Mössbauer spectroscopy: Difference between revisions
fixed misspelling of techniques |
|||
| Line 177: | Line 177: | ||
==Applications of Mössbauer spectroscopy== |
==Applications of Mössbauer spectroscopy== |
||
Mössbauer spectroscopy has been applied in a wide variety of scientific endeavors. As |
Mössbauer spectroscopy has been applied in a wide variety of scientific endeavors. As all other techniques it has both disadvantages and advantages. The drawbacks of the technique are; the limited number of gamma ray sources, and it is only applicable to solid samples, in order to eliminate the recoil of the nucleus. Besides, Mössbauer spectroscopy is very unique in its response as a [[chemical shift]], [[quadrupole splitting]] and [[hyperfine splitting]] to very small changes in the chemical environment of nucleus that includes oxidation state changes, the effect of different ligands on a particular atom, the magnetic environment of the sample, etc. |
||
Mössbauer spectroscopy is used as a useful analytical tool with detection limit as high as particle per 10<sup>11</sup>. Moreover, it has been especially useful in the field of geology for identifying the composition of iron-containing minerals. In particular it has been used to study a number of meteors and moon rocks and has even been used by NASA on Mars.<ref> |
Mössbauer spectroscopy is used as a useful analytical tool with detection limit as high as particle per 10<sup>11</sup>. Moreover, it has been especially useful in the field of geology for identifying the composition of iron-containing minerals. In particular it has been used to study a number of meteors and moon rocks and has even been used by NASA on Mars.<ref> |
||
Revision as of 19:31, 3 May 2010

Mössbauer spectroscopy (Template:Lang-de) is a spectroscopic technique based on the resonant emission and absorption of gamma rays in solids. This resonant emission and absorption was first observed by Rudolf Mössbauer during his graduate studies in 1957, and is called the Mössbauer effect in his honor. Mössbauer spectroscopy is similar to NMR spectroscopy in that it probes nuclear transitions and is thus sensitive to similar electron–nucleus interactions that cause the NMR chemical shift. Moreover, Mössbauer spectroscopy is one of the most sensitive techniques in terms of energy (and hence frequency) resolution, having the capability of detecting changes of just a few parts per 1011 due to the high energy and extremely narrow line widths of gamma rays.
Typical method
In its most common form, Mössbauer absorption spectroscopy, a solid sample is exposed to a beam of gamma radiation, and a detector measures the intensity of the beam transmitted through the sample. The atoms in the source emitting the gamma rays must be of the same isotope as the atoms in the sample absorbing them. In accordance with the Mössbauer effect, a significant fraction (given by the Lamb–Mössbauer factor) of the emitted gamma rays will not lose energy to recoil and thus will have approximately the right energy to be absorbed by the target atoms, the only differences being attributable to the chemical environment of the target, which is what we wish to observe. The gamma-ray energy of the source is varied through the Doppler effect by accelerating it through a range of velocities with a linear motor. A typical range of velocities for 57Fe may be ±11 mm/s (1 mm/s = 48.075 neV).
In the resulting spectra, gamma ray intensity is plotted as a function of the source velocity. At velocities corresponding to the resonant energy levels of the sample, some of the gamma rays are absorbed, resulting in a drop in the measured intensity and a corresponding dip in the spectrum. The number, positions, and intensities of the dips (also called peaks) provide information about the chemical environment of the absorbing nuclei and can be used to characterize the sample.
A major limitation of Mössbauer spectroscopy is finding a suitable gamma-ray source. Usually, this consists of a radioactive parent that decays to the desired isotope. For example, the source for 57Fe consists of 57Co, which undergoes beta decay to an excited state of 57Fe and subsequently decays to the ground state, emitting the desired gamma-ray. Ideally the parent will have a sufficiently long half-life to be usable, but will also have a sufficient decay rate to supply the needed intensity of radiation. Also, the gamma-ray energy should be relatively low, otherwise the system will have a low recoil-free fraction (see Mössbauer effect) resulting in a poor signal-to-noise ratio and requiring long collection times. The periodic table below indicates those elements having an isotope suitable for Mössbauer spectroscopy. Of these, 57Fe is by far the most common element studied using the technique, although 129I, 119Sn, and 121Sb are also frequently studied.
| H | He | |||||||||||||||||||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | |||||||||||||||||||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | |||||||||||||||||||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | |||||||||||||||||
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | |||||||||||||||||
| Cs | Ba | La | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn | |||||||||||||||||
| Fr | Ra | Ac | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Uut | Uuq | Uup | Uuh | Uus | Uuo | |||||||||||||||||
| Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | |||||||||||||||||||||
| Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | |||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||
Analysis of Mössbauer spectra
As described above, Mössbauer spectroscopy has an extremely fine energy resolution and can detect even subtle changes in the nuclear environment of the relevant atoms. Typically, there are three types of nuclear interactions that are observed, isomer shift (or chemical shift), quadrupole splitting and hyperfine splitting (or Zeeman splitting):
Isomer shift arises due to the non-zero probability of existence of electron charge density on nucleus. Electrons in s orbitals are the only one that has non-zero probability. However, the p, d, and other electrons may influence the s electron density by the screening effect. The s electron
density can also be affected by the oxidation state of and the chemical environment of the atom. These changes in the s electron density shift the whole spectrum positively or negatively.
Isomer shift (chemical shift, C.S.) can be expressed as the formula below, where K is a nuclear constant, the difference between Re2 and Rg2 is the effective nuclear charge difference between excited state and the ground state, and the difference between [Ψs2(0)]a and [Ψs2(0)]b is the electron density difference on the nucleus.
C.S. = K (Re2-Rg2) {[Ψs2(0)]a - [Ψs2(0)]b}

The physical meaning of this equation can be clarified with some examples. An increase in s electron density in 57Fe spectrum gives a negative shift, because the change in the effective nuclear charge is negative. On the other hand, an increase in s electron density gives positive shift in 119Sn because of the opposite reason. Another example is the oxidation state effect on Fe2+ and Fe3+ ions, the isomer shift of Fe3+ is more than Fe2+ because of the less screening effect of d electrons on s electron density[1].
The Quadrupole Splitting reflects the interaction between the nuclear energy levels and surrounding electric field gradient (EFG). The nuclear energy levels (I>1/2) split into mI sublevels in the presence of non-cubic electron or ligand distribution, that is due to the removal of the degeneracy in these sub energy levels. Therefore, the transition from the ground state to exited state in nuclear energy levels split. The quadrupole splitting is measured as the separation between these two peaks and reflects the character of the electric field at the nucleus.
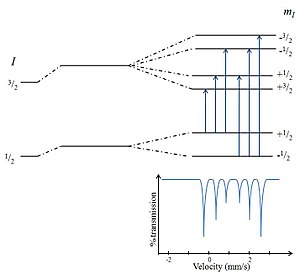
Magnetic Splitting (hyperfine splitting) is a result of the interaction between the nucleus and surrounding magnetic field. A nucleus with I spin splits into 2I + 1 sub-energy levels in the presence of magnetic field. For example, the nucleus spin state I= 3/2 splits into 4 non-degenerate sub-states with mI values of +3/2, +1/2, -1/2 and -3/2. These splitting are in the order of 10-7eV. The restriction rule of magnetic dipoles prevents to have the all transitions from ground states to excited states. A transition is allowed only when Δm = 0, ±1. Therefore, generally 6 peaks can be monitored in a spectrum of hyperfine splitting nucleus.
The three Mössbauer parameters: isomer shift, quadrupole splitting, and hyperfine splitting can often be used to identify a particular compound. A large database including most of the published Mössbauer parameters available in the literature is maintained by the Mössbauer Effect Data Center.[2] In some cases, a compound may have more than one type of site which the relevant atoms occupy. In such cases, because each site has a unique environment it will have its own set of peaks. For example, hematite (Fe2O3) contains two unique sites for the iron atoms and the corresponding spectrum has twelve peaks, six corresponding to each type of site. Thus, hematite also has two sets of Mössbauer parameters, one for each site.
In addition to identification, the relative intensities of the various peaks reflect the relative concentrations of compounds in the sample and can be used for semi-quantitative analysis. Also, since ferromagnetic phenomena are size-dependent, in some cases spectra can provide insight into the crystallite size and grain structure of a material.
Applications of Mössbauer spectroscopy
Mössbauer spectroscopy has been applied in a wide variety of scientific endeavors. As all other techniques it has both disadvantages and advantages. The drawbacks of the technique are; the limited number of gamma ray sources, and it is only applicable to solid samples, in order to eliminate the recoil of the nucleus. Besides, Mössbauer spectroscopy is very unique in its response as a chemical shift, quadrupole splitting and hyperfine splitting to very small changes in the chemical environment of nucleus that includes oxidation state changes, the effect of different ligands on a particular atom, the magnetic environment of the sample, etc.
Mössbauer spectroscopy is used as a useful analytical tool with detection limit as high as particle per 1011. Moreover, it has been especially useful in the field of geology for identifying the composition of iron-containing minerals. In particular it has been used to study a number of meteors and moon rocks and has even been used by NASA on Mars.[3]
A significant application of Mössbauer spectroscopy is the study of the phase transformations that occur in iron catalysts during Fischer–Tropsch synthesis. While these catalysts initially consist of hematite (Fe2O3), during reaction they are transformed into a mixture of magnetite (Fe3O4) and several iron carbides. The formation of carbides appears to improve catalytic activity, however it can also lead to the mechanical break-up and attrition of the catalyst particles, causing difficulties in the separation of the catalyst and the desired reaction products.[4]
Another application is the determination of the relative concentration change of the antimony (Sb) oxidation state (3+ and 5+) during the selective oxidation of olefins. The calcination of the catalysts, which is antimony-containing tin dioxide, takes all Sb into 5+ oxidation state and after catalytic reaction almost all Sb goes from 5+ oxidation state to 3+ oxidation state. A significant chemical environment change arises for the antimony nucleus during the oxidation state change which can easily be monitored as an isomer shift in the mössbauer spectrum.
This technique has also been used to observe the second-order transverse Doppler effect predicted by the theory of relativity, because of very high energy resolution.[5]
Mössbauer spectroscopy has been instrumental in developing an understanding of the structure and function of iron containing enzymes and the model complexes synthesized to mimic the functions of these enzymes. Examples of these iron containing enzymes are ribonucleotide reductase,[6][7] methane monooxygenase,[8][9] tryptophan dioxygenase,[10] deoxypusine hydroxylase,[11] protocatechuate 2,3 dioxygenase,[12] and cytochrome ba3.[13]
Mossbauer spectroscopy has also been used very successfully to investigate the electronic structure of heterobimetallic complexes.[14][15][16][17]
Mössbauer spectrometers

A Mössbauer spectrometer is a device that performs Mössbauer spectroscopy, or a device that uses the Mössbauer effect to determine the chemical environment of Mössbauer nuclei present in the sample. It is formed by three main parts; asource that moves back and forth to generate doppler effect, a collimator that filters the unparallel gamma rays and a detector.
A miniature Mössbauer Spectrometer, named (MB) MIMOS II, was used by the two rovers in NASA's Mars Exploration Rover missions.[18]
Notes on 57Fe Mössbauer spectroscopy
The Mössbauer parameters—chemical isomer shift and quadrupole splitting—are generally evaluated with respect to a reference material. For example, in iron compounds, the Mössbauer parameters were evaluated using iron foil (thickness less than 40 micrometers). The centroid of the six lines spectrum from metallic iron foil is −0.1 mm/s (for Co/Rh source). All shifts in other iron compounds are computed relative to this −0.10 mm/s (at room temperature), i.e., in this case isomer shifts are relative to Co/Rh source. In other words, the centre point of the Mössbauer spectrum is zero. The shift values may also be reported relative to 0.0 mm/s, here shifts are relative to the iron foil. Calculation of outerline distance from six line iron spectrum:
where c is the velocity of light in m/s, Hint is the internal magnetic field of the metallic iron (33 T), μN is the nuclear magneton (3.1524512326×10−8 eV/T), Eγ is the excitation energy (14.412497 keV), gn is the ground state nuclear splitting factor (0.09062/I, where I = 1⁄2) and g*
n is the excited state splitting factor of 57Fe (0.1549/I, where I = 3⁄2).
By substituting the above values one would get V = 10.62 mm/s. Other values are sometimes used as Laboratories use different qualities of iron foils. In any case that changing this V only affects the quadrupole splitting and not the isomer shift. IBAME, the authority for Mössbauer spectroscopy, does not mention any accurate value. Therefore a value between 10.60 mm/s to 10.67 mm/s is usually taken.
References
- ^ L. R. Walker, G. K. Wertheim, and V. Jaccarino, Phys. Rev. Lett., 6, 98, (1961)
- ^ Mössbauer Effect Data Center
- ^ G. Klingelhöfer (2004). "Mössbauer in situ studies of the surface of Mars". Hyperfine Interactions. 158: 117–124. doi:10.1007/s10751-005-9019-1.
- ^
A. Sarkar; et al. (2007). "Fischer–Tropsch Synthesis: Characterization Rb Promoted Iron Catalyst". Catalysis Letters. 121: 1–11. doi:10.1007/s10562-007-9288-1.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Y.-L. Chen, D.-P. Yang (2007). "Recoilless Fraction and Second-Order Doppler Effect". Mössbauer Effect in Lattice Dynamics. John Wiley & Sons. doi:10.1002/9783527611423.ch5. ISBN 9783527611423.
- ^
J.B. Lynch; et al. (1989). "Mössbauer and EPR studies of the binuclear iron center in ribonucleotide reductase from Escherichia coli. A new iron-to-protein stoichiometry". Journal of Biological Chemistry. 264: 8091–8096.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^
T.E. Elgren; et al. (1990). "Electron Transfer Associated with Oxygen Activation in the B2 Protein of Ribonucleotide Reductase from E. Coli". Journal of Biological Chemistry. 266: 19265–19268.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^
B.G. Fox; et al. (1988). "Evidence for a mu-oxo bridged binuclear iron center in the hydroxylase component of methane monooxygenase. Mossbauer and EPR studies". Journal of Biological Chemistry. 263: 10553–10556.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^
B.G. Fox; et al. (1993). "Mössbauer, EPR, and ENDOR Studies of the Hydroxylase and Reductase Components of Methane Monooxygenase from Methylosinus Trichosporium OB3B". Journal of the American Chemical Society. 115: 3688–3701.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^
R. Gupta; et al. (2010). "EPR and Mossbauer spectroscopy show inequivalent hemes in tryptophan dioxygenase". Journal of the American Chemical Society. 132 (3): 1089–1109.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^
V.V. Vu; et al. (2009). "Human deoxypusine hydroxylase, an enzyme involved in cell growth,activates O2 with a nonheme diiron center". Proceedings of the National Academy of Sciences. 106 (35): 14814–14819.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^
S.A. Volgel; et al. (1993). "Purification and Characterization of Protocatechuate 2,3 Dioxygenase from Bacillus macerans: a New Extradiol Catecholic Dioxygenase". Journal of Bacteriology. 175: 4414–4426.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^
B.H. Zimmermann; et al. (1988). "Properties of a Copper-Containing Cytochrome ba3: A Second Terminal Oxidase from the Extreme Thermophile Thermus thermophilus". Proceedings of the National Academy of Sciences. 85: 5779–7783.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^
C. Juarez-Garcia; et al. (1991). "Combined Moessbauer and EPR studies of the S = 3 state of an exchange-coupled iron(III)-copper(II) complex: test for quantitative EPR analysis of integer spin systems". Journal of the American Chemical Society. 113 (2): 518–525.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^
T.R. Holman; et al. (1990). "Models for iron-oxo proteins. Moessbauer and EPR study of an antiferromagnetically coupled iron(III)-nickel(III) complex". Journal of the American Chemical Society. 112 (21): 7611–7618.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^
S. Menage; et al. (1990). "Models for Iron-Oxo Proteins: Dioxygen Binding to a Diferrous Complex". Journal of the American Chemical Society. 112: 6423–6425.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^
J. Kaizer; et al. (2004). "Stable Nonheme FeIVO Complexes That Can Oxidize C-H Bonds of Cyclohexane at Room Temperature". Journal of the American Chemical Society. 126: 472–473.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^
G. Klingelhöfer; et al. (2002). "The miniaturized Mossbauer spectrometer MIMOS II for extraterrestrial and outdoor terrestrial applications: A status report". Hyperfine Interactions. 144: 371–379. doi:10.1023/A:1025444209059.
{{cite journal}}: Explicit use of et al. in:|author=(help)

