O-Cresol: Difference between revisions
6 parameters confirmed |
No edit summary |
||
| Line 7: | Line 7: | ||
| ImageFileL1 = O-Kresol.svg |
| ImageFileL1 = O-Kresol.svg |
||
| ImageFileL1_Ref = {{chemboximage|correct|??}} |
| ImageFileL1_Ref = {{chemboximage|correct|??}} |
||
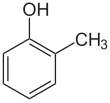
| ImageNameL1 = Kekulé, skeletal formula of o-cresol with some implicit hydrogens shown |
| ImageNameL1 = Kekulé, skeletal formula of ''o''-cresol with some implicit hydrogens shown |
||
| ImageFileR1 = Ortho-cresol-3D-vdW.png |
| ImageFileR1 = Ortho-cresol-3D-vdW.png |
||
| ImageFileR1_Ref = {{chemboximage|correct|??}} |
| ImageFileR1_Ref = {{chemboximage|correct|??}} |
||
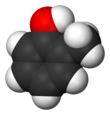
| ImageNameR1 = Spacefill model of o-cresol |
| ImageNameR1 = Spacefill model of ''o''-cresol |
||
| PIN = 2-Methylphenol |
| PIN = 2-Methylphenol |
||
| SystematicName = 2-Methylbenzenol |
| SystematicName = 2-Methylbenzenol |
||
| Line 45: | Line 45: | ||
| Odor = sweet, phenolic odor |
| Odor = sweet, phenolic odor |
||
| C=7 | H=8 | O=1 |
| C=7 | H=8 | O=1 |
||
| Density = 1.0465 g |
| Density = 1.0465 g cm<sup>−3</sup> |
||
| Solubility = 31 g |
| Solubility = 31 g dm<sup>−3</sup> (at 40 °C) |
||
| Solvent1 = ethanol |
| Solvent1 = ethanol |
||
| Solubility1 = Miscible (at 30 |
| Solubility1 = Miscible (at 30 °C) |
||
| Solvent2 = diethyl ether |
| Solvent2 = diethyl ether |
||
| Solubility2 = Miscible (at 30 |
| Solubility2 = Miscible (at 30 °C) |
||
| SolubleOther = soluble in [[chloroform]], [[diethyl ether|ether]], [[carbon tetrachloride|CCl<sub>4</sub>]] |
| SolubleOther = soluble in [[chloroform]], [[diethyl ether|ether]], [[carbon tetrachloride|CCl<sub>4</sub>]] |
||
| MeltingPtK = 304 |
| MeltingPtK = 304 |
||
| Line 56: | Line 56: | ||
| RefractIndex = 1.5353 |
| RefractIndex = 1.5353 |
||
| LogP = 1.962 |
| LogP = 1.962 |
||
| VaporPressure = 40 Pa (at 20 |
| VaporPressure = 40 Pa (at 20 °C) |
||
| pKa = 10.316 |
| pKa = 10.316 |
||
| pKb = 3.681 |
| pKb = 3.681 |
||
| Viscosity = 35.06 cP (at 45 |
| Viscosity = 35.06 cP (at 45 °C) |
||
| MagSus = -72. |
| MagSus = {{val|-72.9e-6|u=cm<sup>3</sup>/mol}} |
||
}} |
}} |
||
|Section3={{Chembox Thermochemistry |
|Section3={{Chembox Thermochemistry |
||
| DeltaHf = |
| DeltaHf = −204.3 kJ mol<sup>−1</sup> |
||
| DeltaHc = |
| DeltaHc = −3.6936 MJ mol<sup>−1</sup> |
||
| Entropy = 165.44 J |
| Entropy = 165.44 J K<sup>−1</sup> mol<sup>−1</sup> |
||
| HeatCapacity = 154.56 J |
| HeatCapacity = 154.56 J K<sup>−1</sup> mol<sup>−1</sup> |
||
}} |
}} |
||
|Section4={{Chembox Hazards |
|Section4={{Chembox Hazards |
||
| Line 81: | Line 81: | ||
| LD50 = 1350 mg/kg (rat, oral)<br/>121 mg/kg (rat, oral)<br/>344 mg/kg (mouse, oral)<ref>{{IDLH|cresol|Cresol (o, m, p isomers)}}</ref> |
| LD50 = 1350 mg/kg (rat, oral)<br/>121 mg/kg (rat, oral)<br/>344 mg/kg (mouse, oral)<ref>{{IDLH|cresol|Cresol (o, m, p isomers)}}</ref> |
||
| PEL = TWA 5 ppm (22 mg/m<sup>3</sup>) [skin]<ref name=PGCH>{{PGCH|0154}}</ref> |
| PEL = TWA 5 ppm (22 mg/m<sup>3</sup>) [skin]<ref name=PGCH>{{PGCH|0154}}</ref> |
||
| ExploLimits = 1.4% |
| ExploLimits = 1.4%–? (148 °C)<ref name=PGCH/> |
||
| IDLH = 250 ppm<ref name=PGCH/> |
| IDLH = 250 ppm<ref name=PGCH/> |
||
| REL = TWA 2.3 ppm (10 mg/m<sup>3</sup>)<ref name=PGCH/> |
| REL = TWA 2.3 ppm (10 mg/m<sup>3</sup>)<ref name=PGCH/> |
||
| Line 94: | Line 94: | ||
== Natural occurrences == |
== Natural occurrences == |
||
''o''-Cresol is one of the chemical compounds found in [[castoreum]]. This compound is gathered from the beaver's castor glands and found in the white cedar consumed by the beaver.<ref>The Beaver: Its Life and Impact. Dietland Muller-Schwarze, 2003, page 43 ([https://books.google.com/books?id=HZ5WjXB5Pr8C&lpg=PA39&ots=WYwTmWi-yJ&dq=Castoreum%20beekeeping&lr&hl=en&pg=PA43#v=onepage&q=Castoreum%20beekeeping&f=false book at google books])</ref> |
''o''-Cresol is one of the chemical compounds found in [[castoreum]]. This compound is gathered from the beaver's castor glands and found in the white cedar consumed by the [[beaver]].<ref>The Beaver: Its Life and Impact. Dietland Muller-Schwarze, 2003, page 43 ([https://books.google.com/books?id=HZ5WjXB5Pr8C&lpg=PA39&ots=WYwTmWi-yJ&dq=Castoreum%20beekeeping&lr&hl=en&pg=PA43#v=onepage&q=Castoreum%20beekeeping&f=false book at google books])</ref> |
||
''o''-Cresol is a constituent of [[tobacco smoke]].<ref>{{cite journal|last1=Talhout|first1=Reinskje|last2=Schulz|first2=Thomas|last3=Florek|first3=Ewa|last4=Van Benthem|first4=Jan|last5=Wester|first5=Piet|last6=Opperhuizen|first6=Antoon|title=Hazardous Compounds in Tobacco Smoke|journal=International Journal of Environmental Research and Public Health|volume=8|issue=12|year=2011|pages=613–628|issn=1660-4601|doi=10.3390/ijerph8020613|pmid=21556207|pmc=3084482}}</ref> |
''o''-Cresol is a constituent of [[tobacco smoke]].<ref>{{cite journal|last1=Talhout|first1=Reinskje|last2=Schulz|first2=Thomas|last3=Florek|first3=Ewa|last4=Van Benthem|first4=Jan|last5=Wester|first5=Piet|last6=Opperhuizen|first6=Antoon|title=Hazardous Compounds in Tobacco Smoke|journal=International Journal of Environmental Research and Public Health|volume=8|issue=12|year=2011|pages=613–628|issn=1660-4601|doi=10.3390/ijerph8020613|pmid=21556207|pmc=3084482}}</ref> |
||
== Production == |
== Production == |
||
Together with many other compounds, o-cresol is traditionally extracted from [[coal tar]], the volatile materials obtained in the production of coke from coal. A similar source material is petroleum residues. These residue contains a few percent by weight of phenol and isomeric |
Together with many other compounds, ''o''-cresol is traditionally extracted from [[coal tar]], the volatile materials obtained in the production of [[coke]] from [[coal]]. A similar source material is petroleum residues. These residue contains a few percent by weight of [[phenol]] and isomeric [[cresol]]s. In addition to the materials derived from these natural sources, about two thirds of the Western world's supply is produced by [[methylation]] of phenol using [[methanol]]. The [[alkylation]] is catalysed by metal oxides: |
||
:C<sub>6</sub>H<sub>5</sub>OH + CH<sub>3</sub>OH → CH<sub>3</sub>C<sub>6</sub>H<sub>4</sub>OH + H<sub>2</sub>O |
:C<sub>6</sub>H<sub>5</sub>OH + CH<sub>3</sub>OH → CH<sub>3</sub>C<sub>6</sub>H<sub>4</sub>OH + H<sub>2</sub>O |
||
Over-methylation gives [[xylenol]]. Many other production methods have been examined, including oxidative decarboxylation of [[salicylic acid]], oxygenation of [[toluene]], and hydrolysis of [[2-chlorotoluene]].<ref name=Ullmann/> |
Over-methylation gives [[xylenol]]. Many other production methods have been examined, including oxidative decarboxylation of [[salicylic acid]], oxygenation of [[toluene]], and hydrolysis of [[2-chlorotoluene]].<ref name=Ullmann/> |
||
== Applications == |
== Applications == |
||
''o''-Cresol is mainly used as a precursor to other compounds. Chlorination and etherification gives members of a commercially important [[herbicide]]s, such as [[2-methyl-4-chlorophenoxyacetic acid]] (MCPA). Nitration gives [[dinitrocresol]], a popular herbicide. [[Kolbe–Schmitt reaction|Kolbe–Schmitt carboxylation]] gives [[o-cresotinic acid|''o''-cresotinic acid]], a pharmaceutical intermediate. [[Carvacrol]], essence of oregano, is derived by alkylation of o-cresol with [[propene]]. The muscle relaxant [[mephenesin]] is an ether derived from ''o''-cresol.<ref name=Ullmann/> |
''o''-Cresol is mainly used as a precursor to other compounds. Chlorination and etherification gives members of a commercially important [[herbicide]]s, such as [[2-methyl-4-chlorophenoxyacetic acid]] (MCPA). Nitration gives [[dinitrocresol]], a popular herbicide. [[Kolbe–Schmitt reaction|Kolbe–Schmitt carboxylation]] gives [[o-cresotinic acid|''o''-cresotinic acid]], a pharmaceutical intermediate. [[Carvacrol]], essence of oregano, is derived by alkylation of ''o''-cresol with [[propene]]. The muscle relaxant [[mephenesin]] is an ether derived from ''o''-cresol.<ref name=Ullmann/> |
||
== Health effects == |
== Health effects == |
||
| Line 110: | Line 110: | ||
== External links == |
== External links == |
||
* [http://www.inchem.org/documents/icsc/icsc/eics0030.htm o-CRESOL (ICSC)] |
* [http://www.inchem.org/documents/icsc/icsc/eics0030.htm ''o''-CRESOL (ICSC)] |
||
* [http://www.syrres.com/esc/smiles_ex_notations.htm Environmental Science - SMILES Examples Notations] |
* [http://www.syrres.com/esc/smiles_ex_notations.htm Environmental Science - SMILES Examples Notations] |
||
* [https://www.cdc.gov/niosh/npg/npgd0154.html CDC - NIOSH Pocket Guide to Chemical Hazards] |
* [https://www.cdc.gov/niosh/npg/npgd0154.html CDC - NIOSH Pocket Guide to Chemical Hazards] |
||
Revision as of 13:47, 14 January 2021
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
2-Methylphenol | |||
| Systematic IUPAC name
2-Methylbenzenol | |||
| Other names
2-Cresol
o-Cresol ortho-Cresol 2-Hydroxytoluene o-Cresylic acid 1-Hydroxy-2-methylbenzene | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 506917 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.002.204 | ||
| EC Number |
| ||
| 101619 | |||
| KEGG | |||
| MeSH | 2-Cresol | ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 2076, 3455 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C7H8O | |||
| Molar mass | 108.140 g·mol−1 | ||
| Appearance | Colorless to white crystals | ||
| Odor | sweet, phenolic odor | ||
| Density | 1.0465 g cm−3 | ||
| Melting point | 31 °C; 88 °F; 304 K | ||
| Boiling point | 191 °C; 376 °F; 464 K | ||
| 31 g dm−3 (at 40 °C) | |||
| Solubility | soluble in chloroform, ether, CCl4 | ||
| Solubility in ethanol | Miscible (at 30 °C) | ||
| Solubility in diethyl ether | Miscible (at 30 °C) | ||
| log P | 1.962 | ||
| Vapor pressure | 40 Pa (at 20 °C) | ||
| Acidity (pKa) | 10.316 | ||
| Basicity (pKb) | 3.681 | ||
| −72.9×10−6 cm3/mol | |||
Refractive index (nD)
|
1.5353 | ||
| Viscosity | 35.06 cP (at 45 °C) | ||
| Thermochemistry | |||
Heat capacity (C)
|
154.56 J K−1 mol−1 | ||
Std molar
entropy (S⦵298) |
165.44 J K−1 mol−1 | ||
Std enthalpy of
formation (ΔfH⦵298) |
−204.3 kJ mol−1 | ||
Std enthalpy of
combustion (ΔcH⦵298) |
−3.6936 MJ mol−1 | ||
| Hazards | |||
| GHS labelling: | |||
 
| |||
| Danger | |||
| H301, H311, H314 | |||
| P260, P264, P270, P280, P301+P310, P301+P330+P331, P302+P352, P303+P361+P353, P304+P340, P305+P351+P338, P310, P312, P321, P322, P330, P361, P363, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 81 °C (178 °F; 354 K) | ||
| 598.9 °C (1,110.0 °F; 872.0 K) | |||
| Explosive limits | 1.4%–? (148 °C)[1] | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
1350 mg/kg (rat, oral) 121 mg/kg (rat, oral) 344 mg/kg (mouse, oral)[2] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
TWA 5 ppm (22 mg/m3) [skin][1] | ||
REL (Recommended)
|
TWA 2.3 ppm (10 mg/m3)[1] | ||
IDLH (Immediate danger)
|
250 ppm[1] | ||
| Safety data sheet (SDS) | External MSDS | ||
| Related compounds | |||
Related phenols
|
m-cresol, p-cresol, phenol | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
ortho-Cresol, also 2-methylphenol, is an organic compound with the formula CH3C6H4(OH). It is a colourless solid that is widely used intermediate in the production of other chemicals. It is a derivative of phenol and is an isomer of p-cresol and m-cresol.[3]
Natural occurrences
o-Cresol is one of the chemical compounds found in castoreum. This compound is gathered from the beaver's castor glands and found in the white cedar consumed by the beaver.[4]
o-Cresol is a constituent of tobacco smoke.[5]
Production
Together with many other compounds, o-cresol is traditionally extracted from coal tar, the volatile materials obtained in the production of coke from coal. A similar source material is petroleum residues. These residue contains a few percent by weight of phenol and isomeric cresols. In addition to the materials derived from these natural sources, about two thirds of the Western world's supply is produced by methylation of phenol using methanol. The alkylation is catalysed by metal oxides:
- C6H5OH + CH3OH → CH3C6H4OH + H2O
Over-methylation gives xylenol. Many other production methods have been examined, including oxidative decarboxylation of salicylic acid, oxygenation of toluene, and hydrolysis of 2-chlorotoluene.[3]
Applications
o-Cresol is mainly used as a precursor to other compounds. Chlorination and etherification gives members of a commercially important herbicides, such as 2-methyl-4-chlorophenoxyacetic acid (MCPA). Nitration gives dinitrocresol, a popular herbicide. Kolbe–Schmitt carboxylation gives o-cresotinic acid, a pharmaceutical intermediate. Carvacrol, essence of oregano, is derived by alkylation of o-cresol with propene. The muscle relaxant mephenesin is an ether derived from o-cresol.[3]
Health effects
Most exposures to cresols are at very low levels that are not harmful although, like phenols, cresols are skin irritants. When cresols are breathed, ingested, or applied to the skin at very high levels, they can be harmful. Breathing high levels of cresols for a short time results in irritation of the nose and throat. Aside from these effects, very little is known about the effects of breathing cresols at lower levels over longer times. The acute LD50 for oral ingestion by mice is 344 mg/kg.[3]
External links
- o-CRESOL (ICSC)
- Environmental Science - SMILES Examples Notations
- CDC - NIOSH Pocket Guide to Chemical Hazards
References
- ^ a b c d NIOSH Pocket Guide to Chemical Hazards. "#0154". National Institute for Occupational Safety and Health (NIOSH).
- ^ "Cresol (o, m, p isomers)". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ a b c d Helmut Fiegein "Cresols and Xylenols" in Ullmann's Encyclopedia of Industrial Chemistry" 2007; Wiley-VCH, Weinheim. doi:10.1002/14356007.a08_025
- ^ The Beaver: Its Life and Impact. Dietland Muller-Schwarze, 2003, page 43 (book at google books)
- ^ Talhout, Reinskje; Schulz, Thomas; Florek, Ewa; Van Benthem, Jan; Wester, Piet; Opperhuizen, Antoon (2011). "Hazardous Compounds in Tobacco Smoke". International Journal of Environmental Research and Public Health. 8 (12): 613–628. doi:10.3390/ijerph8020613. ISSN 1660-4601. PMC 3084482. PMID 21556207.
{{cite journal}}: CS1 maint: unflagged free DOI (link)