Snake venom: Difference between revisions
m Reverted 1 edit by 97.77.152.70 (talk) identified as vandalism to last revision by RE73. (TW) |
|||
| Line 158: | Line 158: | ||
===Vipers=== |
===Vipers=== |
||
The [[Viperidae|viper]]s, which furnish examples of the most highly developed venom delivery apparatus, although inferior to some in its [[toxic]] effects, the venom gland is very large and in intimate relation with the [[masseter]] or [[temporal muscle]], consisting of two bands, the superior arising from behind the eye, the inferior extending from the gland to the mandible. A groove or duct can be located traveling from the modified salivary glands where venom is produced down the length of the fang and out to the tip. In some species, notably the vipers and cobras, this groove is completely closed over. In other species, such as the adders and mambas, this groove is not covered, or only covered partially. From the anterior extremity of the gland the duct passes below the eye and above the [[maxillary bone]], where it makes a bend, to the basal orifice of the venom fang, which is ensheathed in a thick fold of [[mucous membrane]], the vagina dentis. By means of the movable maxillary bone hinged to the prefrontal, and connected with the tranverse bone which is pushed forward by muscles set in action by the opening of the mouth, the tubular fang is erected and the venom discharged through the distal orifice in which it terminates. When the snake bites, the jaws close up, causing the gland to be powerfully wrung, and the venom pressed out into the duct. |
|||
===Elapids=== |
===Elapids=== |
||
Revision as of 22:50, 16 February 2011
| Snake toxin | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||||
| Symbol | Toxin_1 | ||||||||||
| Pfam | PF00087 | ||||||||||
| InterPro | IPR003571 | ||||||||||
| PROSITE | PDOC00245 | ||||||||||
| SCOP2 | 2ctx / SCOPe / SUPFAM | ||||||||||
| OPM superfamily | 55 | ||||||||||
| OPM protein | 1txa | ||||||||||
| |||||||||||
Snake venom is highly modified saliva [1] that is produced by special glands of certain species of snakes. The gland which secretes the zootoxin is a modification of the parotid salivary gland of other vertebrates, and is usually situated on each side of the head below and behind the eye, invested in a muscular sheath. It is provided with a large alveoli in which the venom is stored before being conveyed by a duct to the base of the channelled or tubular fang through which it is ejected. Snake venom is a combination of many different proteins and enzymes. Many of these proteins are harmless to humans, but some are toxins.
Snake venoms are generally not dangerous when ingested, and are therefore not technically poisons.[2]
Chemistry
Snake venom consists of proteins, enzymes, substances with a cytotoxic effect, neurotoxins and coagulants.
- Phosphodiesterases are used to interfere with the prey's cardiac system, mainly to lower the blood pressure.
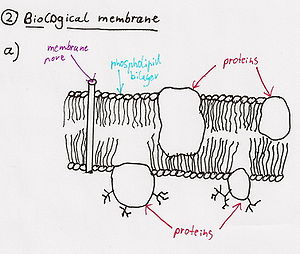
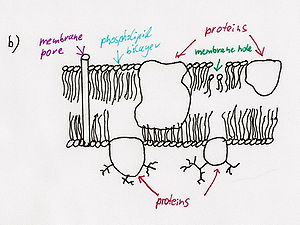
- Phospholipase A2 causes hemolysis by lysing the phospholipid cell membranes of red blood cells.[3]
- Snake venom inhibits cholinesterase to make the prey lose muscle control.
- Hyaluronidase increases tissue permeability to increase the rate that other enzymes are absorbed into the prey's tissues.
- Amino acid oxidases and proteases are used for digestion. Amino acid oxidase also triggers some other enzymes and is responsible for the yellow color of the venom of some species.
- Snake venom often contains ATPase, an enzyme which catalyzes the hydrolysis of ATP to ADP and a free phosphate ion, or to AMP and diphosphate.[4][5]
Snake toxins have a great variety in their function. The two major families are neurotoxins (those that attack the nervous system) and cytotoxins (those that attack cells).[6] They can be further subdivided as follows:
- Neurotoxins
- Fasciculins
- Dendrotoxins
- α-neurotoxins
- Cytotoxins
- Phospholipases
- Cardiotoxins
- Haemotoxins
Neurotoxins
The beginning of a new impulse:
A) An exchange of ions (charged atoms) across the nerve cell membrane sends a depolarising current towards the end of the nerve cell (cell terminus).
B) When the depolarising current arrives at the nerve cell terminus, the neurotransmitter acetylcholine (ACh), which is held in vesicles, is released into the space between the two nerves (synapse). It moves across the synapse to the postsynaptic receptors.
C) If ACh remains at the receptor, the nerve stays stimulated, causing incontrollable muscle contractions. This condition is called tetany. So an enzyme called acetylcholinesterase destroys the ACh so tetany does not occur.
1) Fasciculins:
These toxins attack cholinergic neurons (those that use ACh as a transmitter) by destroying acetylcholinesterase (AChE). ACh therefore cannot be broken down and stays in the receptor. This causes tetany, which can lead to death.
Snake example: Black Mamba
2) Dendrotoxins:
Dendrotoxins inhibit neurotransmissions by blocking the exchange of + and – ions across the neuronal membrane ==> no nerve impulse. So it paralyses the nerves.
Snake example: Mambas
3) α-neurotoxins:
α-neurotoxins also attack cholinergic neurons. They mimic the shape of the acetylcholine molecule and therefore fit into the receptors → they block the ACh flow → feeling of numbness and paralysis.
Snake examples:
- Kraits use erabutoxin (the Many-banded krait uses Bungarotoxin)
- Cobras use cobratoxin,
Cytotoxins
1) Phospholipases:
Phospholipase is an enzyme that transforms the phospholipid molecule into a lysophospholipid (soap) ==> the new molecule attracts and binds fat and rips a hole in the cell membrane. Consequently water flows into the cell and destroys the molecules in it. That is called necrosis.
Snake example: The Japanese Habu snakes (low toxicity)
2) Cardiotoxins:
Actually cardiotoxins are muscle venoms. They bind to particular sites on the surface of muscle cells causing depolarisation ==> the toxin prevents muscle contraction. For example the heart muscle: the heart will beat irregularly and stop beating, which will cause death.
Snake example: King Cobra and some other cobras
3) Haemotoxins:
The toxin destroys red blood cells (erythrocytes). This symptom is called haemolysis. As it is a very slowly progressing venom it would probably not kill a human - another toxin in the snake’s venom would most certainly have caused death by then.
Snake example: most Vipers and the members of Naja genus
Snake cytotoxin InterPro: IPR003572
List of snake venom toxins
- Piscivorin from the Eastern Cottonmouth
- Triflin from the Habu snake
- Ophanin from the King Cobra
- Latisemin from the Erabu snake
- Ablomin from the Mamushi snake
Evolution
The presence of enzymes in snake venom has led to the belief that it was an adaptation to assist in the digestion of prey, but, studies of the western diamondback rattlesnake, a snake with highly proteolytic venom, show that envenomation has no impact on the time food takes to pass through the gut. More research is needed to determine the selective pressures that have armed snakes in this way.[7]
Injection
Vipers
The vipers, which furnish examples of the most highly developed venom delivery apparatus, although inferior to some in its toxic effects, the venom gland is very large and in intimate relation with the masseter or temporal muscle, consisting of two bands, the superior arising from behind the eye, the inferior extending from the gland to the mandible. A groove or duct can be located traveling from the modified salivary glands where venom is produced down the length of the fang and out to the tip. In some species, notably the vipers and cobras, this groove is completely closed over. In other species, such as the adders and mambas, this groove is not covered, or only covered partially. From the anterior extremity of the gland the duct passes below the eye and above the maxillary bone, where it makes a bend, to the basal orifice of the venom fang, which is ensheathed in a thick fold of mucous membrane, the vagina dentis. By means of the movable maxillary bone hinged to the prefrontal, and connected with the tranverse bone which is pushed forward by muscles set in action by the opening of the mouth, the tubular fang is erected and the venom discharged through the distal orifice in which it terminates. When the snake bites, the jaws close up, causing the gland to be powerfully wrung, and the venom pressed out into the duct.
Elapids
In the proteroglyphous elapids, the fangs are tubular, but are short and do not possess the mobility seen in vipers.
Colubrids
In many opisthoglyphous colubrids, with grooved teeth situated at the posterior extremity of the maxilla, a small posterior portion of the upper labial or salivary gland is converted into a venom-secreting organ, distinguished by a light yellow colour, provided with a duct larger than any of those of the labial gland, and proceeding inward and downward to the base of the grooved fang; the duct is not in direct connection with the groove, but the two communicate through the mediation of the cavity enclosed by the folds of mucous membrane surrounding the tooth, and united in front.
Mechanics of biting

The reserve or successional teeth, which are always present just behind or on the side of the functional fang of all venomous snakes, are in no way connected with the duct until called upon to replace a fang that has been lost. It could not be otherwise, since the duct would require a new terminal portion for each new fang; and as the replacement takes place alternately from two parallel series, the new venom-conveying tooth does not occupy exactly the same position as its predecessor.
Two genera, Doliophis among the elapids and Causus among the viperids, are highly remarkable for having the venom gland and its duct of a great length, extending along each side of the body and terminating in front of the heart. Instead of the muscles of the temporal region serving to press out the venom into the duct, this action is performed by those of the side of the body.
When biting, a viperid snake merely strikes, discharging the venom the moment the fangs penetrate the skin, and then immediately lets it go. A proteroglyph or opisthoglyph, on the contrary, closes its jaws like a dog on the part bitten, often holding on firmly for a considerable time. The venom, which is mostly a clear, limpid fluid of a pale straw or amber colour, or rarely greenish, sometimes with a certain amount of suspended matter, is exhausted after several bites, and the glands have to recuperate.
Mechanics of spitting
Venom can be ejected otherwise than by a bite, as in the so-called spitting cobras of the genera Naja and Hemachatus. Some of these deadly snakes, when irritated, are capable of shooting venom from the mouth, at a distance of 4 to 8 feet. These snakes' fangs have been modified for the purposes of spitting: inside the fangs of a spitting cobra is a channel which makes a ninety degree bend to the lower front of the fang. When the snake is threatened the muscles of the venom gland squeeze the venom sac and as a result venom is projected forward. Spitters may spit thirty or forty times in succession, and even then the snake is still able to deliver a fatal bite.
Spitting is a defensive reaction only. The snake tends to aim for the eyes of a perceived threat; a direct hit can cause temporary shock and blindness through severe inflammation of the cornea and conjunctiva. While there are no serious results if the venom is washed away at once with plenty of water, the blindness caused by a successful spit can become permanent if left untreated. Contact with the skin is not in itself dangerous, but open wounds may become envenomated.
Some Effects
There are four distinct types of venom that act on the body differently.
- Proteolytic venom dismantles the molecular structure of the area surrounding and including the bite.
- Hemotoxic venoms act on the heart and cardiovascular system.
- Neurotoxic venom acts on the nervous system and brain.
- Cytotoxic venom has a localized action at the site of the bite.
It is noteworthy that the size of the venom fangs is in no relation to the virulence of the venom. The comparatively innocent Indo-Malay Lachesis alluded to above have enormous fangs, whilst the smallest fangs are found in the Hydrophids which possess very potent venom.
Proteroglyphous snakes
The effect of the venom of proteroglyphous snakes(Hydrophids, Bungarus, Dendroaspis, Elaps, Pseudechis, Notechis, Acanthophis) is mainly on the nervous system, respiratory paralysis being quickly produced by bringing the venom into contact with the central nervous mechanism which controls respiration; the pain and local swelling which follow a bite are not usually severe.
The bite of all the proteroglyphous elapids, even of the smallest and gentlest, such as the Elaps or coral snakes, is, so far as known, deadly to humans.
Vipers
Viper venom (Daboia, Echis, Lachesis, Crotalus) acts more on the vascular system, bringing about coagulation of the blood and clotting of the pulmonary arteries; its action on the nervous system is not great, no individual group of nerve-cells appears to be picked out, and the effect upon respiration is not so direct; the influence upon the circulation explains the great depression which is a symptom of viperine envenomation. The pain of the wound is severe, and is speedily followed by swelling and discoloration. The symptoms produced by the bite of the European vipers are thus described by the best authorities on snake venom (Martin and Lamb):
The bite is immediately followed by local pain of a burning character; the limb soon swells and becomes discoloured, and within one to three hours great prostration, accompanied by vomiting, and often diarrhoea, sets in. Cold, clammy perspiration is usual. The pulse becomes extremely feeble, and slight dyspnoea and restlessness may be seen. In severe cases, which occur mostly in children, the pulse may become imperceptible and the extremities cold; the patient may pass into coma. In from twelve to twenty-four hours these severe constitutional symptoms usually pass off; but in the meantime the swelling and discoloration have spread enormously. The limb becomes phlegmonous, and occasionally suppurates. Within a few days recovery usually occurs somewhat suddenly, but death may result from the severe depression or from the secondary effects of suppuration. That cases of death, in adults as well as in children, are not infrequent in some parts of the Continent is mentioned in the last chapter of this Introduction.
The Viperidae differ much among themselves in the toxicity of their venom. Some, such as the Indian Daboia russelli and Echis carinatus; the American vipers Crotalus, Lachesis muta and Bothrops lanceolatus; and the African Causus, Bitis, and Cerastes, cause fatal results unless a remedy is speedily applied. On the other hand, the Indian and Malay Lachesis seldom cause the death of humans, their bite in some instances being no worse than the sting of a hornet. The bite of the larger European vipers may be very dangerous, and followed by fatal results, especially in children, at least in the hotter parts of the Continent; whilst the small Vipera ursinii, which hardly ever bites unless roughly handled, does not seem to be possessed of a very virulent venom, and, although very common in some parts of Austria-Hungary, is not known to have ever caused a serious accident.
Opisthoglyphous colubrids
Biologists had long known that some snakes had rear fangs, 'inferior' venom injection mechanisms that might immobilize prey; although a few fatalities were on record, until 1957 the possibility that such snakes were deadly to humans seemed at most remote. The deaths of two prominent herpetologists from African colubrid bites changed that assessment, and recent events reveal that several other species of rear-fanged snakes have venoms that are potentially lethal to large vertebrates.
Boomslang and vine snake venom are toxic to blood cells and thin the blood (hemotoxic, hemorrhagic). Early symptoms include headaches, nausea, diarrhea, lethargy, mental disorientation, bruising and bleeding at the site and all body openings. Exsanguination is the main cause of death from such a bite.
The Groen Boomslang's venom is the most potent of all rear-fanged snakes in the world. Although it has venom more potent that many vipers and some elapids, it causes fewer fatalities. This is because the Groen Boomslang only secretes a small amount of venom when it bites and compared to the more aggressive Black Mamba it is much less aggressive.
Symptoms of a bite from these snakes are nausea and internal bleeding, and one could die from a brain hemorrhage and respiratory collapse.
Aglyphous snakes
Experiments made with the secretion of the parotid gland of Tropidonotus and Zamenis have shown that even aglyphous snakes are not entirely devoid of venom, and point to the conclusion that the physiological difference between so-called harmless and venomous snakes is only one of degree, just as there are various steps in the transformation of an ordinary parotid gland into a venom gland or of a solid tooth into a tubular or grooved fang.
Immunity
Among snakes
The question whether individual snakes are immune to their own venom is not yet definitely settled, though there is a known example of a cobra which self-envenomated, resulting in a large abscess requiring surgical intervention but showing none of the other effects that would have proven rapidly lethal in prey species or humans.[9] Furthermore, certain harmless species, such as the North American Coronella getula and the Brazilian Rhacidelus brazili, are proof against the venom of the crotalines which frequent the same districts, and which they are able to overpower and feed upon. The Tropical Rat Snake, Spilotes variabilis, is the enemy of the Fer-de-lance in St. Lucia, and it is said[who?] that in their encounters the Cribo is invariably the victor. Repeated experiments have shown the European Common Snake, Tropidonotus natrix, not to be affected by the bite of Vipera berus and Vipera aspis, this being due to the presence, in the blood of the harmless snake, of toxic principles secreted by the parotid and labial glands, and analogous to those of the venom of these vipers. Several North American species of Rat snakes as well as King snakes have proven to be immune or highly resistant to the venom of Rattle snake species.
Among other animals
The Hedgehog, the Mongoose, the Secretary Bird, the Honey Badger and a few other birds feeding on snakes, are known to be immune to an ordinary dose of snake venom; whether the pig may be considered so is still uncertain, although it is well known that, owing to its subcutaneous layer of fat, it is often bitten with impunity. The garden dormouse (Eliomys quercinus) has recently been added to the list of animals refractory to viper venom. Some populations of California Ground Squirrel are at least partially immune to Rattlesnake venom as adults.
Among Humans
The acquisition of human immunity against snake venom is one of the oldest forms of vaccinology known to date (about AD 60, Psylli Tribe). Since then many humans have attempted to inoculate themselves with snake venom in order to achieve immunity.[10] Charles Tanner and Herschel Flowers studied with dried snake venom and achieved strong immunity (1). Joel La Rocque self injected Eastern diamondback venom and developed a high IgG neutralizing antibody for several rattlesnake species. Harold Mierkey has done so for years. Tim Friede has studied twice with a self-directed vaccine experiment using pure venom and achieved very high IgG neutralizing antibodies with mamba and cobra venom (1). The present goal is to develop a DNA-based vaccine for the Old World using the genes that encode the venom with an electroporation device for DNA delivery (1). If successful, some of the over 100,000 people that die each year from snakebite in the Old World will be saved. (1,2) [11]
Studies
The subject of snake venoms is one which has always attracted much attention and which has made great progress within the last quarter of a century. Plants used to treat snakebites in Trinidad and Tobago are made into tinctures with alcohol or olive oil and kept in rum flasks called 'snake bottles'. Snakes bottles contain several different plants and/ or insects. The plants used include the vine called monkey ladder (Bauhinia cumanensis or Bauhinia excisa, Fabaceae) is pounded and put on the bite. Alternatively a tincture is made with a piece of the vine and kept in a snake bottle. Other plants used include: mat root (Aristolochia rugosa), cat's claw (Pithocellobium unguis-cati), tobacco (Nicotiana tabacum), snake bush (Barleria lupulina), obie seed (Cola nitida), and wild gri gri root (Acrocomia ierensis). Some snake bottles also contain the caterpillars (Battus polydamus, Papilionidae) that eat tree leaves (Aristolochia trilobata). Emergency snake medicines are obtained by chewing a three-inch piece of the root of bois canôt (Cecropia peltata) and administering this chewed-root solution to the bitten(usually hunting dogs). This is a common native plant of Latin America and the Caribbean which makes it appropriate as an emergency remedy. Another native plant used is mardi gras (Renealmia alpinia)(berries), which are crushed together with the juice of wild cane (Costus scaber) and given to the bitten. Quick fixes have included applying chewed tobacco from cigarettes, cigars or pipes as well. Making cuts around the puncture or sucking out the venom has also been helpful.
Serotherapy
Especially noteworthy is progress regarding the defensive reaction by which the blood may be rendered proof against their effect, by processes similar to vaccination—antipoisonous serotherapy.
The studies to which we allude have not only conduced to a method of treatment against snake-bites, but have thrown a new light on the great problem of immunity.
They have shown that the antitoxic sera do not act as chemical antidotes in destroying the venom, but as physiological antidotes; that, in addition to the venom glands, snakes possess other glands supplying their blood with substances antagonistic to the venom, such as also exist in various animals refractory to snake venom, the hedgehog and the mongoose for instance.
Regional venom specificity
Unfortunately, the specificity of the different snake venoms is such that, even when the physiological action appears identical, serum injections or graduated direct inoculations confer immunity towards one species or a few allied species only.
Thus, a European in Australia who had become immune to the poison of the deadly Australian Tiger Snake, Notechis scutatus, manipulating these snakes with impunity, and was under the impression that his immunity extended also to other species, when bitten by a Denisonia superba, an allied elapine, died the following day.
In India, the serum prepared with the venom of Naja tripudians has been found to be without effect on the venom of the two species of kraits of the genus Bungarus, and the Old World vipers Daboia russelli and Echis carinatus, and the pit viper Trimeresurus popeiorum. Daboia russelli serum is without effect on colubrine venoms, or those of Echis and Trimeresurus.
In Brazil, serum prepared with the venom of the New World pit viper Lachesis lanceolatus is without action on Crotalus venom.
Antivenom snakebite treatment must be matched as the type of envenomation that has occurred.
In the Americas, polyvalent antivenoms are available that are effective against the bites of most pit vipers.
These are not effective against coral snake envenomation, which requires a specific antivenom to their neurotoxic venom.
The situation is even more complex in countries like India, with its rich mix of vipers (family Viperidae) and highly neurotoxic cobras and kraits of the family Elapidae.
This article is based on the 1913 book The Snakes of Europe, by G. A. Boulenger, which is now in the public domain in the United States (and possibly elsewhere) because of its age. Because of its age, the text in this article should not necessarily be viewed as reflecting the current knowledge of snake venom.
See also
References
- ^ "Reptile Venom Research". Australian Reptile Park. Retrieved 21 December 2010.
- ^ "The Modern Myth or Are You A Man Or A Mouse?" (PDF). Bushwalking.org.au.
- ^ CONDREA E, DEVRIES A, MAGER J (1964). "HEMOLYSIS AND SPLITTING OF HUMAN ERYTHROCYTE PHOSPHOLIPIDS BY SNAKE VENOMS". Biochim. Biophys. Acta. 84: 60–73. PMID 14124757.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ "ENZYME entry 3.6.1.3".
- ^ "ENZYME entry 3.6.1.8".
- ^ Dufton MJ (1984). "Classification of elapid snake neurotoxins and cytotoxins according to chain length: evolutionary implications". J. Mol. Evol. 20 (2): 128–34. PMID 6433031.
- ^ M.D. McCue (2007). "Prey envenomation does not improve digestive performance in western diamondback rattlesnakes (Crotalus atrox)". J. Exp. Zool. A. 307a (online early): 568. doi:10.1002/jez.411. PMID 17671964.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ [1]
- ^ [2]. Accessed 2 April 2009.
- ^ (Bill Haast, Charles Tanner, Joel La Rocque, Harold Mierkey, Herschel Flowers, Ray Hunter, Tim Friede, Burma Toxoid Project, Habu Toxoid Project, Pakokku Snake Clan, Wanyamwesi Tribe, Dr. Eizenberger[2]).
- ^ http://dnavaccine.com/modules.php?name=News&file=article&sid=1413. Friede, Tim. Venomous Snake Vaccinology, 5th World Congress of Herpetology in Africa.
External links
- UMich Orientation of Proteins in Membranes families/superfamily-55 - Calculated orientations of snake venome toxins in the lipid bilayer
- UMich Orientation of Proteins in Membranes families/superfamily-90 - Calculated orientations of snake venom phospholipases A2 and myotoxins in the lipid bilayer
- LD50's for most toxic venoms.
- Australian Venom Research Unit - a general source of information for venomous creatures in Australasia
- biomedcentral.com - Medicinal and ethnoveterinary remedies of hunters in Trinidad
- reptilis.net - How venom works
- snakevenom.net - Drying and storage of snake venom
- Natural Toxins Research Center at Texas A&M University-Kingsville - For over three decades, our mission has been to provide global research, training & resources that will lead to the discovery of medically important toxins found in snake venoms. We also provide snake venoms, venom fractions and tissue for biomedical research.
- Jonassen I, Collins JF, Higgins DG (1995). "Finding flexible patterns in unaligned protein sequences". Protein Sci. 4 (8): 1587–95. doi:10.1002/pro.5560040817. PMC 2143188. PMID 8520485.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Shaw IC (2007). "Chapter 19: Snake Toxins". In Waring RH, Steventon GB, Mitchell SC (ed.). Molecules of Death (Second Edition ed.). River Edge, N.J: Imperial College Press. pp. 329–344. ISBN 1-86094-815-4.
{{cite book}}:|edition=has extra text (help)CS1 maint: multiple names: editors list (link)