Lorenzo's oil: Difference between revisions
No edit summary |
added Category:Eponyms in biology using HotCat |
||
| (306 intermediate revisions by more than 100 users not shown) | |||
| Line 1: | Line 1: | ||
{{Short description|Mixture of modified vegetable oils used in treating adrenoleukodystrophy}} |
|||
{{dablink|This article is about Lorenzo's oil as a treatment for [[adrenoleukodystrophy]] (ALD). For the movie of the same name, see [[Lorenzo's Oil]].}} |
|||
{{For|the film|Lorenzo's Oil}} |
|||
[[File:LorenzoOil.png|thumb|Bottle of Lorenzo's oil]] |
|||
{{multiple image |
|||
| direction = vertical |
|||
| width = 350 |
|||
| footer = The two component oils |
|||
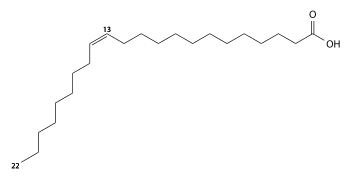
| image1 = Erucic acid.svg |
|||
| alt1 = Erucic acid |
|||
| caption1 = [[Erucic acid]] |
|||
| image2 =Oleic-acid-skeletal.svg |
|||
| alt2 = Oleic acid |
|||
| caption2 = [[Oleic acid]] |
|||
}} |
|||
'''Lorenzo's oil''' is a liquid solution made of 4 parts [[triolein|glycerol trioleate]] and 1 part glycerol trierucate, which are the [[triglyceride|triacylglycerol]] forms of [[oleic acid]] and [[erucic acid]].<ref>{{cite web|url=https://ulf.org/resources/lorenzos-oil/|year=2024|access-date=23 July 2024|title=Lorenzo’s Oil|publisher=United Leukodystrophy Foundation}}</ref> It is prepared from [[olive oil]] and [[rapeseed oil]].<ref name="Vedantam">{{cite news|url=https://www.washingtonpost.com/wp-dyn/content/article/2007/01/27/AR2007012701542_pf.html |title=A Real-Life Sequel to 'Lorenzo's Oil' – washingtonpost.com |access-date=2007-12-10 |
|||
|author=Shankar Vedantam |publisher=Washington Post 2007-01-28|pages= A01}}</ref> |
|||
It is used in the investigational treatment of asymptomatic patients with [[adrenoleukodystrophy]] (ALD), a nervous system disorder. |
|||
The development of the oil was led by [[Augusto, Michaela, and Lorenzo Odone|Augusto and Michaela Odone]] after their son Lorenzo was diagnosed with the disease in 1984, at the age of five. Lorenzo was predicted to die within a few years. His parents sought experimental treatment options, and the initial formulation of the oil was developed by retired British scientist Don Suddaby (formerly of [[Croda International]]).<ref>{{cite news|url=https://www.telegraph.co.uk/news/obituaries/2062622/Obituary-Lorenzo-Odone.html|title=Lorenzo Odone|date=1 June 2008|periodical=The Telegraph}}</ref> Suddaby and his colleague, Keith Coupland, received a U.S. patent (since expired) for invention of the oil.<ref>{{cite web|url=https://patents.google.com/patent/US5331009A/en?oq=US5331009+ |title=U.S. Patent 5,331,009: Pharmaceutical compositions for treating adrenoleukodystrophy. Issued July 19, 1994}}</ref> The [[royalties]] received by Augusto were paid to [[The Myelin Project]] which he and Michaela founded to further research treatments for ALD and similar disorders.{{citation needed|date=September 2013|reason=And did Michaela just pocket the money?}} The Odones and their invention obtained widespread publicity in 1992 because of the film ''[[Lorenzo's Oil]]''. |
|||
Research on the effectiveness of Lorenzo's oil has seen mixed results, with possible benefit for asymptomatic ALD patients but of unpredictable or no benefit to those with symptoms, suggesting its possible role as a preventative measure in families identified as ALD dominant. Lorenzo Odone died on May 30, 2008, at the age of 30; he was bedridden with paralysis and died from [[aspiration pneumonia]], likely caused by having inhaled food.<ref>{{Cite news |url=http://news.bbc.co.uk/2/hi/americas/7429221.stm |title=Lorenzo's Oil boy is dead at 30 |work=[[BBC News]] |date=31 May 2008 |access-date=2008-06-02}}</ref><ref>{{Cite news|url=http://edition.cnn.com/2008/US/05/30/lorenzo.odone.ap/ |title=Subject of 'Lorenzo's Oil' dies at 30 |work=[[CNN]] |agency=[[Associated Press]] |date=2008-05-30 |access-date=2008-07-12 |url-status=dead |archive-url=https://web.archive.org/web/20080717115741/http://edition.cnn.com/2008/US/05/30/lorenzo.odone.ap/ |archive-date=July 17, 2008 }}</ref> |
|||
==Cost and reimbursement== |
|||
Lorenzo's oil is expensive, costing approximately US$440 for a month's treatment. Most insurance companies will not pay for it since it is considered an experimental treatment by the [[Food and Drug Administration|FDA]]. |
|||
== |
==Treatment cost== |
||
In 2012, Lorenzo's oil cost approximately $400 USD for a month's treatment.<ref>{{cite web|title=Lorenzo's Oil – The Oil|url=http://www.myelin.org/lorenzosoil/lorenzosoiltheoil.html|archive-url=https://web.archive.org/web/20121030091217/http://www.myelin.org/lorenzosoil/lorenzosoiltheoil.html|url-status=dead|archive-date=October 30, 2012|publisher=The Myelin Project|access-date=3 November 2012}}</ref> |
|||
This mixture of [[fatty acid]]s reduces the levels of very long chain fatty acids (VLCFA) known to cause ALD. It does so by [[competitive inhibition|competitively inhibiting]] the [[enzyme]] that forms VLCFAs. |
|||
==Proposed mechanism of action== |
|||
The mixture of [[fatty acid]]s purportedly reduces the levels of [[very long chain fatty acids]] (VLCFAs), which are elevated in [[adrenoleukodystrophy|ALD]]. It does so by [[competitive inhibition|competitively inhibiting]] the [[enzyme]] that forms VLCFAs.<ref>{{cite web |url=http://www.myelin.org/images/scishow.swf |title=Archived copy |website=www.myelin.org |access-date=17 January 2022 |archive-url=https://web.archive.org/web/20131104132748/http://www.myelin.org/images/scishow.swf |archive-date=4 November 2013 |url-status=dead}}</ref> |
|||
==Effectiveness== |
==Effectiveness== |
||
{{main|Adrenoleukodystrophy#Treatments}} |
|||
Lorenzo's oil has not been clinically proven to be effective against the progression of ALD. Of five published studies, four have failed to demonstrate benefit in symptomatic patients. It does, however, reduce the probability of developing symptoms if its administration is started before they begin. Its efficiency is lessened once symptoms start. |
|||
Lorenzo's oil, in combination with a diet low in VLCFA, has been investigated for its possible effects on the progression of ALD. Clinical results have been mixed and the use of Lorenzo's oil has been controversial due to uncertainties regarding its clinical efficacy and the clinical indications for its use.<ref name=Berger>{{cite journal |vauthors=Berger J, Pujol A, Aubourg P, Forss-Petter S |title=Current and Future Pharmacological Treatment Strategies in X-Linked Adrenoleukodystrophy |journal=Brain Pathol. |volume=20 |issue=4 |pages=845–56 |date=July 2010 |pmid=20626746 |pmc=2967711 |doi=10.1111/j.1750-3639.2010.00393.x }}</ref> |
|||
[[Hugo Moser (scientist)|Hugo Moser]] played a prominent role in both the treatment of Lorenzo Odone and the scientific evaluation of Lorenzo's oil. In 2005, Moser published a controlled study concluding that Lorenzo's oil does not alter the course of the illness in symptomatic patients, but asymptomatic patients had a reduced risk of developing ALD while on the dietary therapy.<ref name="Moser2005">{{cite journal |vauthors=Moser HW, Raymond GV, Lu SE, Muenz LR, Moser AB, Xu J, Jones RO, Loes DJ, Melhem ER, Dubey P, Bezman L, Brereton NH, Odone A|date=July 2005|title=Follow-up of 89 asymptomatic patients with adrenoleukodystrophy treated with Lorenzo's Oil|journal=Archives of Neurology|volume=62|issue=7|pages=1073–80|pmid=16009761|doi=10.1001/archneur.62.7.1073|doi-access=free}}</ref> Moser appraised Lorenzo's oil again in a 2007 report.<ref name="Moser2007">{{cite journal |vauthors=Moser HW, Moser AB, Hollandsworth K, Brereton NH, Raymond GV |title="Lorenzo's oil" therapy for X-linked adrenoleukodystrophy: rationale and current assessment of efficacy |journal=J. Mol. Neurosci. |volume=33 |issue=1 |pages=105–13 |date=September 2007 |pmid=17901554 |doi=10.1007/s12031-007-0041-4|s2cid=21333247 }}</ref> |
|||
===Studies=== |
|||
In an early study, Duchesne studied eight males and reported improvements in follow-up magnetic resonance imaging of the brain. |
|||
Moser's findings, that Lorenzo's oil did not help symptomatic ALD patients, are consistent with prior studies published in 2003<ref>{{cite journal |vauthors=Aubourg P, Adamsbaum C, Lavallard-Rousseau MC, Rocchiccioli F, Cartier N, Jambaqué I, Jakobezak C, Lemaitre A, Boureau F, Wolf C |title=A two-year trial of oleic and erucic acids ("Lorenzo's oil") as treatment for adrenomyeloneuropathy |journal=N. Engl. J. Med. |volume=329 |issue=11 |pages=745–52 |date=September 1993 |pmid=8350883 |doi=10.1056/NEJM199309093291101 |doi-access=free }}</ref> and 1999.<ref name=Berger/><ref>{{cite journal |vauthors=van Geel BM, Assies J, Haverkort EB, Koelman JH, Verbeeten B, Wanders RJ, Barth PG |title=Progression of abnormalities in adrenomyeloneuropathy and neurologically asymptomatic X-linked adrenoleukodystrophy despite treatment with "Lorenzo's oil" |journal=J. Neurol. Neurosurg. Psychiatry |volume=67 |issue=3 |pages=290–9 |date=September 1999 |pmid=10449548 |pmc=1736534 |doi= 10.1136/jnnp.67.3.290|url=}}</ref> |
|||
In the only clinical study with positive findings, Moser tried Lorenzo's oil in 53 asymptomatic patients with ALD. Analysis of the effect of the oil indicated that there was only a slight but statistically significant slowing of clinical progression and delay of death. |
|||
A study by Poulos published in 1994 found that Lorenzo's oil is of limited value in correcting the accumulation of saturated VLCFAs in the brain of patients with ALD.<ref name="Poulos">{{cite journal |vauthors=Poulos A, Gibson R, Sharp P, Beckman K, Grattan-Smith P |title=Very long chain fatty acids in X-linked adrenoleukodystrophy brain after treatment with Lorenzo's oil |journal=Ann. Neurol. |volume=36 |issue=5 |pages=741–6 |year=1994 |pmid=7979219 |doi=10.1002/ana.410360509|s2cid=41340913 }}</ref> Comparative autopsies showed that treatment enriched erucic acid in plasma and tissues, but not in the brain.<ref name=Rasmussen1>{{cite journal |title= Brain, liver, and adipose tissue erucic and very long chain fatty acid levels in adrenoleukodystrophy patients treated with glyceryl trierucate and trioleate oils (Lorenzo's Oil) |journal=Neurochemical Research |publisher=Springer Netherlands |volume =19 |date= Aug 1994 |doi=10.1007/BF00968719 | pages=1073–1082 |author1=Magnhild Rasmussen |author2=Ann B. Moser |author3=Janet Borel |author4=Surinder Khangoora |author5=Hugo W. Moser |issue= 8 |pmid= 7800117|s2cid=11658824 }}</ref> |
|||
However a controlled study by Moser concluded that Lorenzo's oil does not alter the course of the illness in symptomatic patients; however, dietary therapy started before the development of symptoms may reduce the frequency and severity of subsequent neurological disability. |
|||
==Side effects== |
|||
A study by Poulos found that Lorenzo's oil is of limited value in correcting the accumulation of saturated VLCFAs in the brain of patients with ALD. |
|||
The oil has been shown to cause a lowered [[platelet]] count,<ref name="pmid7847331">{{cite journal |vauthors=Crowther MA, Barr RD, Kelton J, Whelan D, Greenwald M | title = Profound thrombocytopenia complicating dietary erucic acid therapy for adrenoleukodystrophy | journal = [[American Journal of Hematology]] | volume = 48 | issue = 2 | pages = 132–3 |date=February 1995 | pmid = 7847331 | doi = 10.1002/ajh.2830480217| s2cid = 29556389 }}</ref> which can lead to [[thrombocytopenia]] and [[lymphopenia]].<ref name=Hayes>Luger CL et al. Food Safety and Foodborne Toxicants. Chapter 14 in Hayes' Principles and Methods of Toxicology, Sixth Edition. Eds A. Wallace Hayes, Claire L. Kruger. CRC Press, 2014 {{ISBN|9781842145371}}. Quote: "In humans. however. although the long-term use of Lorenzo's oil (oleic acid and erucic acid) in the treatment of adrenoleukodystrophy or adrenomyeloneuropathy leads to thrombocytopenia and lymphopenia (Unkrig et al. 1994), adverse effects from dietary consumption of erucic acid have not been reported."</ref>{{rp|646–657}} |
|||
There are no reports of toxicity from dietary consumption of erucic acid.<ref name=Hayes/><ref name=fsa>Food Standards Australia New Zealand (June 2003) [http://www.foodstandards.gov.au/_srcfiles/Erucic%20acid%20monograph.pdf Erucic acid in food] {{webarchive|url=https://web.archive.org/web/20081203120159/http://www.foodstandards.gov.au/_srcfiles/Erucic%20acid%20monograph.pdf |date=2008-12-03 }} : ''A Toxicological Review and Risk Assessment .'' Technical report series No. 21; Page 4 paragraph 1; {{ISBN|0-642-34526-0}}, ISSN 1448-3017</ref><ref>{{cite web|url=http://www.food.gov.uk/news/newsarchive/2004/sep/erucic|title=Food Standards Agency - Agency issues warning on erucic acid|access-date=2007-11-02|date=2 September 2004}}</ref> |
|||
In the best study so far, Aubourg reported in the ''[[New England Journal of Medicine]]'' the results of an open trial of treatment with Lorenzo's oil in 14 men with adrenomyeloneuropathy, five symptomatic heterozygous women, and five boys (mean age, 13 years) with preclinical ALD. Over a mean follow-up of 33 months, none of the 14 men with ALD improved, one of the five asymptomatic boys developed signs of myelopathy, and there were no changes in the symptomatic heterozygous women. The authors concluded that this open trial found no evidence of a clinically relevant benefit from dietary treatment with Lorenzo's oil in patients with ALD. |
|||
==Current state== |
==Current state== |
||
Dietary manipulation using Lorenzo's oil has been shown to lower blood levels of very long chain fatty acids, but it is ineffective in symptomatic ALD. Moser's 2005 study has found "strongly suggestive, albeit not fully definitive, evidence of a preventive effect" of Lorenzo's oil on the onset of symptoms when used by asymptomatic patients.<ref name="Moser2005"/> |
|||
In the U.S., Lorenzo’s oil is currently only available to patients taking part in a clinical trial under the direction of Dr. Hugo Moser of the [[Kennedy Krieger Institute]]. |
|||
==References== |
==References== |
||
{{Reflist}} |
|||
* [http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=10449544 Treatment of X-linked adrenoleukodystrophy with Lorenzo's oil] |
|||
* [http://news.bbc.co.uk/2/hi/health/3907559.stm Lorenzo's oil, a program in the series "Medical Mysteries"] |
|||
* [http://www.usatoday.com/news/health/2005-07-11-lorenzos-oil_x.htm Study: Lorenzo's Oil protects against ailment] |
|||
* [http://www.myelin.org The Myelin Project] |
|||
* [http://www.myelinproject.co.uk The Myelin Project UK] |
|||
* [http://www.aldlife.org/ ALDLife] |
|||
* [http://www.AMNhelp.com/ AMNhelp.com] |
|||
* [http://www.myelin.org/atlantajournal.htm ''Lorenzo's Oil Lives Up to Its Billing'' Atlanta Journal Constitution – July 12, 2005] |
|||
* [http://www.jr2.ox.ac.uk/bandolier/booth/neurol/lorenz.html Lorenzo's oil for adrenoleukodystrophy and adrenomyeloneuropathy] |
|||
==External links== |
|||
* [https://aldconnect.org/about-us/the-myelin-project/ The Myelin Project] (international site) |
|||
* [http://www.lorenzoandhisparents.com Lorenzo and His Parents] (official site of book) |
|||
{{DEFAULTSORT:Lorenzo's Oil}} |
|||
{{Pharma-stub}} |
|||
[[Category:Lipids]] |
[[Category:Lipids]] |
||
[[Category:Eponyms in biology]] |
|||
[[de:Lorenzos Öl]] |
|||
[[fr:Huile de Lorenzo]] |
|||
[[ko:로렌조 오일]] |
|||
[[zh:羅倫佐的油]] |
|||
Latest revision as of 09:14, 14 December 2024

Lorenzo's oil is a liquid solution made of 4 parts glycerol trioleate and 1 part glycerol trierucate, which are the triacylglycerol forms of oleic acid and erucic acid.[1] It is prepared from olive oil and rapeseed oil.[2] It is used in the investigational treatment of asymptomatic patients with adrenoleukodystrophy (ALD), a nervous system disorder.
The development of the oil was led by Augusto and Michaela Odone after their son Lorenzo was diagnosed with the disease in 1984, at the age of five. Lorenzo was predicted to die within a few years. His parents sought experimental treatment options, and the initial formulation of the oil was developed by retired British scientist Don Suddaby (formerly of Croda International).[3] Suddaby and his colleague, Keith Coupland, received a U.S. patent (since expired) for invention of the oil.[4] The royalties received by Augusto were paid to The Myelin Project which he and Michaela founded to further research treatments for ALD and similar disorders.[citation needed] The Odones and their invention obtained widespread publicity in 1992 because of the film Lorenzo's Oil.
Research on the effectiveness of Lorenzo's oil has seen mixed results, with possible benefit for asymptomatic ALD patients but of unpredictable or no benefit to those with symptoms, suggesting its possible role as a preventative measure in families identified as ALD dominant. Lorenzo Odone died on May 30, 2008, at the age of 30; he was bedridden with paralysis and died from aspiration pneumonia, likely caused by having inhaled food.[5][6]
Treatment cost
[edit]In 2012, Lorenzo's oil cost approximately $400 USD for a month's treatment.[7]
Proposed mechanism of action
[edit]The mixture of fatty acids purportedly reduces the levels of very long chain fatty acids (VLCFAs), which are elevated in ALD. It does so by competitively inhibiting the enzyme that forms VLCFAs.[8]
Effectiveness
[edit]Lorenzo's oil, in combination with a diet low in VLCFA, has been investigated for its possible effects on the progression of ALD. Clinical results have been mixed and the use of Lorenzo's oil has been controversial due to uncertainties regarding its clinical efficacy and the clinical indications for its use.[9]
Hugo Moser played a prominent role in both the treatment of Lorenzo Odone and the scientific evaluation of Lorenzo's oil. In 2005, Moser published a controlled study concluding that Lorenzo's oil does not alter the course of the illness in symptomatic patients, but asymptomatic patients had a reduced risk of developing ALD while on the dietary therapy.[10] Moser appraised Lorenzo's oil again in a 2007 report.[11]
Moser's findings, that Lorenzo's oil did not help symptomatic ALD patients, are consistent with prior studies published in 2003[12] and 1999.[9][13]
A study by Poulos published in 1994 found that Lorenzo's oil is of limited value in correcting the accumulation of saturated VLCFAs in the brain of patients with ALD.[14] Comparative autopsies showed that treatment enriched erucic acid in plasma and tissues, but not in the brain.[15]
Side effects
[edit]The oil has been shown to cause a lowered platelet count,[16] which can lead to thrombocytopenia and lymphopenia.[17]: 646–657
There are no reports of toxicity from dietary consumption of erucic acid.[17][18][19]
Current state
[edit]Dietary manipulation using Lorenzo's oil has been shown to lower blood levels of very long chain fatty acids, but it is ineffective in symptomatic ALD. Moser's 2005 study has found "strongly suggestive, albeit not fully definitive, evidence of a preventive effect" of Lorenzo's oil on the onset of symptoms when used by asymptomatic patients.[10]
References
[edit]- ^ "Lorenzo's Oil". United Leukodystrophy Foundation. 2024. Retrieved 23 July 2024.
- ^ Shankar Vedantam. "A Real-Life Sequel to 'Lorenzo's Oil' – washingtonpost.com". Washington Post 2007-01-28. pp. A01. Retrieved 2007-12-10.
- ^ "Lorenzo Odone". The Telegraph. 1 June 2008.
- ^ "U.S. Patent 5,331,009: Pharmaceutical compositions for treating adrenoleukodystrophy. Issued July 19, 1994".
- ^ "Lorenzo's Oil boy is dead at 30". BBC News. 31 May 2008. Retrieved 2008-06-02.
- ^ "Subject of 'Lorenzo's Oil' dies at 30". CNN. Associated Press. 2008-05-30. Archived from the original on July 17, 2008. Retrieved 2008-07-12.
- ^ "Lorenzo's Oil – The Oil". The Myelin Project. Archived from the original on October 30, 2012. Retrieved 3 November 2012.
- ^ "Archived copy". www.myelin.org. Archived from the original on 4 November 2013. Retrieved 17 January 2022.
{{cite web}}: CS1 maint: archived copy as title (link) - ^ a b Berger J, Pujol A, Aubourg P, Forss-Petter S (July 2010). "Current and Future Pharmacological Treatment Strategies in X-Linked Adrenoleukodystrophy". Brain Pathol. 20 (4): 845–56. doi:10.1111/j.1750-3639.2010.00393.x. PMC 2967711. PMID 20626746.
- ^ a b Moser HW, Raymond GV, Lu SE, Muenz LR, Moser AB, Xu J, Jones RO, Loes DJ, Melhem ER, Dubey P, Bezman L, Brereton NH, Odone A (July 2005). "Follow-up of 89 asymptomatic patients with adrenoleukodystrophy treated with Lorenzo's Oil". Archives of Neurology. 62 (7): 1073–80. doi:10.1001/archneur.62.7.1073. PMID 16009761.
- ^ Moser HW, Moser AB, Hollandsworth K, Brereton NH, Raymond GV (September 2007). ""Lorenzo's oil" therapy for X-linked adrenoleukodystrophy: rationale and current assessment of efficacy". J. Mol. Neurosci. 33 (1): 105–13. doi:10.1007/s12031-007-0041-4. PMID 17901554. S2CID 21333247.
- ^ Aubourg P, Adamsbaum C, Lavallard-Rousseau MC, Rocchiccioli F, Cartier N, Jambaqué I, Jakobezak C, Lemaitre A, Boureau F, Wolf C (September 1993). "A two-year trial of oleic and erucic acids ("Lorenzo's oil") as treatment for adrenomyeloneuropathy". N. Engl. J. Med. 329 (11): 745–52. doi:10.1056/NEJM199309093291101. PMID 8350883.
- ^ van Geel BM, Assies J, Haverkort EB, Koelman JH, Verbeeten B, Wanders RJ, Barth PG (September 1999). "Progression of abnormalities in adrenomyeloneuropathy and neurologically asymptomatic X-linked adrenoleukodystrophy despite treatment with "Lorenzo's oil"". J. Neurol. Neurosurg. Psychiatry. 67 (3): 290–9. doi:10.1136/jnnp.67.3.290. PMC 1736534. PMID 10449548.
- ^ Poulos A, Gibson R, Sharp P, Beckman K, Grattan-Smith P (1994). "Very long chain fatty acids in X-linked adrenoleukodystrophy brain after treatment with Lorenzo's oil". Ann. Neurol. 36 (5): 741–6. doi:10.1002/ana.410360509. PMID 7979219. S2CID 41340913.
- ^ Magnhild Rasmussen; Ann B. Moser; Janet Borel; Surinder Khangoora; Hugo W. Moser (Aug 1994). "Brain, liver, and adipose tissue erucic and very long chain fatty acid levels in adrenoleukodystrophy patients treated with glyceryl trierucate and trioleate oils (Lorenzo's Oil)". Neurochemical Research. 19 (8). Springer Netherlands: 1073–1082. doi:10.1007/BF00968719. PMID 7800117. S2CID 11658824.
- ^ Crowther MA, Barr RD, Kelton J, Whelan D, Greenwald M (February 1995). "Profound thrombocytopenia complicating dietary erucic acid therapy for adrenoleukodystrophy". American Journal of Hematology. 48 (2): 132–3. doi:10.1002/ajh.2830480217. PMID 7847331. S2CID 29556389.
- ^ a b Luger CL et al. Food Safety and Foodborne Toxicants. Chapter 14 in Hayes' Principles and Methods of Toxicology, Sixth Edition. Eds A. Wallace Hayes, Claire L. Kruger. CRC Press, 2014 ISBN 9781842145371. Quote: "In humans. however. although the long-term use of Lorenzo's oil (oleic acid and erucic acid) in the treatment of adrenoleukodystrophy or adrenomyeloneuropathy leads to thrombocytopenia and lymphopenia (Unkrig et al. 1994), adverse effects from dietary consumption of erucic acid have not been reported."
- ^ Food Standards Australia New Zealand (June 2003) Erucic acid in food Archived 2008-12-03 at the Wayback Machine : A Toxicological Review and Risk Assessment . Technical report series No. 21; Page 4 paragraph 1; ISBN 0-642-34526-0, ISSN 1448-3017
- ^ "Food Standards Agency - Agency issues warning on erucic acid". 2 September 2004. Retrieved 2007-11-02.
External links
[edit]- The Myelin Project (international site)
- Lorenzo and His Parents (official site of book)