Hispidin: Difference between revisions
Appearance
Content deleted Content added
Citation bot (talk | contribs) m [Cat543-dev]Add: display-authors, author pars. 10-10. You can use this bot yourself. Report bugs here. |
such as phellibaumins |
||
| Line 44: | Line 44: | ||
'''Hispidin''' is a natural substance. It can also be synthetised.<ref>{{Cite journal|pmid=9088624|doi=10.1023/A:1007321227010|year=1997|last1=Gonindard|first1=C.|last2=Bergonzi|first2=C.|last3=Denier|first3=C.|last4=Sergheraert|first4=C.|last5=Klaebe|first5=A.|last6=Chavant|first6=L.|last7=Hollande|first7=E.|journal=Cell Biology and Toxicology|volume=13|issue=3|pages=141–53|title=Synthetic hispidin, a PKC inhibitor, is more cytotoxic toward cancer cells than normal cells in vitro}}</ref> |
'''Hispidin''' is a natural substance. It can also be synthetised.<ref>{{Cite journal|pmid=9088624|doi=10.1023/A:1007321227010|year=1997|last1=Gonindard|first1=C.|last2=Bergonzi|first2=C.|last3=Denier|first3=C.|last4=Sergheraert|first4=C.|last5=Klaebe|first5=A.|last6=Chavant|first6=L.|last7=Hollande|first7=E.|journal=Cell Biology and Toxicology|volume=13|issue=3|pages=141–53|title=Synthetic hispidin, a PKC inhibitor, is more cytotoxic toward cancer cells than normal cells in vitro}}</ref> |
||
| ⚫ | Hispidin 4-O-β-d-glucopyranoside can be found in ''[[Pteris ensiformis]]''<ref>Identification of phenolic antioxidants from Sword Brake fern (Pteris ensiformis Burm.). Yung-Husan Chen, Fang-Rong Chang, Yih-Jer Lin, Lisu Wang, Jinn-Fen Chen, Yang-Chang Wu and Ming-Jiuan Wu, Food Chemistry, Volume 105, Issue 1, 2007, pp. 48-56, {{doi|10.1016/j.foodchem.2007.03.055}}</ref> whereas hispidin derivatives, such as [[phellibaumin]]s, can be found in the edible mushroom ''[[Inonotus xeranticus]]''<ref>Hispidin Derivatives from the Mushroom Inonotus xeranticus and Their Antioxidant Activity. In-Kyoung Lee, Soon-Ja Seok, Wan-Kyu Kim and Bong-Sik Yun, J. Nat. Prod., 2006, 69 (2), pp. 299–301, {{doi|10.1021/np050453n}}</ref> or ''[[Phellinus]]''.<ref>{{Cite journal|doi=10.1016/j.bmc.2007.03.039|title=Highly oxygenated and unsaturated metabolites providing a diversity of hispidin class antioxidants in the medicinal mushrooms Inonotus and Phellinus|year=2007|last1=Lee|first1=In-Kyoung|last2=Yun|first2=Bong-Sik|journal=Bioorganic & Medicinal Chemistry|volume=15|issue=10|pages=3309}}</ref><ref name="pmid18827365">{{cite journal |doi=10.1248/bpb.31.1968 |title=Protein glycation inhibitors from the fruiting body of ''Phellinus linteus'' |journal=Biological & Pharmaceutical Bulletin |volume=31 |issue=10 |pages=1968–72 |date=October 2008 |pmid=18827365 |url=http://joi.jlc.jst.go.jp/JST.JSTAGE/bpb/31.1968?from=PubMed |format={{dead link|date=May 2010}} |last1=Lee |first1=Yeon Sil |last2=Kang |first2=Young-Hee |last3=Jung |first3=Ju-Young |last4=Lee |first4=Sanghyun |last5=Ohuchi |first5=Kazuo |last6=Shin |first6=Kuk Hyun |last7=Kang |first7=Il-Jun |last8=Park |first8=Jung Han Yoon |last9=Shin |first9=Hyun-Kyung|last10=Lim |first10=Soon Sung |display-authors=8 }}</ref> |
||
Hispidin 4-O-β-d-glucopyranoside can be found in ''[[Pteris ensiformis]]''<ref>Identification of phenolic antioxidants from Sword Brake fern (Pteris ensiformis Burm.). Yung-Husan Chen, Fang-Rong Chang, Yih-Jer Lin, Lisu Wang, Jinn-Fen Chen, Yang-Chang Wu and Ming-Jiuan Wu, Food Chemistry |
|||
| ⚫ | Volume 105, Issue 1, 2007, pp. 48-56, {{doi|10.1016/j.foodchem.2007.03.055}}</ref> whereas hispidin derivatives can be found in the edible mushroom ''[[Inonotus xeranticus]]''<ref>Hispidin Derivatives from the Mushroom Inonotus xeranticus and Their Antioxidant Activity. In-Kyoung Lee, Soon-Ja Seok, Wan-Kyu Kim and Bong-Sik Yun, J. Nat. Prod., 2006, 69 (2), pp. 299–301, {{doi|10.1021/np050453n}}</ref> or ''[[Phellinus]]''.<ref>{{Cite journal|doi=10.1016/j.bmc.2007.03.039|title=Highly oxygenated and unsaturated metabolites providing a diversity of hispidin class antioxidants in the medicinal mushrooms Inonotus and Phellinus|year=2007|last1=Lee|first1=In-Kyoung|last2=Yun|first2=Bong-Sik|journal=Bioorganic & Medicinal Chemistry|volume=15|issue=10|pages=3309}}</ref><ref name="pmid18827365">{{cite journal |doi=10.1248/bpb.31.1968 |title=Protein glycation inhibitors from the fruiting body of ''Phellinus linteus'' |journal=Biological & Pharmaceutical Bulletin |volume=31 |issue=10 |pages=1968–72 |date=October 2008 |pmid=18827365 |url=http://joi.jlc.jst.go.jp/JST.JSTAGE/bpb/31.1968?from=PubMed |format={{dead link|date=May 2010}} |last1=Lee |first1=Yeon Sil |last2=Kang |first2=Young-Hee |last3=Jung |first3=Ju-Young |last4=Lee |first4=Sanghyun |last5=Ohuchi |first5=Kazuo |last6=Shin |first6=Kuk Hyun |last7=Kang |first7=Il-Jun |last8=Park |first8=Jung Han Yoon |last9=Shin |first9=Hyun-Kyung|last10=Lim |first10=Soon Sung |display-authors=8 }}</ref> |
||
== See also == |
== See also == |
||
*[[Davallialactone]] |
* [[Davallialactone]] |
||
*[[Phellibaumin]] |
* [[Phellibaumin]] |
||
== References == |
== References == |
||
Revision as of 14:30, 18 June 2014
| |
| Names | |
|---|---|
| IUPAC name
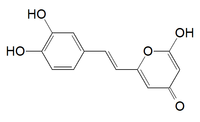
2-[(E)-2-(3,4-dihydroxyphenyl)ethenyl]-6-hydroxypyran-4-one
| |
| Other names
6-(3,4-dihydroxystyrl)-4-hydroxy-2-pyrone
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Molar mass | 246.21 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Hispidin is a natural substance. It can also be synthetised.[1]
Hispidin 4-O-β-d-glucopyranoside can be found in Pteris ensiformis[2] whereas hispidin derivatives, such as phellibaumins, can be found in the edible mushroom Inonotus xeranticus[3] or Phellinus.[4][5]
See also
References
- ^ Gonindard, C.; Bergonzi, C.; Denier, C.; Sergheraert, C.; Klaebe, A.; Chavant, L.; Hollande, E. (1997). "Synthetic hispidin, a PKC inhibitor, is more cytotoxic toward cancer cells than normal cells in vitro". Cell Biology and Toxicology. 13 (3): 141–53. doi:10.1023/A:1007321227010. PMID 9088624.
- ^ Identification of phenolic antioxidants from Sword Brake fern (Pteris ensiformis Burm.). Yung-Husan Chen, Fang-Rong Chang, Yih-Jer Lin, Lisu Wang, Jinn-Fen Chen, Yang-Chang Wu and Ming-Jiuan Wu, Food Chemistry, Volume 105, Issue 1, 2007, pp. 48-56, doi:10.1016/j.foodchem.2007.03.055
- ^ Hispidin Derivatives from the Mushroom Inonotus xeranticus and Their Antioxidant Activity. In-Kyoung Lee, Soon-Ja Seok, Wan-Kyu Kim and Bong-Sik Yun, J. Nat. Prod., 2006, 69 (2), pp. 299–301, doi:10.1021/np050453n
- ^ Lee, In-Kyoung; Yun, Bong-Sik (2007). "Highly oxygenated and unsaturated metabolites providing a diversity of hispidin class antioxidants in the medicinal mushrooms Inonotus and Phellinus". Bioorganic & Medicinal Chemistry. 15 (10): 3309. doi:10.1016/j.bmc.2007.03.039.
- ^ Lee, Yeon Sil; Kang, Young-Hee; Jung, Ju-Young; Lee, Sanghyun; Ohuchi, Kazuo; Shin, Kuk Hyun; Kang, Il-Jun; Park, Jung Han Yoon; et al. (October 2008). "Protein glycation inhibitors from the fruiting body of Phellinus linteus" ([dead link]). Biological & Pharmaceutical Bulletin. 31 (10): 1968–72. doi:10.1248/bpb.31.1968. PMID 18827365.
