Solid solution: Difference between revisions
m +vi |
m examples |
||
| Line 12: | Line 12: | ||
When a solid solution becomes unstable due to a lower temperature for example, exsolution occurs and the two phases separate into distinct microscopic to megascopic lamella. An example of this is the alkali [[feldspar]] mineral variety [[perthite]] in which thin white [[albite]] layers alternate between typically pink [[microcline]]. |
When a solid solution becomes unstable due to a lower temperature for example, exsolution occurs and the two phases separate into distinct microscopic to megascopic lamella. An example of this is the alkali [[feldspar]] mineral variety [[perthite]] in which thin white [[albite]] layers alternate between typically pink [[microcline]]. |
||
==Examples== |
|||
[http://www.uni-graz.at/IEC-7/PDF-files/Chen.pdf Chen, J., Xu Z-Q., Chen Z-Z., Li T-F. & Chen, F-Y., 2005. Pargasite and ilmenite exsolution texture in clinopyroxene from the Hujialing Garnet-Pyroxenite, Su-lu U.H.P. Terrane, chentral China: A geodynamic Implication.] |
|||
[http://www.mines.utah.edu/~wmep/59298/592PDF/rlm2.pdf Petersen, U. Introduction to Ore Microscopy II; Mineral Paragenesis.] |
|||
[[Category:Materials science]] |
[[Category:Materials science]] |
||
Revision as of 01:51, 22 December 2005
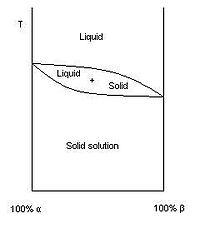
A solid solution is a solid-state solution of one or more solutes in a solvent. Such a mixture is considered a solution rather than a compound when the crystal structure of the solvent remains unchanged by addition of the solutes, and when the mixture remains in a single homogeneous phase. The solute may incorporate into the solvent crystal lattice substitutionally, by replacing a solvent particle in the lattice, or interstitially, by fitting into the space between solvent particles. Both of these types of solid solution affect the properties of the material by distorting the crystal lattice and disrupting the physical and electrical homogeneity of the solvent material.
Some mixtures will readily form solid solutions over a range of concentrations, while other mixtures will not form solid solutions at all. The propensity for any two substances to form a solid solution is a complicated matter involving the chemical, crystallographic, and quantum properties of the substances in question. The phase diagram at right displays an alloy of two metals which forms a solid solution at all relative concentrations of the two species. In this case, the pure phase of each element is of the same crystal structure, and the similar properties of the two elements allow for unbiased substitution through the full range of relative concentrations.
Solid solutions have important commercial and industrial applications, as such mixtures often have superior properties to pure materials. Many metal alloys are solid solutions. Even small amounts of solute can affect the electrical and physical properties of the solvent.
The binary phase diagram at right shows the phases of a mixture of two substances in varying concentrations, alpha and beta. The region labeled "alpha" is a solid solution, with beta acting as the solute in a matrix of alpha. On the other end of the concentration scale, the region labeled "beta" is also a solid solution, with alpha acting as the solute in a matrix of beta. The large solid region in between the alpha and beta solid solutions, labeled "solid alpha and beta", is not a solid solution. Instead, an examination of the microstructure of a mixture in this range would reveal two phases — solid solution alpha-in-beta and solid solution beta-in-alpha would form separate phases, perhaps lamella or grains.
When a solid solution becomes unstable due to a lower temperature for example, exsolution occurs and the two phases separate into distinct microscopic to megascopic lamella. An example of this is the alkali feldspar mineral variety perthite in which thin white albite layers alternate between typically pink microcline.
Examples
Chen, J., Xu Z-Q., Chen Z-Z., Li T-F. & Chen, F-Y., 2005. Pargasite and ilmenite exsolution texture in clinopyroxene from the Hujialing Garnet-Pyroxenite, Su-lu U.H.P. Terrane, chentral China: A geodynamic Implication. Petersen, U. Introduction to Ore Microscopy II; Mineral Paragenesis.
