Rubidium sulfide: Difference between revisions
Appearance
Content deleted Content added
more ids |
hazards upgrade |
||
| Line 28: | Line 28: | ||
| Section7 = {{Chembox Hazards |
| Section7 = {{Chembox Hazards |
||
| MainHazards = toxic |
| MainHazards = toxic |
||
| |
| GHSPictograms = {{GHS05}}{{GHS09}} |
||
| GHSSignalWord = Danger |
|||
| SPhrases = {{S26}}, {{S45}} |
|||
| HPhrases = {{H-phrases|314|400}} |
|||
| PPhrases = {{P-phrases|260|264|273|280|301+330+331|303+361+353|304+340|305+351+338|310|321|363|391|405|501}} |
|||
}} |
}} |
||
| Section8 = {{Chembox Related |
| Section8 = {{Chembox Related |
||
Revision as of 23:35, 2 July 2020
| |
| Names | |
|---|---|
| IUPAC name
Rubidium sulfide
| |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Rb2S | |
| Molar mass | 203.00 |
| Appearance | white crystal |
| Density | 2.912 g/cm3[1] |
| Melting point | 530 °C[2] |
| hydrolyses to rubidium bisulfide[1] | |
| Solubility in ethanol and glycerol | soluble |
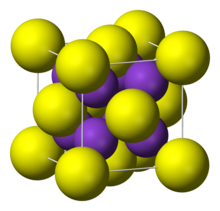
| Structure | |
| cubic:anti-fluorite | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
toxic |
| GHS labelling: | |
 
| |
| Danger | |
| H314, H400 | |
| P260, P264, P273, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P321, P363, P391, P405, P501 | |
| Related compounds | |
Other anions
|
rubidium oxide rubidium selenide rubidium telluride rubidium polonide |
Other cations
|
lithium sulfide,sodium sulfide,potassium sulfide,caesium sulfide,francium sulfide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Rubidium sulfide is an inorganic compound and a salt with a chemical formula Rb2S.It is a white solid with similar properties as other alkali metal sulfides.
Production
Solve hydrogen sulfide into rubidium hydroxide solution,it will produce rubidium bisulfide,then it will produce rubidium sulfide.[3][4]
Properties
Physical properties
Rubidium sulfide is a cubic crystal similar as lithium sulfide,sodium sulfide and potassium sulfide,they were anti-fluorite.Their space groups were .Rubidium sulfide have a crystal lattice a =765.0 pm.[1]
Chemical properties
Rubidium sulfide will reacts with sulfur in hydrogen gas to form rubidium pentasulfide(Rb2S5)。[4][5]
References
- ^ a b c Jean D'Ans, Ellen Lax: Taschenbuch für Chemiker und Physiker. 3. Elemente, anorganische Verbindungen und Materialien, Minerale, Band 3. 4. Auflage, Springer, 1997, ISBN 978-3-5406-0035-0, S. 692 ([1], p. 692, at Google Books).
- ^ Dale L. Perry, Sidney L. Phillips: Handbook of inorganic compounds. CRC Press, 1995, ISBN 978-0-8493-8671-8, S. 336 ([2], p. 336, at Google Books).
- ^ Wilhelm Blitz, Ernst Wilke-Dörfurt: "Über Sulfide des Rubidiums und Cäsiums" in Zeitschr. f. anorg. Chem. 1906. 48, S. 297–317. Volltext
- ^ a b R. Abegg, F. Auerbach: 'Handbuch der anorganischen Chemie'. Verlag S. Hirzel, Bd. 2, 1908. S. 430.Volltext
- ^ Wilhelm Blitz, Ernst Wilke-Dörfurt: Ueber die Pentasulfide des Rubidiums und Cäsiums. In Ber. d. dt. chem. Ges. 1905, 38, 1, S. 123–130, doi:10.1002/cber.19050380114.



