E-4 process: Difference between revisions
m v2.03b - Bot T20 CW#61 - WP:WCW project (Reference before punctuation) |
m Clean up spacing around commas and other punctuation fixes, replaced: ,F → , F, ,T → , T |
||
| (11 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
{{More citations needed|date=April 2021}} |
|||
:''See also [[Ektachrome]] for full details of Kodak E-series processes.'' |
:''See also [[Ektachrome]] for full details of Kodak E-series processes.'' |
||
The '''E-4 process''' is a now outdated process for developing [[transparency (photography)|color reversal (transparency)]] photographic film, |
The '''E-4 process''' is a now outdated process for developing [[transparency (photography)|color reversal (transparency)]] photographic film, which was introduced in 1966. |
||
==Drawbacks== |
|||
The process is infamous for two reasons: |
The process is infamous for two reasons: |
||
First, |
First, it uses the highly toxic [[boron hydride]]-based reversal agent [[tertiary butyl-amine borane]] (TBAB).{{efn|Not to be confused with [[tetra-n-butylammonium bromide]], which also is abbreviated as TBAB.}}<ref name=Jacobson80/>{{rp|379, Table LXVI}} Early releases of the consumer-sized version of the chemistry provided the TBAB in the form of a tablet, possibly to avoid the possibility of inhalation.<ref name=Talbert/> This was later changed to loose powder, likely as a countermeasure against inadvertent ingestion of the substance. |
||
Second, the |
Second, the prehardener agent contains [[formaldehyde]] and 2,5-dimethoxy[[tetrahydrofuran]],<ref name=Jacobson80/>{{rp|377, Formula 269}} which when mixed generates [[succinaldehyde]], a noxious gas which has been likened to tear gas.<ref name=Talbert/> Process E-6 films are hardened during manufacture, eliminating the prehardener step altogether and allowing them to be processed at {{cvt|100|F}}. |
||
==Steps== |
|||
The process is faster than E-3 and runs at 30°C (85°F, ± .5°F),<ref>{{Cite web | url=https://books.google.com/?id=kyYDAAAAMBAJ&pg=PA130&dq=ektachrome#v=onepage&q=ektachrome&f=false |title = Popular Science|date = April 1968}}</ref> about 6°C (10°F) higher than E-3. The ME-4 process was a motion picture variation of the E-4 process. |
|||
{{multiple image |align=right |direction=horizontal |title=Ektachrome film structure and exposure |
|||
|image1=E-6 step 00.svg |caption1=Structure |
|||
|image2=E-6 step 01.svg |caption2=Sample exposure to various colors}} |
|||
Ektachrome film has three separate light-sensitive layers; each layer is sensitive to a different group of wavelengths corresponding to red, green, and blue colors. When the film is exposed, each layer records a latent image based on its sensitivity. A yellow filter prevents blue light from exposing the green- and red-sensitive layers, which have some sensitivity to blue light.<ref name=Z119-1>{{cite web |url=http://www.kodak.com/global/plugins/acrobat/en/service/Zmanuals/z119-1.pdf |title=Process E-6 Using KODAK Chemicals, Process E-6 Publication Z-119 {{!}} Chapter 1: Processing solutions and their effects |publisher=[[Kodak]] |archive-url=https://web.archive.org/web/20050825145808/http://kodak.com/global/plugins/acrobat/en/service/Zmanuals/z119-1.pdf |archive-date=August 25, 2005 |url-status=dead}}</ref> |
|||
The E-4 process is faster than E-3; whereas E-3 required 15 steps and up to 70 minutes from start to finish,<ref name=Talbert>{{cite web |url=https://www.photomemorabilia.co.uk/Colour_Darkroom/Early_Kodak_Ektachrome.html |title=Kodak Ektachrome Colour Transparency films |author=Talbert, Michael |website=Photo Memorabilia |access-date=24 August 2023}}</ref><ref name=E-13>{{cite book |url=https://archive.org/details/kodakektachromef00east/ |title=Kodak Ektachrome Film, Publication No. E-13 |date=1955 |publisher=Eastman Kodak Company |url-access=registration}}</ref>{{rp|30–31}} E-4 was completed in approximately 50 minutes over 13 steps.<ref name=PS-1968/> E-4 runs at {{cvt|85|F}},<ref name=PS-1968>{{Cite magazine | url=https://books.google.com/books?id=kyYDAAAAMBAJ&pg=PA130 |title = Kodak's new E-4 kit: 50-Minute Cure for People Afraid to Develop Their Own Color Film |author=Wahl, Paul |magazine=Popular Science |date = April 1968 |pages=130–131}}</ref> about 10 °F (6 °C) higher than E-3. The temperature tolerance is ±1 °F for prehardener, ±{{frac|2}}°F for the first developer, and ±2–5 °F for all other steps.<ref name=PS-1968/> The ME-4 process was a motion picture variation of the E-4 process. |
|||
Process E-4 consisted of nine chemicals: prehardener, neutralizer, first developer, First Stop Bath, Color Developer, Second Stop Bath, bleach, fixer, stabilizer |
|||
The major change for E-4 was the inclusion of a chemical reversal agent, which permits processing of the film without the manual re-exposure/fogging step required by the predecessor E-1 / E-2 / [[E-3 process]]es.<ref name=Talbert/><ref name=PS-1968/> |
|||
Total darkness was required during the first four chemicals; normal room light was used for the remaining five. The temperature tolerance was 1°F for prehardener, 1/2°F for the two developers, and 2°F all other steps. |
|||
Total darkness is required during the first four development steps; normal room light can be used for the remaining steps.<ref name=PS-1968/> |
|||
| ⚫ | |||
{|class="wikitable" style="font-size:100%;text-align:left;" |
|||
| ⚫ | |||
|+E-4 Process<ref name=PS-1968/> |
|||
! colspan=3 | Step !! Schematic !! Time (min.) !! Temp. !! Description |
|||
|- |
|||
| rowspan=4 style="background:#000;" | |
|||
! 1 !! Prehardener |
|||
| rowspan=2 | |
|||
| 3 || {{cvt|85|F}} ±1 °F |
|||
| Tempers film for high-temperature processing |
|||
|- |
|||
! 2 !! Neutralizer |
|||
| 1 || {{cvt|83–87|F}} |
|||
| |
|||
|- |
|||
! 3 !! First developer |
|||
| rowspan=3 | [[File:E-6 step 02.svg|frameless|upright=0.7]] |
|||
| 7 || {{cvt|85|F}} ±{{frac|2}}°F |
|||
| Conventional black-and-white developer used to transform silver halide crystals exposed in all three layers as a negative image. |
|||
|- |
|||
! 4 !! First stop bath |
|||
| 2 || {{cvt|83–87|F}} |
|||
| Solution should not be reused for second stop bath (step 7) |
|||
|- |
|||
| rowspan=9 style="background:#fff;" | |
|||
! 5 !! Wash |
|||
| 4 || {{cvt|80–90|F}} |
|||
| Running water |
|||
|- |
|||
! 6 !! Color developer |
|||
| rowspan=3 | [[File:E-6 step 03.svg|frameless|upright=0.7]] |
|||
| 9 || {{cvt|83–87|F}} |
|||
| |
|||
|- |
|||
! 7 !! Second stop bath |
|||
| 3 || {{cvt|83–87|F}} |
|||
| Solution should not be reused from first stop bath (step 4) |
|||
|- |
|||
! 8 !! Wash |
|||
| 3 || {{cvt|80–90|F}} |
|||
| Running water |
|||
|- |
|||
! 9 !! Bleach |
|||
| rowspan=1 | [[File:E-6 step 04.svg|frameless|upright=0.7]] |
|||
| 5 || {{cvt|83–87|F}} |
|||
| Convert metallic silver to soluble particles |
|||
|- |
|||
! 10!! Fixer |
|||
| rowspan=4 | [[File:E-6 step 05.svg|frameless|upright=0.7]] |
|||
| 6 || {{cvt|83–87|F}} |
|||
| Dissolve silver particles, which can be recovered after processing |
|||
|- |
|||
! 11!! Wash |
|||
| 6 || {{cvt|80–90|F}} |
|||
| Running water |
|||
|- |
|||
! 12!! Stabilizer |
|||
| 1 || {{cvt|83–87|F}} |
|||
| |
|||
|- |
|||
! 13!! Dry |
|||
| var. || <{{cvt|110|F}} |
|||
| |
|||
|} |
|||
==History== |
|||
The process has been discontinued but was used up until 1996 for Kodak IE color infrared film, due to legal commitment by Kodak to provide the process for 30 years. |
|||
[[File:Kodak Ektachrome IE 135-20 Infrared Slide Film.jpg|thumb|right|Kodak Ektachrome Infrared film using E-4 process]] |
|||
| ⚫ | |||
| ⚫ | |||
The E-4 process has been discontinued since 1996; after 1976 it was used solely for Kodak IE color [[Infrared photography|infrared film]],<ref>{{cite magazine |url=https://books.google.com/books?id=_eMDAAAAMBAJ&pg=PA100 |title=Inner Visions |author=Ensanian, Armand |date=July 1988 |magazine=Popular Mechanics |pages=100–101 |access-date=24 August 2023 |quote=Color IR film has one drawback. It is not readily processed because it requires the old E-4 chemistry.}}</ref> due to a legal commitment by Kodak to provide process support for 30 years after introduction. Kodak discontinued E-4 processing in 1985, but independent photofinishers continued to support the process.<ref>{{cite magazine |url=https://books.google.com/books?id=LxmeZrp-lukC&pg=PA114 |title=Pop Photo Snapshots: Bad and good news from Kodak |author=Rothschild, Norman |date=December 1985 |magazine=Popular Photography |pages=28–32;114 |access-date=24 August 2023 |quote=Eastman Kodak no longer offers processing for E-4 films such as Ektachrome Infrared and Kodak Microphotography color-slide films. However, there are more than a dozen independent labs in the U.S. that offer this service.}}</ref> The E-4 chemicals were reverse-engineered and substitute formulae were published in the ''[[British Journal of Photography]] Annual'' in 1977.<ref name=Jacobson80>{{cite book |chapter-url=https://archive.org/details/DevelopingTheNegativeTechnique/page/n375/mode/2up |title=Developing: The Negative Technique |author1=Jacobson, Kurt I. |author2=Jacobson, Ralph Eric |date=1980 |edition=Eighteenth revised |publisher=Focal Press |location=London |isbn=0-240-44770-0 |access-date=24 August 2023 |chapter=Processing Colour Films |pages=363–383}}</ref>{{rp|374}} |
|||
==Notes== |
|||
{{notelist}} |
|||
== References == |
== References == |
||
| Line 26: | Line 103: | ||
== External links == |
== External links == |
||
* [http://www.kodak.com/global/en/professional/support/techPubs/cis111/cis111.jhtml Kodak specifications for hand mixing of chemistry] |
* [http://www.kodak.com/global/en/professional/support/techPubs/cis111/cis111.jhtml Kodak specifications for hand mixing of chemistry] |
||
* {{cite web |url=https://www.mat.uc.pt/~rps/photos/FAQ_e4.txt |title=More than you want to know about E-4 |date=19 May 1995}} |
|||
===Processing of older Ektachrome films (including Process E-4)=== |
===Processing of older Ektachrome films (including Process E-4)=== |
||
| Line 32: | Line 110: | ||
[[Category:Photographic film processes]] |
[[Category:Photographic film processes]] |
||
{{photography-stub}} |
{{photography-stub}} |
||
Latest revision as of 00:01, 7 September 2024
This article needs additional citations for verification. (April 2021) |
- See also Ektachrome for full details of Kodak E-series processes.
The E-4 process is a now outdated process for developing color reversal (transparency) photographic film, which was introduced in 1966.
Drawbacks
[edit]The process is infamous for two reasons:
First, it uses the highly toxic boron hydride-based reversal agent tertiary butyl-amine borane (TBAB).[a][1]: 379, Table LXVI Early releases of the consumer-sized version of the chemistry provided the TBAB in the form of a tablet, possibly to avoid the possibility of inhalation.[2] This was later changed to loose powder, likely as a countermeasure against inadvertent ingestion of the substance.
Second, the prehardener agent contains formaldehyde and 2,5-dimethoxytetrahydrofuran,[1]: 377, Formula 269 which when mixed generates succinaldehyde, a noxious gas which has been likened to tear gas.[2] Process E-6 films are hardened during manufacture, eliminating the prehardener step altogether and allowing them to be processed at 100 °F (38 °C).
Steps
[edit]Ektachrome film has three separate light-sensitive layers; each layer is sensitive to a different group of wavelengths corresponding to red, green, and blue colors. When the film is exposed, each layer records a latent image based on its sensitivity. A yellow filter prevents blue light from exposing the green- and red-sensitive layers, which have some sensitivity to blue light.[3]
The E-4 process is faster than E-3; whereas E-3 required 15 steps and up to 70 minutes from start to finish,[2][4]: 30–31 E-4 was completed in approximately 50 minutes over 13 steps.[5] E-4 runs at 85 °F (29 °C),[5] about 10 °F (6 °C) higher than E-3. The temperature tolerance is ±1 °F for prehardener, ±1⁄2°F for the first developer, and ±2–5 °F for all other steps.[5] The ME-4 process was a motion picture variation of the E-4 process.
The major change for E-4 was the inclusion of a chemical reversal agent, which permits processing of the film without the manual re-exposure/fogging step required by the predecessor E-1 / E-2 / E-3 processes.[2][5]
Total darkness is required during the first four development steps; normal room light can be used for the remaining steps.[5]
| Step | Schematic | Time (min.) | Temp. | Description | ||
|---|---|---|---|---|---|---|
| 1 | Prehardener | 3 | 85 °F (29 °C) ±1 °F | Tempers film for high-temperature processing | ||
| 2 | Neutralizer | 1 | 83–87 °F (28–31 °C) | |||
| 3 | First developer | 
|
7 | 85 °F (29 °C) ±1⁄2°F | Conventional black-and-white developer used to transform silver halide crystals exposed in all three layers as a negative image. | |
| 4 | First stop bath | 2 | 83–87 °F (28–31 °C) | Solution should not be reused for second stop bath (step 7) | ||
| 5 | Wash | 4 | 80–90 °F (27–32 °C) | Running water | ||
| 6 | Color developer | 
|
9 | 83–87 °F (28–31 °C) | ||
| 7 | Second stop bath | 3 | 83–87 °F (28–31 °C) | Solution should not be reused from first stop bath (step 4) | ||
| 8 | Wash | 3 | 80–90 °F (27–32 °C) | Running water | ||
| 9 | Bleach | 
|
5 | 83–87 °F (28–31 °C) | Convert metallic silver to soluble particles | |
| 10 | Fixer | 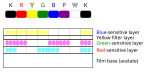
|
6 | 83–87 °F (28–31 °C) | Dissolve silver particles, which can be recovered after processing | |
| 11 | Wash | 6 | 80–90 °F (27–32 °C) | Running water | ||
| 12 | Stabilizer | 1 | 83–87 °F (28–31 °C) | |||
| 13 | Dry | var. | <110 °F (43 °C) | |||
History
[edit]
E-4 processed film is color stable for about 30 years.[6]
The process largely was phased out in 1976 with the introduction of the E-6 process, which is more environmentally friendly due to its lack of toxic chemicals. E-6 avoids the use of TBAB by adding a separate reversal bath containing the tin salt stannous chloride.
The E-4 process has been discontinued since 1996; after 1976 it was used solely for Kodak IE color infrared film,[7] due to a legal commitment by Kodak to provide process support for 30 years after introduction. Kodak discontinued E-4 processing in 1985, but independent photofinishers continued to support the process.[8] The E-4 chemicals were reverse-engineered and substitute formulae were published in the British Journal of Photography Annual in 1977.[1]: 374
Notes
[edit]- ^ Not to be confused with tetra-n-butylammonium bromide, which also is abbreviated as TBAB.
References
[edit]- ^ a b c Jacobson, Kurt I.; Jacobson, Ralph Eric (1980). "Processing Colour Films". Developing: The Negative Technique (Eighteenth revised ed.). London: Focal Press. pp. 363–383. ISBN 0-240-44770-0. Retrieved 24 August 2023.
- ^ a b c d Talbert, Michael. "Kodak Ektachrome Colour Transparency films". Photo Memorabilia. Retrieved 24 August 2023.
- ^ "Process E-6 Using KODAK Chemicals, Process E-6 Publication Z-119 | Chapter 1: Processing solutions and their effects" (PDF). Kodak. Archived from the original (PDF) on August 25, 2005.
- ^ Kodak Ektachrome Film, Publication No. E-13. Eastman Kodak Company. 1955.
- ^ a b c d e f Wahl, Paul (April 1968). "Kodak's new E-4 kit: 50-Minute Cure for People Afraid to Develop Their Own Color Film". Popular Science. pp. 130–131.
- ^ "Ektachrome: A Look Back". 25 January 2017.
- ^ Ensanian, Armand (July 1988). "Inner Visions". Popular Mechanics. pp. 100–101. Retrieved 24 August 2023.
Color IR film has one drawback. It is not readily processed because it requires the old E-4 chemistry.
- ^ Rothschild, Norman (December 1985). "Pop Photo Snapshots: Bad and good news from Kodak". Popular Photography. pp. 28–32, 114. Retrieved 24 August 2023.
Eastman Kodak no longer offers processing for E-4 films such as Ektachrome Infrared and Kodak Microphotography color-slide films. However, there are more than a dozen independent labs in the U.S. that offer this service.
External links
[edit]- Kodak specifications for hand mixing of chemistry
- "More than you want to know about E-4". 19 May 1995.
Processing of older Ektachrome films (including Process E-4)
[edit]- Film Rescue USA and Canada
- Rocky Mountain USA


