Titanium tetrafluoride: Difference between revisions
m Added FDA UNII |
M97uzivatel (talk | contribs) "hygroscopic" is not appearance; multispace |
||
| Line 27: | Line 27: | ||
| Formula = TiF<sub>4</sub> |
| Formula = TiF<sub>4</sub> |
||
| MolarMass = 123.861 g/mol |
| MolarMass = 123.861 g/mol |
||
| Appearance = white powder |
| Appearance = white powder |
||
| Density = 2.798 g/cm<sup>3</sup> |
| Density = 2.798 g/cm<sup>3</sup> |
||
| MeltingPtC = 377 |
| MeltingPtC = 377 |
||
| BoilingPt = sublimes |
| BoilingPt = sublimes |
||
| ⚫ | |||
| Solubility = |
|||
}} |
|||
|Section3={{Chembox Structure |
|||
| CrystalStruct = |
|||
| ⚫ | |||
|Section7={{Chembox Hazards |
|Section7={{Chembox Hazards |
||
| EUClass = not listed |
| EUClass = not listed |
||
| Line 41: | Line 38: | ||
| NFPA-F = 0 |
| NFPA-F = 0 |
||
| NFPA-R = 0 |
| NFPA-R = 0 |
||
| NFPA-S = |
|||
}} |
}} |
||
|Section8={{Chembox Related |
|Section8={{Chembox Related |
||
| OtherAnions = [[Titanium(IV) bromide]] <br/> [[Titanium(IV) chloride]] <br/> [[Titanium(IV) iodide]] |
| OtherAnions = [[Titanium(IV) bromide]] <br/> [[Titanium(IV) chloride]] <br/> [[Titanium(IV) iodide]] |
||
| OtherCompounds = [[Titanium(III) fluoride]] |
| OtherCompounds = [[Titanium(III) fluoride]] |
||
}} |
|||
}} |
}} |
||
'''Titanium(IV) fluoride''' is the [[inorganic compound]] with the [[chemical formula|formula]] [[Titanium|Ti]][[Fluoride|F<sub>4</sub>]]. It is a white [[hygroscopic]] solid. |
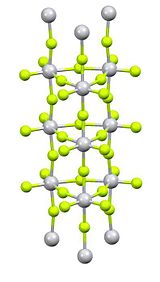
'''Titanium(IV) fluoride''' is the [[inorganic compound]] with the [[chemical formula|formula]] [[Titanium|Ti]][[Fluoride|F<sub>4</sub>]]. It is a white [[hygroscopic]] solid. In contrast to the other tetrahalides of titanium, it adopts a polymeric structure.<ref>{{Greenwood&Earnshaw2nd}}</ref> In common with the other tetrahalides, TiF<sub>4</sub> is a strong [[Lewis acid]]. |
||
==Preparation, structure, reactions== |
==Preparation, structure, reactions== |
||
The traditional method involves treatment of titanium tetrachloride with excess [[hydrogen fluoride]]: |
The traditional method involves treatment of titanium tetrachloride with excess [[hydrogen fluoride]]: |
||
:TiCl<sub>4</sub> |
:TiCl<sub>4</sub> + 4 HF → TiF<sub>4</sub> + 4 HCl |
||
Purification is by sublimation, which involves reversible cracking of the polymeric structure.<ref>Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY. Vol. 1. p. 200.</ref> |
Purification is by sublimation, which involves reversible cracking of the polymeric structure.<ref>Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY. Vol. 1. p. 200.</ref> |
||
X-ray crystallography reveals that the Ti centres are octahedral, but conjoined in an unusual columnar structure.<ref>{{cite journal|author1=Bialowons, H.|author2=Mueller, M.|author3=Mueller, B.G.|title=Titantetrafluorid - Eine Überraschend einfache Kolumnarstruktur|journal=Zeitschrift für Anorganische und Allgemeine Chemie|year=1995|volume= 621|pages=1227–1231|doi=10.1002/zaac.19956210720}}</ref> |
X-ray crystallography reveals that the Ti centres are octahedral, but conjoined in an unusual columnar structure.<ref>{{cite journal|author1=Bialowons, H.|author2=Mueller, M.|author3=Mueller, B.G.|title=Titantetrafluorid - Eine Überraschend einfache Kolumnarstruktur|journal=Zeitschrift für Anorganische und Allgemeine Chemie|year=1995|volume= 621|pages=1227–1231|doi=10.1002/zaac.19956210720}}</ref> |
||
| Line 68: | Line 64: | ||
[[Category:Metal halides]] |
[[Category:Metal halides]] |
||
[[Category:Titanium compounds]] |
[[Category:Titanium compounds]] |
||
{{inorganic-compound-stub}} |
{{inorganic-compound-stub}} |
||
Revision as of 18:20, 14 January 2021
| |
| Names | |
|---|---|
| IUPAC name
Titanium(IV) fluoride
| |
| Other names
Titanium tetrafluoride
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.029.106 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| TiF4 | |
| Molar mass | 123.861 g/mol |
| Appearance | white powder |
| Density | 2.798 g/cm3 |
| Melting point | 377 °C (711 °F; 650 K) |
| Boiling point | sublimes |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
|Section7=! colspan=2 style="background: #f8eaba; text-align: center;" |Hazards
|-
| NFPA 704 (fire diamond)
|
|- |Section8=! colspan=2 style="background: #f8eaba; text-align: center;" |Related compounds
|-
|
| Titanium(IV) bromide
Titanium(IV) chloride
Titanium(IV) iodide
|-
|
| Titanium(III) fluoride |- }}
Titanium(IV) fluoride is the inorganic compound with the formula TiF4. It is a white hygroscopic solid. In contrast to the other tetrahalides of titanium, it adopts a polymeric structure.[1] In common with the other tetrahalides, TiF4 is a strong Lewis acid.
Preparation, structure, reactions
The traditional method involves treatment of titanium tetrachloride with excess hydrogen fluoride:
- TiCl4 + 4 HF → TiF4 + 4 HCl
Purification is by sublimation, which involves reversible cracking of the polymeric structure.[2] X-ray crystallography reveals that the Ti centres are octahedral, but conjoined in an unusual columnar structure.[3]
TiF4 forms adducts with many ligands. One example is cis-TiF4(MeCN)2, which is formed by treatment with acetonitrile.[4]
References
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ^ Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY. Vol. 1. p. 200.
- ^ Bialowons, H.; Mueller, M.; Mueller, B.G. (1995). "Titantetrafluorid - Eine Überraschend einfache Kolumnarstruktur". Zeitschrift für Anorganische und Allgemeine Chemie. 621: 1227–1231. doi:10.1002/zaac.19956210720.
- ^ Nikiforov, Grigory B.; Roesky, Herbert W.; Koley, Debasis (2014). "A survey of titanium fluoride complexes, their preparation, reactivity, and applications". Coordination Chemistry Reviews. 258–259: 16–57. doi:10.1016/j.ccr.2013.09.002.

