |
|||
{{div col end}} |
{{div col end}} |
||
== |
== Notes == |
||
{{ |
{{notelist}} |
||
== References == |
|||
{{Reflist}} |
|||
== Further reading == |
== Further reading == |
||
{{refbegin}} |
{{refbegin}} |
||
* {{cite book | last = Tymoczko | first = John L. | name-list- |
* {{cite book | last = Tymoczko | first = John L. | name-list-style = vanc | year = 2012 | title = Biochemistry | url = https://archive.org/details/biochemistryseve00berg | url-access = limited | publisher = W. H. Freeman and company | location = New York | chapter = Protein Composition and Structure | pages = 28–31 | chapter-url = https://archive.org/details/biochemistryseve00berg/page/n61 | isbn = 9781429229364}} |
||
* {{cite book | |
* {{cite book | vauthors = Doolittle RF | author-link = Russell Doolittle | veditors = Fasman GD | year = 1989 | title = Predictions of Protein Structure and the Principles of Protein Conformation | publisher = [[Plenum Press]] | location = New York | chapter = Redundancies in protein sequences | pages = 599–623 | isbn = 978-0-306-43131-9 | lccn = 89008555}} |
||
* {{cite book | last1 = Nelson | first1 = David L. | last2 = Cox | first2 = Michael M. | name-list- |
* {{cite book | last1 = Nelson | first1 = David L. | last2 = Cox | first2 = Michael M. | name-list-style = vanc | year = 2000 | title = Lehninger Principles of Biochemistry | publisher = [[Worth Publishers]] | edition = 3rd | isbn = 978-1-57259-153-0 | lccn = 99049137 | url-access = registration | url = https://archive.org/details/lehningerprincip01lehn}} |
||
* {{cite book|last=Meierhenrich |first=Uwe |name-list- |
* {{cite book | last = Meierhenrich | first = Uwe | name-list-style = vanc | author-link = Uwe Meierhenrich | year = 2008 | title = Amino acids and the asymmetry of life | publisher = [[Springer Verlag]] | location = Berlin | isbn = 978-3-540-76885-2 | lccn = 2008930865 | url = http://rogov.zwz.ru/Macroevolution/amino.pdf | url-status = dead | archive-url = https://web.archive.org/web/20120112005425/http://rogov.zwz.ru/Macroevolution/amino.pdf | archive-date = 12 January 2012}} |
||
{{refend}} |
{{refend}} |
||
| Line 728: | Line 727: | ||
{{Amino acids}} |
{{Amino acids}} |
||
{{Chemical bonds}} |
|||
{{Protein primary structure}} |
{{Protein primary structure}} |
||
{{Amino acid metabolism enzymes}} |
{{Amino acid metabolism enzymes}} |
||
{{Authority control}} |
{{Authority control}} |
||
{{Good article}} |
|||
{{DEFAULTSORT:Amino Acid}} |
{{DEFAULTSORT:Amino Acid}} |
||
Latest revision as of 02:36, 8 January 2025

Amino acids are organic compounds that contain both amino and carboxylic acid functional groups.[1] Although over 500 amino acids exist in nature, by far the most important are the 22 α-amino acids incorporated into proteins.[2] Only these 22 appear in the genetic code of life.[3][4]
Amino acids can be classified according to the locations of the core structural functional groups (alpha- (α-), beta- (β-), gamma- (γ-) amino acids, etc.); other categories relate to polarity, ionization, and side-chain group type (aliphatic, acyclic, aromatic, polar, etc.). In the form of proteins, amino-acid residues form the second-largest component (water being the largest) of human muscles and other tissues.[5] Beyond their role as residues in proteins, amino acids participate in a number of processes such as neurotransmitter transport and biosynthesis. It is thought that they played a key role in enabling life on Earth and its emergence.[6]
Amino acids are formally named by the IUPAC-IUBMB Joint Commission on Biochemical Nomenclature in terms of the fictitious "neutral" structure shown in the illustration. For example, the systematic name of alanine is 2-aminopropanoic acid, based on the formula CH3−CH(NH2)−COOH. The Commission justified this approach as follows:[7]
The systematic names and formulas given refer to hypothetical forms in which amino groups are unprotonated and carboxyl groups are undissociated. This convention is useful to avoid various nomenclatural problems but should not be taken to imply that these structures represent an appreciable fraction of the amino-acid molecules.
History
[edit]The first few amino acids were discovered in the early 1800s.[8][9] In 1806, French chemists Louis-Nicolas Vauquelin and Pierre Jean Robiquet isolated a compound from asparagus that was subsequently named asparagine, the first amino acid to be discovered.[10][11] Cystine was discovered in 1810,[12] although its monomer, cysteine, remained undiscovered until 1884.[13][11][a] Glycine and leucine were discovered in 1820.[14] The last of the 20 common amino acids to be discovered was threonine in 1935 by William Cumming Rose, who also determined the essential amino acids and established the minimum daily requirements of all amino acids for optimal growth.[15][16]
The unity of the chemical category was recognized by Wurtz in 1865, but he gave no particular name to it.[17] The first use of the term "amino acid" in the English language dates from 1898,[18] while the German term, Aminosäure, was used earlier.[19] Proteins were found to yield amino acids after enzymatic digestion or acid hydrolysis. In 1902, Emil Fischer and Franz Hofmeister independently proposed that proteins are formed from many amino acids, whereby bonds are formed between the amino group of one amino acid with the carboxyl group of another, resulting in a linear structure that Fischer termed "peptide".[20]
General structure
[edit]
2-, alpha-, or α-amino acids[21] have the generic formula H2NCHRCOOH in most cases,[b] where R is an organic substituent known as a "side chain".[22]
Of the many hundreds of described amino acids, 22 are proteinogenic ("protein-building").[23][24][25] It is these 22 compounds that combine to give a vast array of peptides and proteins assembled by ribosomes.[26] Non-proteinogenic or modified amino acids may arise from post-translational modification or during nonribosomal peptide synthesis.
Chirality
[edit]The carbon atom next to the carboxyl group is called the α–carbon. In proteinogenic amino acids, it bears the amine and the R group or side chain specific to each amino acid, as well as a hydrogen atom. With the exception of glycine, for which the side chain is also a hydrogen atom, the α–carbon is stereogenic. All chiral proteogenic amino acids have the L configuration. They are "left-handed" enantiomers, which refers to the stereoisomers of the alpha carbon.
A few D-amino acids ("right-handed") have been found in nature, e.g., in bacterial envelopes, as a neuromodulator (D-serine), and in some antibiotics.[27][28] Rarely, D-amino acid residues are found in proteins, and are converted from the L-amino acid as a post-translational modification.[29][c]
Side chains
[edit]Polar charged side chains
[edit]Five amino acids possess a charge at neutral pH. Often these side chains appear at the surfaces on proteins to enable their solubility in water, and side chains with opposite charges form important electrostatic contacts called salt bridges that maintain structures within a single protein or between interfacing proteins.[32] Many proteins bind metal into their structures specifically, and these interactions are commonly mediated by charged side chains such as aspartate, glutamate and histidine. Under certain conditions, each ion-forming group can be charged, forming double salts.[33]
The two negatively charged amino acids at neutral pH are aspartate (Asp, D) and glutamate (Glu, E). The anionic carboxylate groups behave as Brønsted bases in most circumstances.[32] Enzymes in very low pH environments, like the aspartic protease pepsin in mammalian stomachs, may have catalytic aspartate or glutamate residues that act as Brønsted acids.

There are three amino acids with side chains that are cations at neutral pH: arginine (Arg, R), lysine (Lys, K) and histidine (His, H). Arginine has a charged guanidino group and lysine a charged alkyl amino group, and are fully protonated at pH 7. Histidine's imidazole group has a pKa of 6.0, and is only around 10% protonated at neutral pH. Because histidine is easily found in its basic and conjugate acid forms it often participates in catalytic proton transfers in enzyme reactions.[32]
Polar uncharged side chains
[edit]The polar, uncharged amino acids serine (Ser, S), threonine (Thr, T), asparagine (Asn, N) and glutamine (Gln, Q) readily form hydrogen bonds with water and other amino acids.[32] They do not ionize in normal conditions, a prominent exception being the catalytic serine in serine proteases. This is an example of severe perturbation, and is not characteristic of serine residues in general. Threonine has two chiral centers, not only the L (2S) chiral center at the α-carbon shared by all amino acids apart from achiral glycine, but also (3R) at the β-carbon. The full stereochemical specification is (2S,3R)-L-threonine.
Hydrophobic side chains
[edit]Nonpolar amino acid interactions are the primary driving force behind the processes that fold proteins into their functional three dimensional structures.[32] None of these amino acids' side chains ionize easily, and therefore do not have pKas, with the exception of tyrosine (Tyr, Y). The hydroxyl of tyrosine can deprotonate at high pH forming the negatively charged phenolate. Because of this one could place tyrosine into the polar, uncharged amino acid category, but its very low solubility in water matches the characteristics of hydrophobic amino acids well.
Special case side chains
[edit]Several side chains are not described well by the charged, polar and hydrophobic categories. Glycine (Gly, G) could be considered a polar amino acid since its small size means that its solubility is largely determined by the amino and carboxylate groups. However, the lack of any side chain provides glycine with a unique flexibility among amino acids with large ramifications to protein folding.[32] Cysteine (Cys, C) can also form hydrogen bonds readily, which would place it in the polar amino acid category, though it can often be found in protein structures forming covalent bonds, called disulphide bonds, with other cysteines. These bonds influence the folding and stability of proteins, and are essential in the formation of antibodies. Proline (Pro, P) has an alkyl side chain and could be considered hydrophobic, but because the side chain joins back onto the alpha amino group it becomes particularly inflexible when incorporated into proteins. Similar to glycine this influences protein structure in a way unique among amino acids. Selenocysteine (Sec, U) is a rare amino acid not directly encoded by DNA, but is incorporated into proteins via the ribosome. Selenocysteine has a lower redox potential compared to the similar cysteine, and participates in several unique enzymatic reactions.[34] Pyrrolysine (Pyl, O) is another amino acid not encoded in DNA, but synthesized into protein by ribosomes.[35] It is found in archaeal species where it participates in the catalytic activity of several methyltransferases.
β- and γ-amino acids
[edit]Amino acids with the structure NH+3−CXY−CXY−CO−2, such as β-alanine, a component of carnosine and a few other peptides, are β-amino acids. Ones with the structure NH+3−CXY−CXY−CXY−CO−2 are γ-amino acids, and so on, where X and Y are two substituents (one of which is normally H).[7]
Zwitterions
[edit]
The common natural forms of amino acids have a zwitterionic structure, with −NH+3 (−NH+2− in the case of proline) and −CO−2 functional groups attached to the same C atom, and are thus α-amino acids, and are the only ones found in proteins during translation in the ribosome. In aqueous solution at pH close to neutrality, amino acids exist as zwitterions, i.e. as dipolar ions with both NH+3 and CO−2 in charged states, so the overall structure is NH+3−CHR−CO−2. At physiological pH the so-called "neutral forms" −NH2−CHR−CO2H are not present to any measurable degree.[36] Although the two charges in the zwitterion structure add up to zero it is misleading to call a species with a net charge of zero "uncharged".
In strongly acidic conditions (pH below 3), the carboxylate group becomes protonated and the structure becomes an ammonio carboxylic acid, NH+3−CHR−CO2H. This is relevant for enzymes like pepsin that are active in acidic environments such as the mammalian stomach and lysosomes, but does not significantly apply to intracellular enzymes. In highly basic conditions (pH greater than 10, not normally seen in physiological conditions), the ammonio group is deprotonated to give NH2−CHR−CO−2.
Although various definitions of acids and bases are used in chemistry, the only one that is useful for chemistry in aqueous solution is that of Brønsted:[37][38] an acid is a species that can donate a proton to another species, and a base is one that can accept a proton. This criterion is used to label the groups in the above illustration. The carboxylate side chains of aspartate and glutamate residues are the principal Brønsted bases in proteins. Likewise, lysine, tyrosine and cysteine will typically act as a Brønsted acid. Histidine under these conditions can act both as a Brønsted acid and a base.
Isoelectric point
[edit]
For amino acids with uncharged side-chains the zwitterion predominates at pH values between the two pKa values, but coexists in equilibrium with small amounts of net negative and net positive ions. At the midpoint between the two pKa values, the trace amount of net negative and trace of net positive ions balance, so that average net charge of all forms present is zero.[39] This pH is known as the isoelectric point pI, so pI = 1/2(pKa1 + pKa2).
For amino acids with charged side chains, the pKa of the side chain is involved. Thus for aspartate or glutamate with negative side chains, the terminal amino group is essentially entirely in the charged form −NH+3, but this positive charge needs to be balanced by the state with just one C-terminal carboxylate group is negatively charged. This occurs halfway between the two carboxylate pKa values: pI = 1/2(pKa1 + pKa(R)), where pKa(R) is the side chain pKa.[38]
Similar considerations apply to other amino acids with ionizable side-chains, including not only glutamate (similar to aspartate), but also cysteine, histidine, lysine, tyrosine and arginine with positive side chains.
Amino acids have zero mobility in electrophoresis at their isoelectric point, although this behaviour is more usually exploited for peptides and proteins than single amino acids. Zwitterions have minimum solubility at their isoelectric point, and some amino acids (in particular, with nonpolar side chains) can be isolated by precipitation from water by adjusting the pH to the required isoelectric point.
Physicochemical properties
[edit]The 20 canonical amino acids can be classified according to their properties. Important factors are charge, hydrophilicity or hydrophobicity, size, and functional groups.[28] These properties influence protein structure and protein–protein interactions. The water-soluble proteins tend to have their hydrophobic residues (Leu, Ile, Val, Phe, and Trp) buried in the middle of the protein, whereas hydrophilic side chains are exposed to the aqueous solvent. (In biochemistry, a residue refers to a specific monomer within the polymeric chain of a polysaccharide, protein or nucleic acid.) The integral membrane proteins tend to have outer rings of exposed hydrophobic amino acids that anchor them in the lipid bilayer. Some peripheral membrane proteins have a patch of hydrophobic amino acids on their surface that sticks to the membrane. In a similar fashion, proteins that have to bind to positively charged molecules have surfaces rich in negatively charged amino acids such as glutamate and aspartate, while proteins binding to negatively charged molecules have surfaces rich in positively charged amino acids like lysine and arginine. For example, lysine and arginine are present in large amounts in the low-complexity regions of nucleic-acid binding proteins.[40] There are various hydrophobicity scales of amino acid residues.[41]
Some amino acids have special properties. Cysteine can form covalent disulfide bonds to other cysteine residues. Proline forms a cycle to the polypeptide backbone, and glycine is more flexible than other amino acids.
Glycine and proline are strongly present within low complexity regions of both eukaryotic and prokaryotic proteins, whereas the opposite is the case with cysteine, phenylalanine, tryptophan, methionine, valine, leucine, isoleucine, which are highly reactive, or complex, or hydrophobic.[40][42][43]
Many proteins undergo a range of posttranslational modifications, whereby additional chemical groups are attached to the amino acid residue side chains sometimes producing lipoproteins (that are hydrophobic),[44] or glycoproteins (that are hydrophilic)[45] allowing the protein to attach temporarily to a membrane. For example, a signaling protein can attach and then detach from a cell membrane, because it contains cysteine residues that can have the fatty acid palmitic acid added to them and subsequently removed.[46]
Table of standard amino acid abbreviations and properties
[edit]Although one-letter symbols are included in the table, IUPAC–IUBMB recommend[7] that "Use of the one-letter symbols should be restricted to the comparison of long sequences".
The one-letter notation was chosen by IUPAC-IUB based on the following rules:[47]
- Initial letters are used where there is no ambuiguity: C cysteine, H histidine, I isoleucine, M methionine, S serine, V valine,[47]
- Where arbitrary assignment is needed, the structurally simpler amino acids are given precedence: A Alanine, G glycine, L leucine, P proline, T threonine,[47]
- F PHenylalanine and R aRginine are assigned by being phonetically suggestive,[47]
- W tryptophan is assigned based on the double ring being visually suggestive to the bulky letter W,[47]
- K lysine and Y tyrosine are assigned as alphabetically nearest to their initials L and T (note that U was avoided for its similarity with V, while X was reserved for undetermined or atypical amino acids); for tyrosine the mnemonic tYrosine was also proposed,[48]
- D aspartate was assigned arbitrarily, with the proposed mnemonic asparDic acid;[49] E glutamate was assigned in alphabetical sequence being larger by merely one methylene –CH2– group,[48]
- N asparagine was assigned arbitrarily, with the proposed mnemonic asparagiNe;[49] Q glutamine was assigned in alphabetical sequence of those still available (note again that O was avoided due to similarity with D), with the proposed mnemonic Qlutamine.[49]
| Amino acid | 3- and 1-letter symbols | Side chain | Hydropathy index[50] |
Molar absorptivity[51] | Molecular mass | Abundance in proteins (%)[52] | Standard genetic coding, IUPAC notation | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 | 1 | Class | Chemical polarity[53] | Net charge at pH 7.4[53] |
Wavelength, λmax (nm) |
Coefficient ε (mM−1·cm−1) | |||||
| Alanine | Ala | A | Aliphatic | Nonpolar | Neutral | 1.8 | 89.094 | 8.76 | GCN | ||
| Arginine | Arg | R | Fixed cation | Basic polar | Positive | −4.5 | 174.203 | 5.78 | MGR, CGY[d] | ||
| Asparagine | Asn | N | Amide | Polar | Neutral | −3.5 | 132.119 | 3.93 | AAY | ||
| Aspartate | Asp | D | Anion | Brønsted base | Negative | −3.5 | 133.104 | 5.49 | GAY | ||
| Cysteine | Cys | C | Thiol | Brønsted acid | Neutral | 2.5 | 250 | 0.3 | 121.154 | 1.38 | UGY |
| Glutamine | Gln | Q | Amide | Polar | Neutral | −3.5 | 146.146 | 3.9 | CAR | ||
| Glutamate | Glu | E | Anion | Brønsted base | Negative | −3.5 | 147.131 | 6.32 | GAR | ||
| Glycine | Gly | G | Aliphatic | Nonpolar | Neutral | −0.4 | 75.067 | 7.03 | GGN | ||
| Histidine | His | H | Cationic | Brønsted acid and base | Positive, 10% Neutral, 90% |
−3.2 | 211 | 5.9 | 155.156 | 2.26 | CAY |
| Isoleucine | Ile | I | Aliphatic | Nonpolar | Neutral | 4.5 | 131.175 | 5.49 | AUH | ||
| Leucine | Leu | L | Aliphatic | Nonpolar | Neutral | 3.8 | 131.175 | 9.68 | YUR, CUY[e] | ||
| Lysine | Lys | K | Cation | Brønsted acid | Positive | −3.9 | 146.189 | 5.19 | AAR | ||
| Methionine | Met | M | Thioether | Nonpolar | Neutral | 1.9 | 149.208 | 2.32 | AUG | ||
| Phenylalanine | Phe | F | Aromatic | Nonpolar | Neutral | 2.8 | 257, 206, 188 | 0.2, 9.3, 60.0 | 165.192 | 3.87 | UUY |
| Proline | Pro | P | Cyclic | Nonpolar | Neutral | −1.6 | 115.132 | 5.02 | CCN | ||
| Serine | Ser | S | Hydroxylic | Polar | Neutral | −0.8 | 105.093 | 7.14 | UCN, AGY | ||
| Threonine | Thr | T | Hydroxylic | Polar | Neutral | −0.7 | 119.119 | 5.53 | ACN | ||
| Tryptophan | Trp | W | Aromatic | Nonpolar | Neutral | −0.9 | 280, 219 | 5.6, 47.0 | 204.228 | 1.25 | UGG |
| Tyrosine | Tyr | Y | Aromatic | Brønsted acid | Neutral | −1.3 | 274, 222, 193 | 1.4, 8.0, 48.0 | 181.191 | 2.91 | UAY |
| Valine | Val | V | Aliphatic | Nonpolar | Neutral | 4.2 | 117.148 | 6.73 | GUN | ||
Two additional amino acids are in some species coded for by codons that are usually interpreted as stop codons:
| 21st and 22nd amino acids | 3-letter | 1-letter | Molecular mass |
|---|---|---|---|
| Selenocysteine | Sec | U | 168.064 |
| Pyrrolysine | Pyl | O | 255.313 |
In addition to the specific amino acid codes, placeholders are used in cases where chemical or crystallographic analysis of a peptide or protein cannot conclusively determine the identity of a residue. They are also used to summarize conserved protein sequence motifs. The use of single letters to indicate sets of similar residues is similar to the use of abbreviation codes for degenerate bases.[54][55]
| Ambiguous amino acids | 3-letter | 1-letter | Amino acids included | Codons included |
|---|---|---|---|---|
| Any / unknown | Xaa | X | All | NNN |
| Asparagine or aspartate | Asx | B | D, N | RAY |
| Glutamine or glutamate | Glx | Z | E, Q | SAR |
| Leucine or isoleucine | Xle | J | I, L | YTR, ATH, CTY[f] |
| Hydrophobic | Φ | V, I, L, F, W, Y, M | NTN, TAY, TGG | |
| Aromatic | Ω | F, W, Y, H | YWY, TTY, TGG[g] | |
| Aliphatic (non-aromatic) | Ψ | V, I, L, M | VTN, TTR[h] | |
| Small | π | P, G, A, S | BCN, RGY, GGR | |
| Hydrophilic | ζ | S, T, H, N, Q, E, D, K, R | VAN, WCN, CGN, AGY[i] | |
| Positively-charged | + | K, R, H | ARR, CRY, CGR | |
| Negatively-charged | − | D, E | GAN |
Unk is sometimes used instead of Xaa, but is less standard.
Ter or * (from termination) is used in notation for mutations in proteins when a stop codon occurs. It corresponds to no amino acid at all.[56]
In addition, many nonstandard amino acids have a specific code. For example, several peptide drugs, such as Bortezomib and MG132, are artificially synthesized and retain their protecting groups, which have specific codes. Bortezomib is Pyz–Phe–boroLeu, and MG132 is Z–Leu–Leu–Leu–al. To aid in the analysis of protein structure, photo-reactive amino acid analogs are available. These include photoleucine (pLeu) and photomethionine (pMet).[57]
Occurrence and functions in biochemistry
[edit]Proteinogenic amino acids
[edit]Amino acids are the precursors to proteins.[26] They join by condensation reactions to form short polymer chains called peptides or longer chains called either polypeptides or proteins. These chains are linear and unbranched, with each amino acid residue within the chain attached to two neighboring amino acids. In nature, the process of making proteins encoded by RNA genetic material is called translation and involves the step-by-step addition of amino acids to a growing protein chain by a ribozyme that is called a ribosome.[58] The order in which the amino acids are added is read through the genetic code from an mRNA template, which is an RNA derived from one of the organism's genes.
Twenty-two amino acids are naturally incorporated into polypeptides and are called proteinogenic or natural amino acids.[28] Of these, 20 are encoded by the universal genetic code. The remaining 2, selenocysteine and pyrrolysine, are incorporated into proteins by unique synthetic mechanisms. Selenocysteine is incorporated when the mRNA being translated includes a SECIS element, which causes the UGA codon to encode selenocysteine instead of a stop codon.[59] Pyrrolysine is used by some methanogenic archaea in enzymes that they use to produce methane. It is coded for with the codon UAG, which is normally a stop codon in other organisms.[60]
Several independent evolutionary studies have suggested that Gly, Ala, Asp, Val, Ser, Pro, Glu, Leu, Thr may belong to a group of amino acids that constituted the early genetic code, whereas Cys, Met, Tyr, Trp, His, Phe may belong to a group of amino acids that constituted later additions of the genetic code.[61][62][63]
Standard vs nonstandard amino acids
[edit]The 20 amino acids that are encoded directly by the codons of the universal genetic code are called standard or canonical amino acids. A modified form of methionine (N-formylmethionine) is often incorporated in place of methionine as the initial amino acid of proteins in bacteria, mitochondria and plastids (including chloroplasts). Other amino acids are called nonstandard or non-canonical. Most of the nonstandard amino acids are also non-proteinogenic (i.e. they cannot be incorporated into proteins during translation), but two of them are proteinogenic, as they can be incorporated translationally into proteins by exploiting information not encoded in the universal genetic code.
The two nonstandard proteinogenic amino acids are selenocysteine (present in many non-eukaryotes as well as most eukaryotes, but not coded directly by DNA) and pyrrolysine (found only in some archaea and at least one bacterium). The incorporation of these nonstandard amino acids is rare. For example, 25 human proteins include selenocysteine in their primary structure,[64] and the structurally characterized enzymes (selenoenzymes) employ selenocysteine as the catalytic moiety in their active sites.[65] Pyrrolysine and selenocysteine are encoded via variant codons. For example, selenocysteine is encoded by stop codon and SECIS element.[66][67][68]
N-formylmethionine (which is often the initial amino acid of proteins in bacteria, mitochondria, and chloroplasts) is generally considered as a form of methionine rather than as a separate proteinogenic amino acid. Codon–tRNA combinations not found in nature can also be used to "expand" the genetic code and form novel proteins known as alloproteins incorporating non-proteinogenic amino acids.[69][70][71]
Non-proteinogenic amino acids
[edit]Aside from the 22 proteinogenic amino acids, many non-proteinogenic amino acids are known. Those either are not found in proteins (for example carnitine, GABA, levothyroxine) or are not produced directly and in isolation by standard cellular machinery. For example, hydroxyproline, is synthesised from proline. Another example is selenomethionine).
Non-proteinogenic amino acids that are found in proteins are formed by post-translational modification. Such modifications can also determine the localization of the protein, e.g., the addition of long hydrophobic groups can cause a protein to bind to a phospholipid membrane.[72] Examples:
- the carboxylation of glutamate allows for better binding of calcium cations,[73]
- Hydroxyproline, generated by hydroxylation of proline, is a major component of the connective tissue collagen.[74]
- Hypusine in the translation initiation factor EIF5A, contains a modification of lysine.[75]
Some non-proteinogenic amino acids are not found in proteins. Examples include 2-aminoisobutyric acid and the neurotransmitter gamma-aminobutyric acid. Non-proteinogenic amino acids often occur as intermediates in the metabolic pathways for standard amino acids – for example, ornithine and citrulline occur in the urea cycle, part of amino acid catabolism (see below).[76] A rare exception to the dominance of α-amino acids in biology is the β-amino acid beta alanine (3-aminopropanoic acid), which is used in plants and microorganisms in the synthesis of pantothenic acid (vitamin B5), a component of coenzyme A.[77]
In mammalian nutrition
[edit]
Amino acids are not typical component of food: animals eat proteins. The protein is broken down into amino acids in the process of digestion. They are then used to synthesize new proteins, other biomolecules, or are oxidized to urea and carbon dioxide as a source of energy.[78] The oxidation pathway starts with the removal of the amino group by a transaminase; the amino group is then fed into the urea cycle. The other product of transamidation is a keto acid that enters the citric acid cycle.[79] Glucogenic amino acids can also be converted into glucose, through gluconeogenesis.[80]
Of the 20 standard amino acids, nine (His, Ile, Leu, Lys, Met, Phe, Thr, Trp and Val) are called essential amino acids because the human body cannot synthesize them from other compounds at the level needed for normal growth, so they must be obtained from food.[81][82][83]
Semi-essential and conditionally essential amino acids, and juvenile requirements
[edit]In addition, cysteine, tyrosine, and arginine are considered semiessential amino acids, and taurine a semi-essential aminosulfonic acid in children. Some amino acids are conditionally essential for certain ages or medical conditions. Essential amino acids may also vary from species to species.[j] The metabolic pathways that synthesize these monomers are not fully developed.[84][85]
Non-protein functions
[edit]Many proteinogenic and non-proteinogenic amino acids have biological functions beyond being precursors to proteins and peptides.In humans, amino acids also have important roles in diverse biosynthetic pathways. Defenses against herbivores in plants sometimes employ amino acids.[89] Examples:
Standard amino acids
[edit]- Tryptophan is a precursor of the neurotransmitter serotonin.[90]
- Tyrosine (and its precursor phenylalanine) are precursors of the catecholamine neurotransmitters dopamine, epinephrine and norepinephrine and various trace amines.
- Phenylalanine is a precursor of phenethylamine and tyrosine in humans. In plants, it is a precursor of various phenylpropanoids, which are important in plant metabolism.
- Glycine is a precursor of porphyrins such as heme.[91]
- Arginine is a precursor of nitric oxide.[92]
- Ornithine and S-adenosylmethionine are precursors of polyamines.[93]
- Aspartate, glycine, and glutamine are precursors of nucleotides.[94]
Roles for nonstandard amino acids
[edit]- Carnitine is used in lipid transport.
- gamma-aminobutyric acid is a neurotransmitter.[95]
- 5-HTP (5-hydroxytryptophan) is used for experimental treatment of depression.[96]
- L-DOPA (L-dihydroxyphenylalanine) for Parkinson's treatment,[97]
- Eflornithine inhibits ornithine decarboxylase and used in the treatment of sleeping sickness.[98]
- Canavanine, an analogue of arginine found in many legumes is an antifeedant, protecting the plant from predators.[99]
- Mimosine found in some legumes, is another possible antifeedant.[100] This compound is an analogue of tyrosine and can poison animals that graze on these plants.
However, not all of the functions of other abundant nonstandard amino acids are known.
Uses in industry
[edit]Animal feed
[edit]Amino acids are sometimes added to animal feed because some of the components of these feeds, such as soybeans, have low levels of some of the essential amino acids, especially of lysine, methionine, threonine, and tryptophan.[101] Likewise amino acids are used to chelate metal cations in order to improve the absorption of minerals from feed supplements.[102]
Food
[edit]The food industry is a major consumer of amino acids, especially glutamic acid, which is used as a flavor enhancer,[103] and aspartame (aspartylphenylalanine 1-methyl ester), which is used as an artificial sweetener.[104] Amino acids are sometimes added to food by manufacturers to alleviate symptoms of mineral deficiencies, such as anemia, by improving mineral absorption and reducing negative side effects from inorganic mineral supplementation.[105]
Chemical building blocks
[edit]Amino acids are low-cost feedstocks used in chiral pool synthesis as enantiomerically pure building blocks.[106][107]
Amino acids are used in the synthesis of some cosmetics.[101]
Aspirational uses
[edit]Fertilizer
[edit]The chelating ability of amino acids is sometimes used in fertilizers to facilitate the delivery of minerals to plants in order to correct mineral deficiencies, such as iron chlorosis. These fertilizers are also used to prevent deficiencies from occurring and to improve the overall health of the plants.[108]
Biodegradable plastics
[edit]Amino acids have been considered as components of biodegradable polymers, which have applications as environmentally friendly packaging and in medicine in drug delivery and the construction of prosthetic implants.[109] An interesting example of such materials is polyaspartate, a water-soluble biodegradable polymer that may have applications in disposable diapers and agriculture.[110] Due to its solubility and ability to chelate metal ions, polyaspartate is also being used as a biodegradable antiscaling agent and a corrosion inhibitor.[111][112]
Synthesis
[edit]Chemical synthesis
[edit]The commercial production of amino acids usually relies on mutant bacteria that overproduce individual amino acids using glucose as a carbon source. Some amino acids are produced by enzymatic conversions of synthetic intermediates. 2-Aminothiazoline-4-carboxylic acid is an intermediate in one industrial synthesis of L-cysteine for example. Aspartic acid is produced by the addition of ammonia to fumarate using a lyase.[105]
Biosynthesis
[edit]In plants, nitrogen is first assimilated into organic compounds in the form of glutamate, formed from alpha-ketoglutarate and ammonia in the mitochondrion. For other amino acids, plants use transaminases to move the amino group from glutamate to another alpha-keto acid. For example, aspartate aminotransferase converts glutamate and oxaloacetate to alpha-ketoglutarate and aspartate.[113] Other organisms use transaminases for amino acid synthesis, too.
Nonstandard amino acids are usually formed through modifications to standard amino acids. For example, homocysteine is formed through the transsulfuration pathway or by the demethylation of methionine via the intermediate metabolite S-adenosylmethionine,[114] while hydroxyproline is made by a post translational modification of proline.[115]
Microorganisms and plants synthesize many uncommon amino acids. For example, some microbes make 2-aminoisobutyric acid and lanthionine, which is a sulfide-bridged derivative of alanine. Both of these amino acids are found in peptidic lantibiotics such as alamethicin.[116] However, in plants, 1-aminocyclopropane-1-carboxylic acid is a small disubstituted cyclic amino acid that is an intermediate in the production of the plant hormone ethylene.[117]
Primordial synthesis
[edit]The formation of amino acids and peptides is assumed to have preceded and perhaps induced the emergence of life on earth. Amino acids can form from simple precursors under various conditions.[118] Surface-based chemical metabolism of amino acids and very small compounds may have led to the build-up of amino acids, coenzymes and phosphate-based small carbon molecules.[119][additional citation(s) needed] Amino acids and similar building blocks could have been elaborated into proto-peptides, with peptides being considered key players in the origin of life.[120]

In the famous Urey-Miller experiment, the passage of an electric arc through a mixture of methane, hydrogen, and ammonia produces a large number of amino acids. Since then, scientists have discovered a range of ways and components by which the potentially prebiotic formation and chemical evolution of peptides may have occurred, such as condensing agents, the design of self-replicating peptides and a number of non-enzymatic mechanisms by which amino acids could have emerged and elaborated into peptides.[120] Several hypotheses invoke the Strecker synthesis whereby hydrogen cyanide, simple aldehydes, ammonia, and water produce amino acids.[118]
According to a review, amino acids, and even peptides, "turn up fairly regularly in the various experimental broths that have been allowed to be cooked from simple chemicals. This is because nucleotides are far more difficult to synthesize chemically than amino acids." For a chronological order, it suggests that there must have been a 'protein world' or at least a 'polypeptide world', possibly later followed by the 'RNA world' and the 'DNA world'.[121] Codon–amino acids mappings may be the biological information system at the primordial origin of life on Earth.[122] While amino acids and consequently simple peptides must have formed under different experimentally probed geochemical scenarios, the transition from an abiotic world to the first life forms is to a large extent still unresolved.[123]
Reactions
[edit]Amino acids undergo the reactions expected of the constituent functional groups.[124][125]
Peptide bond formation
[edit]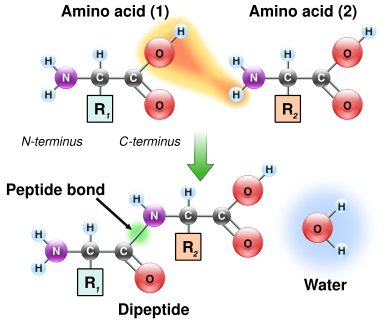
As both the amine and carboxylic acid groups of amino acids can react to form amide bonds, one amino acid molecule can react with another and become joined through an amide linkage. This polymerization of amino acids is what creates proteins. This condensation reaction yields the newly formed peptide bond and a molecule of water. In cells, this reaction does not occur directly; instead, the amino acid is first activated by attachment to a transfer RNA molecule through an ester bond. This aminoacyl-tRNA is produced in an ATP-dependent reaction carried out by an aminoacyl tRNA synthetase.[126] This aminoacyl-tRNA is then a substrate for the ribosome, which catalyzes the attack of the amino group of the elongating protein chain on the ester bond.[127] As a result of this mechanism, all proteins made by ribosomes are synthesized starting at their N-terminus and moving toward their C-terminus.
However, not all peptide bonds are formed in this way. In a few cases, peptides are synthesized by specific enzymes. For example, the tripeptide glutathione is an essential part of the defenses of cells against oxidative stress. This peptide is synthesized in two steps from free amino acids.[128] In the first step, gamma-glutamylcysteine synthetase condenses cysteine and glutamate through a peptide bond formed between the side chain carboxyl of the glutamate (the gamma carbon of this side chain) and the amino group of the cysteine. This dipeptide is then condensed with glycine by glutathione synthetase to form glutathione.[129]
In chemistry, peptides are synthesized by a variety of reactions. One of the most-used in solid-phase peptide synthesis uses the aromatic oxime derivatives of amino acids as activated units. These are added in sequence onto the growing peptide chain, which is attached to a solid resin support.[130] Libraries of peptides are used in drug discovery through high-throughput screening.[131]
The combination of functional groups allow amino acids to be effective polydentate ligands for metal–amino acid chelates.[132] The multiple side chains of amino acids can also undergo chemical reactions.
Catabolism
[edit]
* Glucogenic, with the products having the ability to form glucose by gluconeogenesis
* Ketogenic, with the products not having the ability to form glucose. These products may still be used for ketogenesis or lipid synthesis.
* Amino acids catabolized into both glucogenic and ketogenic products.
Degradation of an amino acid often involves deamination by moving its amino group to α-ketoglutarate, forming glutamate. This process involves transaminases, often the same as those used in amination during synthesis. In many vertebrates, the amino group is then removed through the urea cycle and is excreted in the form of urea. However, amino acid degradation can produce uric acid or ammonia instead. For example, serine dehydratase converts serine to pyruvate and ammonia.[94] After removal of one or more amino groups, the remainder of the molecule can sometimes be used to synthesize new amino acids, or it can be used for energy by entering glycolysis or the citric acid cycle, as detailed in image at right.
Complexation
[edit]Amino acids are bidentate ligands, forming transition metal amino acid complexes.[134]
Chemical analysis
[edit]The total nitrogen content of organic matter is mainly formed by the amino groups in proteins. The Total Kjeldahl Nitrogen (TKN) is a measure of nitrogen widely used in the analysis of (waste) water, soil, food, feed and organic matter in general. As the name suggests, the Kjeldahl method is applied. More sensitive methods are available.[135][136]
See also
[edit]Notes
[edit]- ^ The late discovery is explained by the fact that cysteine becomes oxidized to cystine in air.
- ^ Proline and other cyclic amino acids are an exception to this general formula. Cyclization of the α-amino acid creates the corresponding secondary amine. These are occasionally referred to as imino acids.
- ^ The L and D convention for amino acid configuration refers not to the optical activity of the amino acid itself but rather to the optical activity of the isomer of glyceraldehyde from which that amino acid can, in theory, be synthesized (D-glyceraldehyde is dextrorotatory; L-glyceraldehyde is levorotatory). An alternative convention is to use the (S) and (R) designators to specify the absolute configuration.[30] Almost all of the amino acids in proteins are (S) at the α carbon, with cysteine being (R) and glycine non-chiral.[31] Cysteine has its side chain in the same geometric location as the other amino acids, but the R/S terminology is reversed because sulfur has higher atomic number compared to the carboxyl oxygen which gives the side chain a higher priority by the Cahn-Ingold-Prelog sequence rules.
- ^ Codons can also be expressed by: CGN, AGR.
- ^ Codons can also be expressed by: CUN, UUR.
- ^ Codons can also be expressed by: CTN, ATH, TTR; MTY, YTR, ATA; MTY, HTA, YTG.
- ^ Codons can also be expressed by: TWY, CAY, TGG.
- ^ Codons can also be expressed by: NTR, VTY.
- ^ Codons can also be expressed by: VAN, WCN, MGY, CGP.
- ^ For example, ruminants such as cows obtain a number of amino acids via microbes in the first two stomach chambers.
References
[edit]- ^ Nelson DL, Cox MM (2005). Principles of Biochemistry (4th ed.). New York: W. H. Freeman. ISBN 0-7167-4339-6.
- ^ Flissi, Areski; Ricart, Emma; Campart, Clémentine; Chevalier, Mickael; Dufresne, Yoann; Michalik, Juraj; Jacques, Philippe; Flahaut, Christophe; Lisacek, Frédérique; Leclère, Valérie; Pupin, Maude (2020). "Norine: update of the nonribosomal peptide resource". Nucleic Acids Research. 48 (D1): D465 – D469. doi:10.1093/nar/gkz1000. PMC 7145658. PMID 31691799.
- ^ Richard Cammack, ed. (2009). "Newsletter 2009". Biochemical Nomenclature Committee of IUPAC and NC-IUBMB. Pyrrolysine. Archived from the original on 12 September 2017. Retrieved 16 April 2012.
- ^ Rother, Michael; Krzycki, Joseph A. (1 January 2010). "Selenocysteine, Pyrrolysine, and the Unique Energy Metabolism of Methanogenic Archaea". Archaea. 2010: 1–14. doi:10.1155/2010/453642. ISSN 1472-3646. PMC 2933860. PMID 20847933.
- ^ Latham MC (1997). "Chapter 8. Body composition, the functions of food, metabolism and energy". Human nutrition in the developing world. Food and Nutrition Series – No. 29. Rome: Food and Agriculture Organization of the United Nations. Archived from the original on 8 October 2012. Retrieved 9 September 2012.
- ^
Luisi, Pier Luigi (13 July 2006). The Emergence of Life: From Chemical Origins to Synthetic Biology. Cambridge University Press. p. 13. ISBN 9781139455640. Retrieved 5 August 2024.
Of course if on Earth there had only been diketopiperazines and not amino acids; or if sugars did not have the size they have; or if lipids were three times shorter, then we would not have life.
- ^ a b c "Nomenclature and Symbolism for Amino Acids and Peptides". IUPAC-IUB Joint Commission on Biochemical Nomenclature. 1983. Archived from the original on 9 October 2008. Retrieved 17 November 2008.
- ^ Vickery HB, Schmidt CL (1931). "The history of the discovery of the amino acids". Chem. Rev. 9 (2): 169–318. doi:10.1021/cr60033a001.
- ^ Hansen S (May 2015). "Die Entdeckung der proteinogenen Aminosäuren von 1805 in Paris bis 1935 in Illinois" (PDF) (in German). Berlin. Archived from the original (PDF) on 1 December 2017.
- ^ Vauquelin LN, Robiquet PJ (1806). "The discovery of a new plant principle in Asparagus sativus". Annales de Chimie. 57: 88–93.
- ^ a b Anfinsen CB, Edsall JT, Richards FM (1972). Advances in Protein Chemistry. New York: Academic Press. pp. 99, 103. ISBN 978-0-12-034226-6.
- ^ Wollaston WH (1810). "On cystic oxide, a new species of urinary calculus". Philosophical Transactions of the Royal Society. 100: 223–230. doi:10.1098/rstl.1810.0015. S2CID 110151163.
- ^ Baumann E (1884). "Über cystin und cystein". Z Physiol Chem. 8 (4): 299–305. Archived from the original on 14 March 2011. Retrieved 28 March 2011.
- ^ Braconnot HM (1820). "Sur la conversion des matières animales en nouvelles substances par le moyen de l'acide sulfurique". Annales de Chimie et de Physique. 2nd Series. 13: 113–125.
- ^ Simoni RD, Hill RL, Vaughan M (September 2002). "The discovery of the amino acid threonine: the work of William C. Rose [classical article]". The Journal of Biological Chemistry. 277 (37): 56–58. doi:10.1016/S0021-9258(20)74369-3. PMID 12218068.
- ^ McCoy RH, Meyer CE, Rose WC (1935). "Feeding Experiments with Mixtures of Highly Purified Amino Acids. VIII. Isolation and Identification of a New Essential Amino Acid". Journal of Biological Chemistry. 112: 283–302. doi:10.1016/S0021-9258(18)74986-7.
- ^ Menten, P. Dictionnaire de chimie: Une approche étymologique et historique. De Boeck, Bruxelles. link Archived 28 December 2019 at the Wayback Machine.
- ^ Harper D. "amino-". Online Etymology Dictionary. Archived from the original on 2 December 2017. Retrieved 19 July 2010.
- ^ Paal C (1894). "Ueber die Einwirkung von Phenyl-i-cyanat auf organische Aminosäuren". Berichte der Deutschen Chemischen Gesellschaft. 27: 974–979. doi:10.1002/cber.189402701205. Archived from the original on 25 July 2020.
- ^ Fruton JS (1990). "Chapter 5- Emil Fischer and Franz Hofmeister". Contrasts in Scientific Style: Research Groups in the Chemical and Biochemical Sciences. Vol. 191. American Philosophical Society. pp. 163–165. ISBN 978-0-87169-191-0.
- ^ "Alpha amino acid". Merriam-Webster Medical. Archived from the original on 3 January 2015. Retrieved 3 January 2015..
- ^ Clark, Jim (August 2007). "An introduction to amino acids". chemguide. Archived from the original on 30 April 2015. Retrieved 4 July 2015.
- ^ Jakubke HD, Sewald N (2008). "Amino acids". Peptides from A to Z: A Concise Encyclopedia. Germany: Wiley-VCH. p. 20. ISBN 9783527621170. Archived from the original on 17 May 2016. Retrieved 5 January 2016 – via Google Books.
- ^ Pollegioni L, Servi S, eds. (2012). Unnatural Amino Acids: Methods and Protocols. Methods in Molecular Biology. Vol. 794. Humana Press. p. v. doi:10.1007/978-1-61779-331-8. ISBN 978-1-61779-331-8. OCLC 756512314. S2CID 3705304.
- ^ Hertweck C (October 2011). "Biosynthesis and Charging of Pyrrolysine, the 22nd Genetically Encoded Amino Acid". Angewandte Chemie International Edition. 50 (41): 9540–9541. doi:10.1002/anie.201103769. PMID 21796749. S2CID 5359077.

- ^ a b "Chapter 1: Proteins are the Body's Worker Molecules". The Structures of Life. National Institute of General Medical Sciences. 27 October 2011. Archived from the original on 7 June 2014. Retrieved 20 May 2008.
- ^ Michal G, Schomburg D, eds. (2012). Biochemical Pathways: An Atlas of Biochemistry and Molecular Biology (2nd ed.). Oxford: Wiley-Blackwell. p. 5. ISBN 978-0-470-14684-2.
- ^ a b c Creighton TH (1993). "Chapter 1". Proteins: structures and molecular properties. San Francisco: W. H. Freeman. ISBN 978-0-7167-7030-5.
- ^ Genchi, Giuseppe (1 September 2017). "An overview on d-amino acids". Amino Acids. 49 (9): 1521–1533. doi:10.1007/s00726-017-2459-5. ISSN 1438-2199. PMID 28681245. S2CID 254088816.
- ^ Cahn, R.S.; Ingold, C.K.; Prelog, V. (1966). "Specification of Molecular Chirality". Angewandte Chemie International Edition. 5 (4): 385–415. doi:10.1002/anie.196603851.
- ^ Hatem SM (2006). "Gas chromatographic determination of Amino Acid Enantiomers in tobacco and bottled wines". University of Giessen. Archived from the original on 22 January 2009. Retrieved 17 November 2008.
- ^ a b c d e f Garrett, Reginald H.; Grisham, Charles M. (2010). Biochemistry (4th ed.). Belmont, CA: Brooks/Cole, Cengage Learning. pp. 74, 134–176, 430–442. ISBN 978-0-495-10935-8. OCLC 297392560.
- ^ Novikov, Anton P.; Safonov, Alexey V.; German, Konstantin E.; Grigoriev, Mikhail S. (1 December 2023). "What kind of interactions we may get moving from zwitter to "dritter" ions: C–O⋯Re(O4) and Re–O⋯Re(O4) anion⋯anion interactions make structural difference between L-histidinium perrhenate and pertechnetate". CrystEngComm. 26: 61–69. doi:10.1039/D3CE01164J. ISSN 1466-8033. S2CID 265572280.
- ^ Papp, Laura Vanda; Lu, Jun; Holmgren, Arne; Khanna, Kum Kum (1 July 2007). "From Selenium to Selenoproteins: Synthesis, Identity, and Their Role in Human Health". Antioxidants & Redox Signaling. 9 (7): 775–806. doi:10.1089/ars.2007.1528. ISSN 1523-0864. PMID 17508906.
- ^ Hao, Bing; Gong, Weimin; Ferguson, Tsuneo K.; James, Carey M.; Krzycki, Joseph A.; Chan, Michael K. (24 May 2002). "A New UAG-Encoded Residue in the Structure of a Methanogen Methyltransferase". Science. 296 (5572): 1462–1466. Bibcode:2002Sci...296.1462H. doi:10.1126/science.1069556. ISSN 0036-8075. PMID 12029132. S2CID 35519996.
- ^ Steinhardt, J.; Reynolds, J. A. (1969). Multiple equilibria in proteins. New York: Academic Press. pp. 176–21. ISBN 978-0126654509.
- ^ Brønsted, J. N. (1923). "Einige Bemerkungen über den Begriff der Säuren und Basen" [Remarks on the concept of acids and bases]. Recueil des Travaux Chimiques des Pays-Bas. 42 (8): 718–728. doi:10.1002/recl.19230420815.
- ^ a b Vollhardt, K. Peter C. (2007). Organic chemistry : structure and function. Neil Eric Schore (5th ed.). New York: W.H. Freeman. pp. 58–66. ISBN 978-0-7167-9949-8. OCLC 61448218.
- ^ Fennema OR (19 June 1996). Food Chemistry 3rd Ed. CRC Press. pp. 327–328. ISBN 978-0-8247-9691-4.
- ^ a b Ntountoumi C, Vlastaridis P, Mossialos D, Stathopoulos C, Iliopoulos I, Promponas V, et al. (November 2019). "Low complexity regions in the proteins of prokaryotes perform important functional roles and are highly conserved". Nucleic Acids Research. 47 (19): 9998–10009. doi:10.1093/nar/gkz730. PMC 6821194. PMID 31504783.
- ^ Urry DW (2004). "The change in Gibbs free energy for hydrophobic association: Derivation and evaluation by means of inverse temperature transitions". Chemical Physics Letters. 399 (1–3): 177–183. Bibcode:2004CPL...399..177U. doi:10.1016/S0009-2614(04)01565-9.
- ^ Marcotte EM, Pellegrini M, Yeates TO, Eisenberg D (October 1999). "A census of protein repeats". Journal of Molecular Biology. 293 (1): 151–60. doi:10.1006/jmbi.1999.3136. PMID 10512723.
- ^ Haerty W, Golding GB (October 2010). Bonen L (ed.). "Low-complexity sequences and single amino acid repeats: not just "junk" peptide sequences". Genome. 53 (10): 753–62. doi:10.1139/G10-063. PMID 20962881.
- ^ Magee T, Seabra MC (April 2005). "Fatty acylation and prenylation of proteins: what's hot in fat". Current Opinion in Cell Biology. 17 (2): 190–196. doi:10.1016/j.ceb.2005.02.003. PMID 15780596.
- ^ Pilobello KT, Mahal LK (June 2007). "Deciphering the glycocode: the complexity and analytical challenge of glycomics". Current Opinion in Chemical Biology. 11 (3): 300–305. doi:10.1016/j.cbpa.2007.05.002. PMID 17500024.
- ^ Smotrys JE, Linder ME (2004). "Palmitoylation of intracellular signaling proteins: regulation and function". Annual Review of Biochemistry. 73 (1): 559–587. doi:10.1146/annurev.biochem.73.011303.073954. PMID 15189153.
- ^ a b c d e "IUPAC-IUB Commission on Biochemical Nomenclature A One-Letter Notation for Amino Acid Sequences". Journal of Biological Chemistry. 243 (13): 3557–3559. 10 July 1968. doi:10.1016/S0021-9258(19)34176-6.
- ^ a b Saffran, M. (April 1998). "Amino acid names and parlor games: from trivial names to a one-letter code, amino acid names have strained students' memories. Is a more rational nomenclature possible?". Biochemical Education. 26 (2): 116–118. doi:10.1016/S0307-4412(97)00167-2.
- ^ a b c Adoga, Godwin I; Nicholson, Bh (January 1988). "Letters to the editor". Biochemical Education. 16 (1): 49. doi:10.1016/0307-4412(88)90026-X.
- ^ Kyte J, Doolittle RF (May 1982). "A simple method for displaying the hydropathic character of a protein". Journal of Molecular Biology. 157 (1): 105–132. CiteSeerX 10.1.1.458.454. doi:10.1016/0022-2836(82)90515-0. PMID 7108955.
- ^ Freifelder D (1983). Physical Biochemistry (2nd ed.). W. H. Freeman and Company. ISBN 978-0-7167-1315-9.[page needed]
- ^ Kozlowski LP (January 2017). "Proteome-pI: proteome isoelectric point database". Nucleic Acids Research. 45 (D1): D1112 – D1116. doi:10.1093/nar/gkw978. PMC 5210655. PMID 27789699.
- ^ a b Hausman RE, Cooper GM (2004). The cell: a molecular approach. Washington, D.C.: ASM Press. p. 51. ISBN 978-0-87893-214-6.
- ^ Aasland R, Abrams C, Ampe C, Ball LJ, Bedford MT, Cesareni G, Gimona M, Hurley JH, Jarchau T, Lehto VP, Lemmon MA, Linding R, Mayer BJ, Nagai M, Sudol M, Walter U, Winder SJ (February 2002). "Normalization of nomenclature for peptide motifs as ligands of modular protein domains". FEBS Letters. 513 (1): 141–144. doi:10.1111/j.1432-1033.1968.tb00350.x. PMID 11911894.
- ^ IUPAC–IUB Commission on Biochemical Nomenclature (1972). "A one-letter notation for amino acid sequences". Pure and Applied Chemistry. 31 (4): 641–645. doi:10.1351/pac197231040639. PMID 5080161.
- ^ "HGVS: Sequence Variant Nomenclature, Protein Recommendations". Archived from the original on 24 September 2021. Retrieved 23 September 2021.
- ^ Suchanek M, Radzikowska A, Thiele C (April 2005). "Photo-leucine and photo-methionine allow identification of protein–protein interactions in living cells". Nature Methods. 2 (4): 261–267. doi:10.1038/nmeth752. PMID 15782218.
- ^ Rodnina MV, Beringer M, Wintermeyer W (January 2007). "How ribosomes make peptide bonds". Trends in Biochemical Sciences. 32 (1): 20–26. doi:10.1016/j.tibs.2006.11.007. PMID 17157507.
- ^ Driscoll DM, Copeland PR (2003). "Mechanism and regulation of selenoprotein synthesis". Annual Review of Nutrition. 23 (1): 17–40. doi:10.1146/annurev.nutr.23.011702.073318. PMID 12524431.
- ^ Krzycki JA (December 2005). "The direct genetic encoding of pyrrolysine". Current Opinion in Microbiology. 8 (6): 706–712. doi:10.1016/j.mib.2005.10.009. PMID 16256420.
- ^ Wong, J. T.-F. (1975). "A Co-Evolution Theory of the Genetic Code". Proceedings of the National Academy of Sciences. 72 (5): 1909–1912. Bibcode:1975PNAS...72.1909T. doi:10.1073/pnas.72.5.1909. PMC 432657. PMID 1057181.
- ^ Trifonov EN (December 2000). "Consensus temporal order of amino acids and evolution of the triplet code". Gene. 261 (1): 139–151. doi:10.1016/S0378-1119(00)00476-5. PMID 11164045.
- ^ Higgs PG, Pudritz RE (June 2009). "A thermodynamic basis for prebiotic amino acid synthesis and the nature of the first genetic code". Astrobiology. 9 (5): 483–90. arXiv:0904.0402. Bibcode:2009AsBio...9..483H. doi:10.1089/ast.2008.0280. PMID 19566427. S2CID 9039622.
- ^ Kryukov GV, Castellano S, Novoselov SV, Lobanov AV, Zehtab O, Guigó R, Gladyshev VN (May 2003). "Characterization of mammalian selenoproteomes". Science. 300 (5624): 1439–1443. Bibcode:2003Sci...300.1439K. doi:10.1126/science.1083516. PMID 12775843. S2CID 10363908.
- ^ Gromer S, Urig S, Becker K (January 2004). "The thioredoxin system—from science to clinic". Medicinal Research Reviews. 24 (1): 40–89. doi:10.1002/med.10051. PMID 14595672. S2CID 1944741.
- ^ Tjong H (2008). Modeling Electrostatic Contributions to Protein Folding and Binding (PhD thesis). Florida State University. p. 1 footnote. Archived from the original on 28 January 2020. Retrieved 28 January 2020.
- ^ Stewart L, Burgin AB (2005). "Whole Gene Synthesis: A Gene-O-Matic Future". Frontiers in Drug Design & Discovery. 1. Bentham Science Publishers: 299. doi:10.2174/1574088054583318. ISBN 978-1-60805-199-1. ISSN 1574-0889. Archived from the original on 14 April 2021. Retrieved 5 January 2016.
- ^ Elzanowski A, Ostell J (7 April 2008). "The Genetic Codes". National Center for Biotechnology Information (NCBI). Archived from the original on 20 August 2016. Retrieved 10 March 2010.
- ^ Xie J, Schultz PG (December 2005). "Adding amino acids to the genetic repertoire". Current Opinion in Chemical Biology. 9 (6): 548–554. doi:10.1016/j.cbpa.2005.10.011. PMID 16260173.
- ^ Wang Q, Parrish AR, Wang L (March 2009). "Expanding the genetic code for biological studies". Chemistry & Biology. 16 (3): 323–336. doi:10.1016/j.chembiol.2009.03.001. PMC 2696486. PMID 19318213.
- ^ Simon M (2005). Emergent computation: emphasizing bioinformatics. New York: AIP Press/Springer Science+Business Media. pp. 105–106. ISBN 978-0-387-22046-8.
- ^ Blenis J, Resh MD (December 1993). "Subcellular localization specified by protein acylation and phosphorylation". Current Opinion in Cell Biology. 5 (6): 984–989. doi:10.1016/0955-0674(93)90081-Z. PMID 8129952.
- ^ Vermeer C (March 1990). "Gamma-carboxyglutamate-containing proteins and the vitamin K-dependent carboxylase". The Biochemical Journal. 266 (3): 625–636. doi:10.1042/bj2660625. PMC 1131186. PMID 2183788.
- ^ Bhattacharjee A, Bansal M (March 2005). "Collagen Structure: the Madras triple helix and the current scenario". IUBMB Life. 57 (3): 161–172. doi:10.1080/15216540500090710. PMID 16036578. S2CID 7211864.
- ^ Park MH (February 2006). "The post-translational synthesis of a polyamine-derived amino acid, hypusine, in the eukaryotic translation initiation factor 5A (eIF5A)". Journal of Biochemistry. 139 (2): 161–169. doi:10.1093/jb/mvj034. PMC 2494880. PMID 16452303.
- ^ Curis E, Nicolis I, Moinard C, Osowska S, Zerrouk N, Bénazeth S, Cynober L (November 2005). "Almost all about citrulline in mammals". Amino Acids. 29 (3): 177–205. doi:10.1007/s00726-005-0235-4. PMID 16082501. S2CID 23877884.
- ^ Coxon KM, Chakauya E, Ottenhof HH, Whitney HM, Blundell TL, Abell C, Smith AG (August 2005). "Pantothenate biosynthesis in higher plants". Biochemical Society Transactions. 33 (Pt 4): 743–746. doi:10.1042/BST0330743. PMID 16042590.
- ^ Sakami W, Harrington H (1963). "Amino acid metabolism". Annual Review of Biochemistry. 32 (1): 355–398. doi:10.1146/annurev.bi.32.070163.002035. PMID 14144484.
- ^ Brosnan JT (April 2000). "Glutamate, at the interface between amino acid and carbohydrate metabolism". The Journal of Nutrition. 130 (4S Suppl): 988S – 990S. doi:10.1093/jn/130.4.988S. PMID 10736367.
- ^ Young VR, Ajami AM (September 2001). "Glutamine: the emperor or his clothes?". The Journal of Nutrition. 131 (9 Suppl): 2449S – 2459S, 2486S – 2487S. doi:10.1093/jn/131.9.2449S. PMID 11533293.
- ^ Young VR (August 1994). "Adult amino acid requirements: the case for a major revision in current recommendations". The Journal of Nutrition. 124 (8 Suppl): 1517S – 1523S. doi:10.1093/jn/124.suppl_8.1517S. PMID 8064412.
- ^ Fürst P, Stehle P (June 2004). "What are the essential elements needed for the determination of amino acid requirements in humans?". The Journal of Nutrition. 134 (6 Suppl): 1558S – 1565S. doi:10.1093/jn/134.6.1558S. PMID 15173430.
- ^ Reeds PJ (July 2000). "Dispensable and indispensable amino acids for humans". The Journal of Nutrition. 130 (7): 1835S – 1840S. doi:10.1093/jn/130.7.1835S. PMID 10867060.
- ^ Imura K, Okada A (January 1998). "Amino acid metabolism in pediatric patients". Nutrition. 14 (1): 143–148. doi:10.1016/S0899-9007(97)00230-X. PMID 9437700.
- ^ Lourenço R, Camilo ME (2002). "Taurine: a conditionally essential amino acid in humans? An overview in health and disease". Nutricion Hospitalaria. 17 (6): 262–270. PMID 12514918.
- ^ Broadley KJ (March 2010). "The vascular effects of trace amines and amphetamines". Pharmacology & Therapeutics. 125 (3): 363–375. doi:10.1016/j.pharmthera.2009.11.005. PMID 19948186.
- ^ Lindemann L, Hoener MC (May 2005). "A renaissance in trace amines inspired by a novel GPCR family". Trends in Pharmacological Sciences. 26 (5): 274–281. doi:10.1016/j.tips.2005.03.007. PMID 15860375.
- ^ Wang X, Li J, Dong G, Yue J (February 2014). "The endogenous substrates of brain CYP2D". European Journal of Pharmacology. 724: 211–218. doi:10.1016/j.ejphar.2013.12.025. PMID 24374199.
- ^ Hylin JW (1969). "Toxic peptides and amino acids in foods and feeds". Journal of Agricultural and Food Chemistry. 17 (3): 492–496. doi:10.1021/jf60163a003.
- ^ Savelieva KV, Zhao S, Pogorelov VM, Rajan I, Yang Q, Cullinan E, Lanthorn TH (2008). Bartolomucci A (ed.). "Genetic disruption of both tryptophan hydroxylase genes dramatically reduces serotonin and affects behavior in models sensitive to antidepressants". PLOS ONE. 3 (10): e3301. Bibcode:2008PLoSO...3.3301S. doi:10.1371/journal.pone.0003301. PMC 2565062. PMID 18923670.
- ^ Shemin D, Rittenberg D (December 1946). "The biological utilization of glycine for the synthesis of the protoporphyrin of hemoglobin". The Journal of Biological Chemistry. 166 (2): 621–625. doi:10.1016/S0021-9258(17)35200-6. PMID 20276176.
- ^ Tejero J, Biswas A, Wang ZQ, Page RC, Haque MM, Hemann C, Zweier JL, Misra S, Stuehr DJ (November 2008). "Stabilization and characterization of a heme-oxy reaction intermediate in inducible nitric-oxide synthase". The Journal of Biological Chemistry. 283 (48): 33498–33507. doi:10.1074/jbc.M806122200. PMC 2586280. PMID 18815130.
- ^ Rodríguez-Caso C, Montañez R, Cascante M, Sánchez-Jiménez F, Medina MA (August 2006). "Mathematical modeling of polyamine metabolism in mammals". The Journal of Biological Chemistry. 281 (31): 21799–21812. doi:10.1074/jbc.M602756200. hdl:10630/32289. PMID 16709566.
- ^ a b Stryer L, Berg JM, Tymoczko JL (2002). Biochemistry (5th ed.). New York: W.H. Freeman. pp. 693–698. ISBN 978-0-7167-4684-3.
- ^ Petroff OA (December 2002). "GABA and glutamate in the human brain". The Neuroscientist. 8 (6): 562–573. doi:10.1177/1073858402238515. PMID 12467378. S2CID 84891972.
- ^ Turner EH, Loftis JM, Blackwell AD (March 2006). "Serotonin a la carte: supplementation with the serotonin precursor 5-hydroxytryptophan". Pharmacology & Therapeutics. 109 (3): 325–338. doi:10.1016/j.pharmthera.2005.06.004. PMID 16023217. S2CID 2563606.
- ^ Kostrzewa RM, Nowak P, Kostrzewa JP, Kostrzewa RA, Brus R (March 2005). "Peculiarities of L-DOPA treatment of Parkinson's disease". Amino Acids. 28 (2): 157–164. doi:10.1007/s00726-005-0162-4. PMID 15750845. S2CID 33603501.
- ^ Heby O, Persson L, Rentala M (August 2007). "Targeting the polyamine biosynthetic enzymes: a promising approach to therapy of African sleeping sickness, Chagas' disease, and leishmaniasis". Amino Acids. 33 (2): 359–366. doi:10.1007/s00726-007-0537-9. PMID 17610127. S2CID 26273053.
- ^ Rosenthal GA (2001). "L-Canavanine: a higher plant insecticidal allelochemical". Amino Acids. 21 (3): 319–330. doi:10.1007/s007260170017. PMID 11764412. S2CID 3144019.
- ^ Hammond, Andrew C. (1 May 1995). "Leucaena toxicosis and its control in ruminants". Journal of Animal Science. 73 (5): 1487–1492. doi:10.2527/1995.7351487x. PMID 7665380.
- ^ a b Leuchtenberger W, Huthmacher K, Drauz K (November 2005). "Biotechnological production of amino acids and derivatives: current status and prospects". Applied Microbiology and Biotechnology. 69 (1): 1–8. doi:10.1007/s00253-005-0155-y. PMID 16195792. S2CID 24161808.
- ^ Ashmead HD (1993). The Role of Amino Acid Chelates in Animal Nutrition. Westwood: Noyes Publications.
- ^ Garattini S (April 2000). "Glutamic acid, twenty years later". The Journal of Nutrition. 130 (4S Suppl): 901S – 909S. doi:10.1093/jn/130.4.901S. PMID 10736350.
- ^ Stegink LD (July 1987). "The aspartame story: a model for the clinical testing of a food additive". The American Journal of Clinical Nutrition. 46 (1 Suppl): 204–215. doi:10.1093/ajcn/46.1.204. PMID 3300262.
- ^ a b Drauz K, Grayson I, Kleemann A, Krimmer HP, Leuchtenberger W, Weckbecker C (2007). "Amino Acids". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a02_057.pub2. ISBN 978-3527306732.
- ^ Hanessian S (1993). "Reflections on the total synthesis of natural products: Art, craft, logic, and the chiron approach". Pure and Applied Chemistry. 65 (6): 1189–1204. doi:10.1351/pac199365061189. S2CID 43992655.
- ^ Blaser HU (1992). "The chiral pool as a source of enantioselective catalysts and auxiliaries". Chemical Reviews. 92 (5): 935–952. doi:10.1021/cr00013a009.
- ^ Ashmead HD (1986). Foliar Feeding of Plants with Amino Acid Chelates. Park Ridge: Noyes Publications.
- ^ Sanda F, Endo T (1999). "Syntheses and functions of polymers based on amino acids". Macromolecular Chemistry and Physics. 200 (12): 2651–2661. doi:10.1002/(SICI)1521-3935(19991201)200:12<2651::AID-MACP2651>3.0.CO;2-P.
- ^ Gross RA, Kalra B (August 2002). "Biodegradable polymers for the environment". Science. 297 (5582): 803–807. Bibcode:2002Sci...297..803G. doi:10.1126/science.297.5582.803. PMID 12161646. Archived from the original on 25 July 2020. Retrieved 12 June 2019.
- ^ Low KC, Wheeler AP, Koskan LP (1996). Commercial poly(aspartic acid) and Its Uses. Advances in Chemistry Series. Vol. 248. Washington, D.C.: American Chemical Society.
- ^ Thombre SM, Sarwade BD (2005). "Synthesis and Biodegradability of Polyaspartic Acid: A Critical Review". Journal of Macromolecular Science, Part A. 42 (9): 1299–1315. doi:10.1080/10601320500189604. S2CID 94818855.
- ^ Jones RC, Buchanan BB, Gruissem W (2000). Biochemistry & molecular biology of plants. Rockville, Md: American Society of Plant Physiologists. pp. 371–372. ISBN 978-0-943088-39-6.
- ^ Brosnan JT, Brosnan ME (June 2006). "The sulfur-containing amino acids: an overview". The Journal of Nutrition. 136 (6 Suppl): 1636S – 1640S. doi:10.1093/jn/136.6.1636S. PMID 16702333.
- ^ Kivirikko KI, Pihlajaniemi T (1998). "Collagen Hydroxylases and the Protein Disulfide Isomerase Subunit of Prolyl 4-Hydroxylases". Advances in Enzymology and Related Areas of Molecular Biology. Advances in Enzymology – and Related Areas of Molecular Biology. Vol. 72. pp. 325–398. doi:10.1002/9780470123188.ch9. ISBN 9780470123188. PMID 9559057.
- ^ Whitmore L, Wallace BA (May 2004). "Analysis of peptaibol sequence composition: implications for in vivo synthesis and channel formation". European Biophysics Journal. 33 (3): 233–237. doi:10.1007/s00249-003-0348-1. PMID 14534753. S2CID 24638475.
- ^ Alexander L, Grierson D (October 2002). "Ethylene biosynthesis and action in tomato: a model for climacteric fruit ripening". Journal of Experimental Botany. 53 (377): 2039–2055. doi:10.1093/jxb/erf072. PMID 12324528.
- ^ a b Kitadai, Norio; Maruyama, Shigenori (2018). "Origins of building blocks of life: A review". Geoscience Frontiers. 9 (4): 1117–1153. Bibcode:2018GeoFr...9.1117K. doi:10.1016/j.gsf.2017.07.007. S2CID 102659869.
- ^ Danchin, Antoine (12 June 2017). "From chemical metabolism to life: the origin of the genetic coding process". Beilstein Journal of Organic Chemistry. 13 (1): 1119–1135. doi:10.3762/bjoc.13.111. ISSN 1860-5397. PMC 5480338. PMID 28684991.
- ^ a b Frenkel-Pinter, Moran; Samanta, Mousumi; Ashkenasy, Gonen; Leman, Luke J. (10 June 2020). "Prebiotic Peptides: Molecular Hubs in the Origin of Life". Chemical Reviews. 120 (11): 4707–4765. doi:10.1021/acs.chemrev.9b00664. ISSN 0009-2665. PMID 32101414. S2CID 211536416.
- ^ Milner-White, E. James (6 December 2019). "Protein three-dimensional structures at the origin of life". Interface Focus. 9 (6): 20190057. doi:10.1098/rsfs.2019.0057. PMC 6802138. PMID 31641431.
- ^ Chatterjee, Sankar; Yadav, Surya (June 2022). "The Coevolution of Biomolecules and Prebiotic Information Systems in the Origin of Life: A Visualization Model for Assembling the First Gene". Life. 12 (6): 834. Bibcode:2022Life...12..834C. doi:10.3390/life12060834. ISSN 2075-1729. PMC 9225589. PMID 35743865.
- ^ Kirschning, Andreas (26 May 2021). "The coenzyme/protein pair and the molecular evolution of life". Natural Product Reports. 38 (5): 993–1010. doi:10.1039/D0NP00037J. ISSN 1460-4752. PMID 33206101. S2CID 227037164.
- ^ Elmore DT, Barrett GC (1998). Amino acids and peptides. Cambridge, UK: Cambridge University Press. pp. 48–60. ISBN 978-0-521-46827-5.
- ^ Gutteridge A, Thornton JM (November 2005). "Understanding nature's catalytic toolkit". Trends in Biochemical Sciences. 30 (11): 622–629. doi:10.1016/j.tibs.2005.09.006. PMID 16214343.
- ^ Ibba M, Söll D (May 2001). "The renaissance of aminoacyl-tRNA synthesis". EMBO Reports. 2 (5): 382–387. doi:10.1093/embo-reports/kve095. PMC 1083889. PMID 11375928.
- ^ Lengyel P, Söll D (June 1969). "Mechanism of protein biosynthesis". Bacteriological Reviews. 33 (2): 264–301. doi:10.1128/MMBR.33.2.264-301.1969. PMC 378322. PMID 4896351.
- ^ Wu G, Fang YZ, Yang S, Lupton JR, Turner ND (March 2004). "Glutathione metabolism and its implications for health". The Journal of Nutrition. 134 (3): 489–492. doi:10.1093/jn/134.3.489. PMID 14988435.
- ^ Meister A (November 1988). "Glutathione metabolism and its selective modification". The Journal of Biological Chemistry. 263 (33): 17205–17208. doi:10.1016/S0021-9258(19)77815-6. PMID 3053703.
- ^ Carpino LA (1992). "1-Hydroxy-7-azabenzotriazole. An efficient peptide coupling additive". Journal of the American Chemical Society. 115 (10): 4397–4398. doi:10.1021/ja00063a082.
- ^ Marasco D, Perretta G, Sabatella M, Ruvo M (October 2008). "Past and future perspectives of synthetic peptide libraries". Current Protein & Peptide Science. 9 (5): 447–467. doi:10.2174/138920308785915209. PMID 18855697.
- ^ Konara S, Gagnona K, Clearfield A, Thompson C, Hartle J, Ericson C, Nelson C (2010). "Structural determination and characterization of copper and zinc bis-glycinates with X-ray crystallography and mass spectrometry". Journal of Coordination Chemistry. 63 (19): 3335–3347. doi:10.1080/00958972.2010.514336. S2CID 94822047.
- ^ Stipanuk MH (2006). Biochemical, physiological, & molecular aspects of human nutrition (2nd ed.). Saunders Elsevier.
- ^ Dghaym RD, Dhawan R, Arndtsen BA (September 2001). "The Use of Carbon Monoxide and Imines as Peptide Derivative Synthons: A Facile Palladium-Catalyzed Synthesis of α-Amino Acid Derived Imidazolines". Angewandte Chemie. 40 (17): 3228–3230. doi:10.1002/(SICI)1521-3773(19980703)37:12<1634::AID-ANIE1634>3.0.CO;2-C. PMID 29712039.
- ^ Muñoz-Huerta RF, Guevara-Gonzalez RG, Contreras-Medina LM, Torres-Pacheco I, Prado-Olivarez J, Ocampo-Velazquez RV (August 2013). "A review of methods for sensing the nitrogen status in plants: advantages, disadvantages and recent advances". Sensors. 13 (8). Basel, Switzerland: 10823–43. Bibcode:2013Senso..1310823M. doi:10.3390/s130810823. PMC 3812630. PMID 23959242.
- ^ Martin PD, Malley DF, Manning G, Fuller L (2002). "Determination of soil organic carbon and nitrogen at thefield level using near-infrared spectroscopy". Canadian Journal of Soil Science. 82 (4): 413–422. doi:10.4141/S01-054.
Further reading
[edit]- Tymoczko JL (2012). "Protein Composition and Structure". Biochemistry. New York: W. H. Freeman and company. pp. 28–31. ISBN 9781429229364.
- Doolittle RF (1989). "Redundancies in protein sequences". In Fasman GD (ed.). Predictions of Protein Structure and the Principles of Protein Conformation. New York: Plenum Press. pp. 599–623. ISBN 978-0-306-43131-9. LCCN 89008555.
- Nelson DL, Cox MM (2000). Lehninger Principles of Biochemistry (3rd ed.). Worth Publishers. ISBN 978-1-57259-153-0. LCCN 99049137.
- Meierhenrich U (2008). Amino acids and the asymmetry of life (PDF). Berlin: Springer Verlag. ISBN 978-3-540-76885-2. LCCN 2008930865. Archived from the original (PDF) on 12 January 2012.
External links
[edit] Media related to Amino acids at Wikimedia Commons
Media related to Amino acids at Wikimedia Commons