Piroxicam: Difference between revisions
I think it's correct, but it needs a reference, and preferably an explanation in the body |
that link doesn't seem to have any information about this topic |
||
| Line 59: | Line 59: | ||
}} |
}} |
||
<!-- Definition and medical uses --> |
<!-- Definition and medical uses --> |
||
'''Piroxicam''' is a [[nonsteroidal anti-inflammatory drug]] (NSAID) of the [[oxicam]] class used to relieve the symptoms of painful inflammatory conditions like [[arthritis]].<ref name = MD/><ref>{{cite journal | title=TGA Approved Terminology for Medicines, Section 1 – Chemical Substances | date=July 1999 | publisher=Therapeutic Goods Administration, Department of Health and Ageing, Australian Government | page=97 | url=http://www.tga.gov.au/pdf/medicines-approved-terminology-chemical.pdf }}</ref> Piroxicam works by preventing the production of endogenous [[prostaglandins]] which are involved in the mediation of pain, stiffness, tenderness and swelling.<ref name = MD/> The medicine is available as [[capsule (pharmacy)|capsules]], [[tablet (pharmacy)|tablets]] and (not in all countries) as a prescription-free [[gel]] 0.5%.<ref name = BNF/> It is also available in a betadex formulation, which allows a more rapid absorption of piroxicam from the digestive tract.<ref name = MD>{{cite web|title=Piroxicam|work=Martindale: The Complete Drug Reference|publisher=Pharmaceutical Press|date=14 January 2014|access-date=24 June 2014|url=http://www.medicinescomplete.com/mc/martindale/current/ms-2692-v.htm|editor=Brayfield, A|location=London, UK}}</ref> Piroxicam is one of the few NSAIDs that can be given parenteral routes.{{cn}} |
'''Piroxicam''' is a [[nonsteroidal anti-inflammatory drug]] (NSAID) of the [[oxicam]] class used to relieve the symptoms of painful inflammatory conditions like [[arthritis]].<ref name = MD/><ref>{{cite journal | title=TGA Approved Terminology for Medicines, Section 1 – Chemical Substances | date=July 1999 | publisher=Therapeutic Goods Administration, Department of Health and Ageing, Australian Government | page=97 | url=http://www.tga.gov.au/pdf/medicines-approved-terminology-chemical.pdf }}</ref> Piroxicam works by preventing the production of endogenous [[prostaglandins]] which are involved in the mediation of pain, stiffness, tenderness and swelling.<ref name = MD/> The medicine is available as [[capsule (pharmacy)|capsules]], [[tablet (pharmacy)|tablets]] and (not in all countries) as a prescription-free [[gel]] 0.5%.<ref name = BNF/> It is also available in a betadex formulation, which allows a more rapid absorption of piroxicam from the digestive tract.<ref name = MD>{{cite web|title=Piroxicam|work=Martindale: The Complete Drug Reference|publisher=Pharmaceutical Press|date=14 January 2014|access-date=24 June 2014|url=http://www.medicinescomplete.com/mc/martindale/current/ms-2692-v.htm|editor=Brayfield, A|location=London, UK}}{{dead link}}</ref> Piroxicam is one of the few NSAIDs that can be given parenteral routes.{{cn}} |
||
<!-- Society and culture --> |
<!-- Society and culture --> |
||
Revision as of 16:01, 17 September 2022
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /paɪˈrɒksɪˌkæm/ |
| Trade names | Feldene, others[1] |
| Other names | Piroksikam, piroxikam |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a684045 |
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | 99%[4] |
| Metabolism | Liver-mediated hydroxylation and glucuronidation[4] |
| Elimination half-life | 50 hours[4] |
| Excretion | Urine, faeces |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.048.144 |
| Chemical and physical data | |
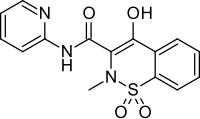
| Formula | C15H13N3O4S |
| Molar mass | 331.35 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Piroxicam is a nonsteroidal anti-inflammatory drug (NSAID) of the oxicam class used to relieve the symptoms of painful inflammatory conditions like arthritis.[4][5] Piroxicam works by preventing the production of endogenous prostaglandins which are involved in the mediation of pain, stiffness, tenderness and swelling.[4] The medicine is available as capsules, tablets and (not in all countries) as a prescription-free gel 0.5%.[6] It is also available in a betadex formulation, which allows a more rapid absorption of piroxicam from the digestive tract.[4] Piroxicam is one of the few NSAIDs that can be given parenteral routes.[citation needed]
It was patented in 1968 by Pfizer and approved for medical use in 1979.[7] It became generic in 1992,[8] and is marketed worldwide under many brandnames.[1]
Medical uses
It is used in the treatment of certain inflammatory conditions like rheumatoid and osteoarthritis, primary dysmenorrhoea, postoperative pain; and act as an analgesic, especially where there is an inflammatory component.[4] The European Medicines Agency issued a review of its use in 2007 and recommended that its use be limited to the treatment of chronic inflammatory conditions, as it is only in these circumstances that its risk-benefit ratio proves to be favourable.[6][9]
Adverse effects
→ As with other NSAIDs the principal side effects include: digestive complaints like nausea, discomfort, diarrhoea and bleeds or ulceration of the stomach, as well as headache, dizziness, nervousness, depression, drowsiness, insomnia, vertigo, hearing disturbances (such as tinnitus), high blood pressure, oedema, light sensitivity, skin reactions (including, albeit rarely, Stevens–Johnson syndrome and toxic epidermal necrolysis) and rarely, kidney failure, pancreatitis, liver damage, visual disturbances, pulmonary eosinophilia and fibrosing alveolitis.[6] Compared to other NSAIDs it is more prone to causing gastrointestinal disturbances and serious skin reactions.[6]
In October 2020, the U.S. Food and Drug Administration (FDA) required the drug label to be updated for all nonsteroidal anti-inflammatory medications to describe the risk of kidney problems in unborn babies that result in low amniotic fluid.[10][11] They recommend avoiding NSAIDs in pregnant women at 20 weeks or later in pregnancy.[10][11]
Mechanism of action
Piroxicam is an NSAID and, as such, is a non-selective COX inhibitor possessing both analgesic and antipyretic properties.[6]
Chemical properties
Piroxicam exists as alkenol tautomer in organic solvents and as zwitterionic form in water.[12]
History
The project that produced piroxicam began in 1962 at Pfizer; the first clinical trial results were reported in 1977, and the product launched in 1980 under the brand name "Feldene".[8][13] Major patents expired in 1992[8] and the drug is marketed worldwide under many brandnames.[1]
See also
References
- ^ a b c Drugs.com Drugs.com international listings for piroxicam Page accessed July 3, 2015
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 Oct 2023.
- ^ https://www.ema.europa.eu/documents/psusa/piroxicam-list-nationally-authorised-medicinal-products-psusa/00002438/202004_en.pdf [bare URL PDF]
- ^ a b c d e f g Brayfield, A, ed. (14 January 2014). "Piroxicam". Martindale: The Complete Drug Reference. London, UK: Pharmaceutical Press. Retrieved 24 June 2014.[dead link]
- ^ "TGA Approved Terminology for Medicines, Section 1 – Chemical Substances" (PDF). Therapeutic Goods Administration, Department of Health and Ageing, Australian Government. July 1999: 97.
{{cite journal}}: Cite journal requires|journal=(help) - ^ a b c d e Joint Formulary Committee (2013). British National Formulary (BNF) (65 ed.). London, UK: Pharmaceutical Press. pp. 665, 673–674. ISBN 978-0-85711-084-8.
- ^ Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 519. ISBN 9783527607495.
- ^ a b c Lombardino, JG; Lowe, JA 3rd (2004). "The role of the medicinal chemist in drug discovery--then and now". Nat Rev Drug Discov. 3 (10): 853–62. doi:10.1038/nrd1523. PMID 15459676. S2CID 11225541.
{{cite journal}}: CS1 maint: numeric names: authors list (link). See: [1] Box 1: Discovery of piroxicam (1962–1980) - ^ "Committee for medicinal products for human use (CHMP) opinion following an Article 31(2) referral for Piroxicam containing medicinal products" (PDF). European Medicines Agency. London, UK: European Medicines Agency. 20 September 2007. Retrieved 24 June 2014.
- ^ a b "FDA Warns that Using a Type of Pain and Fever Medication in Second Half of Pregnancy Could Lead to Complications". U.S. Food and Drug Administration (FDA) (Press release). 15 October 2020. Retrieved 15 October 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ a b "NSAIDs may cause rare kidney problems in unborn babies". U.S. Food and Drug Administration. 21 July 2017. Retrieved 15 October 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ Ivanova D, Deneva V, Nedeltcheva D, Kamounah FS, Gergov G, Hansen PE, Kawauchi S, Antonov L (2015). "Tautomeric transformations of piroxicam in solution: a combined experimental and theoretical study". RSC Advances. 5 (40): 31852–31860. Bibcode:2015RSCAd...531852I. doi:10.1039/c5ra03653d.
- ^ Weintraub M, Jacox RF, Angevine CD, Atwater EC (1977). "Piroxicam (CP 16171) in rheumatoid arthritis: a controlled clinical trial with novel assessment techniques". Journal of Rheumatology. 4 (4): 393–404. PMID 342691.
Further reading
- Dean L (2019). "Piroxicam Therapy and CYP2C9 Genotype". In Pratt VM, McLeod HL, Rubinstein WS, et al. (eds.). Medical Genetics Summaries. National Center for Biotechnology Information (NCBI). PMID 30742401. Bookshelf ID: NBK537367.
