Acetic anhydride: Difference between revisions
Tag: Reverted |
Tag: Reverted |
||
| Line 103: | Line 103: | ||
: CH<sub>3</sub>CO<sub>2</sub>CH<sub>3</sub> + CO → (CH<sub>3</sub>CO)<sub>2</sub>O |
: CH<sub>3</sub>CO<sub>2</sub>CH<sub>3</sub> + CO → (CH<sub>3</sub>CO)<sub>2</sub>O |
||
The [[Monsanto process#Tennessee Eastman acetic anhydride process|Tennessee Eastman acetic anhydride process]] involves the conversion of methyl acetate to [[methyl iodide]] and an acetate salt. Carbonylation of the methyl iodide in turn affords [[acetyl iodide]], which reacts with acetate salts or acetic acid to give the product. [[Rhodium chloride]] in the presence of [[lithium iodide]] is employed as catalysts put catalysts in. Because acetic anhydride is not stable in water, the conversion is conducted under anhydrous conditions. |
The [[Monsanto process#Tennessee Eastman acetic anhydride process|Tennessee Eastman acetic anhydride process]] involves the conversion of methyl acetate to [[methyl iodide]] and an acetate salt. Carbonylation of the methyl iodide in turn affords [[acetyl iodide]], which reacts with acetate salts or acetic acid to give the product. [[Rhodium chloride]] in the presence of [[lithium iodide]] is employed as catalysts put catalysts in your ___. Because acetic anhydride is not stable in water, the conversion is conducted under anhydrous conditions. |
||
To a decreasing extent, acetic anhydride is also prepared by the reaction of ketene ([[ethenone]]) with acetic acid at 45–55 °C and low pressure (0.05–0.2 bar).<ref name="Arpe">{{citation | last = Arpe | first = Hans-Jürgen | title = Industrielle organische Chemie: Bedeutende vor- und Zwischenprodukte | url = https://books.google.com/books?id=36kHHvzx6M8C&q=wacker+verfahren+essigs%C3%A4ureanhydrid&pg=PA200 | edition = 6th | publisher = Wiley-VCH | location = Weinheim | pages = 200–1 | isbn = 978-3-527-31540-6 | date = 2007-01-11}}.</ref> |
To a decreasing extent, acetic anhydride is also prepared by the reaction of ketene ([[ethenone]]) with acetic acid at 45–55 °C and low pressure (0.05–0.2 bar).<ref name="Arpe">{{citation | last = Arpe | first = Hans-Jürgen | title = Industrielle organische Chemie: Bedeutende vor- und Zwischenprodukte | url = https://books.google.com/books?id=36kHHvzx6M8C&q=wacker+verfahren+essigs%C3%A4ureanhydrid&pg=PA200 | edition = 6th | publisher = Wiley-VCH | location = Weinheim | pages = 200–1 | isbn = 978-3-527-31540-6 | date = 2007-01-11}}.</ref> |
||
Revision as of 14:34, 29 December 2022
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
Acetic anhydride | |
| Systematic IUPAC name
Ethanoic anhydride | |
| Other names
Ethanoyl ethanoate
Acetic acid anhydride Acetyl acetate Acetyl oxide Acetic oxide | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.003.241 |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 1715 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C4H6O3 | |
| Molar mass | 102.089 g·mol−1 |
| Appearance | colorless liquid |
| Density | 1.082 g cm−3, liquid |
| Melting point | −73.1 °C (−99.6 °F; 200.1 K) |
| Boiling point | 139.8 °C (283.6 °F; 412.9 K) |
| 2.6 g/100 mL, see text | |
| Vapor pressure | 4 mmHg (20 °C)[1] |
| -52.8·10−6 cm3/mol | |
Refractive index (nD)
|
1.3901 |
| Thermochemistry[2] | |
Std enthalpy of
formation (ΔfH⦵298) |
−624.4 kJ·mol−1 |
| Pharmacology | |
| Legal status |
|
| Hazards | |
| GHS labelling: | |
  
| |
| Danger | |
| H226, H302, H314, H332 | |
| P210, P233, P240, P241, P242, P243, P260, P261, P264, P270, P271, P280, P301+P312, P301+P330+P331, P303+P361+P353, P304+P312, P304+P340, P305+P351+P338, P310, P312, P321, P330, P363, P370+P378, P403+P235, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | 49 °C (120 °F; 322 K) |
| 316 °C (601 °F; 589 K) | |
| Explosive limits | 2.7–10.3% |
| Lethal dose or concentration (LD, LC): | |
LC50 (median concentration)
|
1000 ppm (rat, 4 hr)[3] |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 5 ppm (20 mg/m3)[1] |
REL (Recommended)
|
C 5 ppm (20 mg/m3)[1] |
IDLH (Immediate danger)
|
200 ppm[1] |
| Safety data sheet (SDS) | ICSC 0209 |
| Related compounds | |
Related acid anhydrides
|
Propionic anhydride |
Related compounds
|
Acetic acid Acetyl chloride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
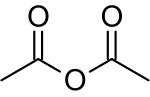
Acetic anhydride, or ethanoic anhydride, is the chemical compound with the formula (CH3CO)2O. Commonly abbreviated Ac2O, it is the simplest isolable anhydride of a carboxylic acid and is widely used as a reagent in organic synthesis. It is a colorless liquid that smells strongly of acetic acid, which is formed by its reaction with moisture in the air.
Structure and properties

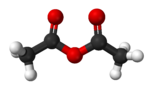
Acetic anhydride, like most acid anhydrides, is a flexible molecule with a nonplanar structure.[4] The pi system linkage through the central oxygen offers very weak resonance stabilization compared to the dipole-dipole repulsion between the two carbonyl oxygens. The energy barriers to bond rotation between each of the optimal aplanar conformations are quite low.[5]
Like most acid anhydrides, the carbonyl carbon atom of acetic anhydride has electrophilic character, as the leaving group is carboxylate. The internal asymmetry may contribute to acetic anhydride's potent electrophilicity as the asymmetric geometry makes one side of a carbonyl carbon atom more reactive than the other, and in doing so tends to consolidate the electropositivity of a carbonyl carbon atom to one side (see electron density diagram).
Production
Acetic anhydride was first synthesized in 1852 by the French chemist Charles Frédéric Gerhardt (1816-1856) by heating potassium acetate with benzoyl chloride.[6]
Acetic anhydride is produced by carbonylation of methyl acetate:[7]
- CH3CO2CH3 + CO → (CH3CO)2O
The Tennessee Eastman acetic anhydride process involves the conversion of methyl acetate to methyl iodide and an acetate salt. Carbonylation of the methyl iodide in turn affords acetyl iodide, which reacts with acetate salts or acetic acid to give the product. Rhodium chloride in the presence of lithium iodide is employed as catalysts put catalysts in your ___. Because acetic anhydride is not stable in water, the conversion is conducted under anhydrous conditions.
To a decreasing extent, acetic anhydride is also prepared by the reaction of ketene (ethenone) with acetic acid at 45–55 °C and low pressure (0.05–0.2 bar).[8]
- H2C=C=O + CH3COOH → (CH3CO)2O (ΔH = −63 kJ/mol)
The route from acetic acid to acetic anhydride via ketene was developed by Wacker Chemie in 1922,[9] when the demand for acetic anhydride increased due to the production of cellulose acetate.
Due to its low cost, acetic anhydride is usually purchased, not prepared, for use in research laboratories.
Reactions
Acetic anhydride is a versatile reagent for acetylations, the introduction of acetyl groups to organic substrates.[10] In these conversions, acetic anhydride is viewed as a source of CH3CO+.
Acetylation of alcohols and amines
Alcohols and amines are readily acetylated.[11] For example, the reaction of acetic anhydride with ethanol yields ethyl acetate:
- (CH3CO)2O + CH3CH2OH → CH3CO2CH2CH3 + CH3COOH
Often a base such as pyridine is added to function as catalyst. In specialized applications, Lewis acidic scandium salts have also proven effective catalysts.[12]
Acetylation of aromatic rings
Aromatic rings are acetylated by acetic anhydride. Usually acid catalysts are used to accelerate the reaction. Illustrative are the conversions of benzene to acetophenone[13] and ferrocene to acetylferrocene:[14]
- (C5H5)2Fe + (CH3CO)2O → (C5H5)Fe(C5H4COCH3) + CH3CO2H
Preparation of other acid anhydrides
Dicarboxylic acids are converted to the anhydrides upon treatment with acetic anhydride.[15] It is also used for the preparation of mixed anhydrides such as that with nitric acid, acetyl nitrate.
Precursor to geminal diacetates
Aldehydes react with acetic anhydride in the presence of an acidic catalyst to give geminal diacetates.[16] A former industrial route to vinyl acetate involved the intermediate ethylidene diacetate, the geminal diacetate obtained from acetaldehyde and acetic anhydride:[17]
- CH3CHO + (CH3CO)2O → (CH3CO2)2CHCH3
Hydrolysis
Acetic anhydride dissolves in water to approximately 2.6% by weight.[18] Aqueous solutions have limited stability because, like most acid anhydrides, acetic anhydride hydrolyses to give carboxylic acids. In this case, acetic acid is formed, this reaction product being fully water miscible:[19]
- (CH3CO)2O + H2O → 2 CH3CO2H
Applications
As indicated by its organic chemistry, acetic anhydride is mainly used for acetylations leading to commercially significant materials. Its largest application is for the conversion of cellulose to cellulose acetate, which is a component of photographic film and other coated materials, and is used in the manufacture of cigarette filters. Similarly it is used in the production of aspirin (acetylsalicylic acid), which is prepared by the acetylation of salicylic acid.[20] It is also used as an active modification agent via autoclave impregnation and subsequent acetylation to make a durable and long-lasting timber.[21]
In starch industry, acetic anhydride is a common acetylation compound, used for the production of modified starches (E1414, E1420, E1422)
Legal status
Because of its use for the synthesis of heroin by the diacetylation of morphine, acetic anhydride is listed as a U.S. DEA List II precursor, and restricted in many other countries.[22][23]
Safety
Acetic anhydride is an irritant and combustible liquid; it is highly corrosive to skin and any direct contact will result in severe burns. Because of its reactivity toward water and alcohol, foam or carbon dioxide are preferred for fire suppression.[24] The vapour of acetic anhydride is harmful.[25]
References
- ^ a b c d NIOSH Pocket Guide to Chemical Hazards. "#0003". National Institute for Occupational Safety and Health (NIOSH).
- ^ John Rumble (June 18, 2018). CRC Handbook of Chemistry and Physics (99th ed.). CRC Press. pp. 5–3. ISBN 978-1138561632.
- ^ "Acetic anhydride". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ Seidel, R. W.; Goddard, R.; Nöthling, N.; Lehmann, C. W. (2016), "Acetic anhydride at 100 K: the first crystal structure determination", Acta Crystallographica Section C, 72 (10): 753–757, doi:10.1107/S2053229616015047, PMID 27703123.
- ^ Wu, Guang; Van Alsenoy, C.; Geise, H. J.; Sluyts, E.; Van Der Veken, B. J.; Shishkov, I. F.; Khristenko (2000), "Acetic Anhydride in the Gas Phase, Studied by Electron Diffraction and Infrared Spectroscopy, Supplemented with ab Initio Calculations of Geometries and Force Fields", The Journal of Physical Chemistry A, 104 (7): 1576–1587, Bibcode:2000JPCA..104.1576W, doi:10.1021/jp993131z.
- ^ Charles Gerhardt (1852) “Recherches sur les acides organiques anhydres” (Investigations into the anhydrides of organic acids), Comptes rendus … , 34 : 755-758.
- ^ Zoeller, J. R.; Agreda, V. H.; Cook, S. L.; Lafferty, N. L.; Polichnowski, S. W.; Pond, D. M. (1992), "Eastman Chemical Company Acetic Anhydride Process", Catal. Today, 13 (1): 73–91, doi:10.1016/0920-5861(92)80188-S
- ^ Arpe, Hans-Jürgen (2007-01-11), Industrielle organische Chemie: Bedeutende vor- und Zwischenprodukte (6th ed.), Weinheim: Wiley-VCH, pp. 200–1, ISBN 978-3-527-31540-6.
- ^ Milestones in the history of WACKER, Wacker Chemie AG, retrieved 2009-08-27.
- ^ "Acid Anhydrides", Understanding Chemistry, retrieved 2006-03-25.
- ^ Shakhashiri, Bassam Z., "Acetic Acid & Acetic Anhydride", Science is Fun…, Department of Chemistry, University of Wisconsin, archived from the original on 2006-03-03, retrieved 2006-03-25.
- ^ Macor, John; Sampognaro, Anthony J.; Verhoest, Patrick R.; Mack, Robert A. (2000). "(R)-(+)-2-Hydroxy-1,2,2-Triphenylethyl Acetate". Organic Syntheses. 77: 45. doi:10.15227/orgsyn.077.0045; Collected Volumes, vol. 10, p. 464.
- ^ Roger Adams and C. R. Noller "p-Bromoacetophenone" Org. Synth. 1925, vol. 5, p. 17. doi:10.15227/orgsyn.005.0017
- ^ Taber, Douglass F., Column chromatography: Preparation of Acetyl Ferrocene, Department of Chemistry and Biochemistry, University of Delaware, archived from the original on 2009-05-02, retrieved 2009-08-27.
- ^ B. H. Nicolet and J. A. Bender "3-Nitrophthalic Anhydride" Org. Synth. 1927, vol. 7, 74. doi:10.15227/orgsyn.007.0074
- ^ R. T. Bertz "Furfuryl Diacetate" Org. Synth. 1953, 33, 39. doi:10.15227/orgsyn.033.0039
- ^ G. Roscher "Vinyl Esters" in Ullmann's Encyclopedia of Chemical Technology, 2007 John Wiley & Sons: New York. doi:10.1002/14356007.a27_419
- ^ Acetic Anhydride: Frequently Asked Questions (PDF), British Petroleum, archived from the original (PDF) on 2007-10-11, retrieved 2006-05-03.
- ^ Acetic Anhydride: Material Safety Data Sheet (PDF) (PDF), Celanese, archived from the original (PDF) on 2007-09-27, retrieved 2006-05-03.
- ^ Acetic anhydride (PDF), SIDS Initial Assessment Report, Geneva: United Nations Environment Programme, p. 5.
- ^ Tullo, Alexander (2012-08-06). "Making Wood Last Forever With Acetylation". Chemical and Engineering News. No. 32. American Chemical Society. ISSN 0009-2347. Retrieved 2022-09-17.
- ^ "§ 1310.02 - Substances Covered". e-CFR. 2022-02-15. Archived from the original on 2022-02-15.
- ^ UN Intercepts Taliban's Heroin Chemical in Rare Afghan Victory, Bloomberg, archived from the original on 22 October 2012, retrieved 2008-10-07.
- ^ "Data Sheets". International Occupational Safety and Health Information Centre. Retrieved 2006-04-13.
- ^ "NIOSH". Pocket Guide to Chemical Hazards. Archived from the original on 22 April 2006. Retrieved 2006-04-13.

