Trimethylamine N-oxide: Difference between revisions
m Dating maintenance tags: {{By whom}} |
→Controversy: Funding sources alone do not necessarily mean that the results of a study are biased or invalid. Without further evidence, we can only say that they "may" be biased and advise the reader of the funding relationship. Tags: Mobile edit Mobile web edit |
||
| Line 96: | Line 96: | ||
====Controversy==== |
====Controversy==== |
||
Clouatre et al. argue that choline sources and dietary L-carnitine do not contribute to a significant elevation of blood TMAO. However, the study used to raise this dispute was sponsored by Lonza, Inc., who manufactures and sells an L-Carnitine supplement, so their dispute |
Clouatre et al. argue that choline sources and dietary L-carnitine do not contribute to a significant elevation of blood TMAO. However, the study used to raise this dispute was sponsored by Lonza, Inc., who manufactures and sells an L-Carnitine supplement, so their dispute may be considered biased. Instead the main source of TMAO in the diet is fish.<ref name="JohriHeyland2014">{{cite journal|last1=Johri|first1=A.M.|last2=Heyland|first2=D.K.|last3=Hétu|first3=M.-F.|last4=Crawford|first4=B.|last5=Spence|first5=J.D.|title=Carnitine therapy for the treatment of metabolic syndrome and cardiovascular disease: Evidence and controversies|journal=Nutrition, Metabolism and Cardiovascular Diseases|volume=24|issue=8|year=2014|pages=808–814|issn=0939-4753|doi=10.1016/j.numecd.2014.03.007|pmid=24837277}}</ref> And the link between cardiovascular diseases and TMAO is disputed in a mouse study.<ref name="CollinsDrazul-Schrader2016">{{cite journal|last1=Collins|first1=Heidi L.|last2=Drazul-Schrader|first2=Denise|last3=Sulpizio|first3=Anthony C.|last4=Koster|first4=Paul D.|last5=Williamson|first5=Yuping|last6=Adelman|first6=Steven J.|last7=Owen|first7=Kevin|last8=Sanli|first8=Toran|last9=Bellamine|first9=Aouatef|title=L-Carnitine intake and high trimethylamine N-oxide plasma levels correlate with low aortic lesions in ApoE−/− transgenic mice expressing CETP|journal=Atherosclerosis|volume=244|year=2016|pages=29–37|issn=0021-9150|doi=10.1016/j.atherosclerosis.2015.10.108|pmid=26584136|doi-access=free}}</ref> |
||
Another source of TMAO is dietary [[phosphatidylcholine]], again by way of bacterial action in the gut. Phosphatidyl choline is present at high concentration in egg yolks and some meats. The strongest evidence to contradict the apparent causal relationship between TMAO and cardiovascular disease comes from a Mendelian randomization study that failed to detect a significant association between circulating TMAO levels and myocardial infarction or coronary artery disease.<ref>{{Cite journal|last1=Jia|first1=Jinzhu|last2=Dou|first2=Pan|last3=Gao|first3=Meng|last4=Kong|first4=Xuejun|last5=Li|first5=Changwei|last6=Liu|first6=Zhonghua|last7=Huang|first7=Tao|date=2019-09-01|title=Assessment of Causal Direction Between Gut Microbiota–Dependent Metabolites and Cardiometabolic Health: A Bidirectional Mendelian Randomization Analysis|url=https://diabetes.diabetesjournals.org/content/68/9/1747|journal=Diabetes|language=en|volume=68|issue=9|pages=1747–1755|doi=10.2337/db19-0153|issn=0012-1797|pmid=31167879|doi-access=free|access-date=2020-05-14|archive-date=2021-03-20|archive-url=https://web.archive.org/web/20210320222409/https://diabetes.diabetesjournals.org/content/68/9/1747|url-status=live}}</ref> |
Another source of TMAO is dietary [[phosphatidylcholine]], again by way of bacterial action in the gut. Phosphatidyl choline is present at high concentration in egg yolks and some meats. The strongest evidence to contradict the apparent causal relationship between TMAO and cardiovascular disease comes from a Mendelian randomization study that failed to detect a significant association between circulating TMAO levels and myocardial infarction or coronary artery disease.<ref>{{Cite journal|last1=Jia|first1=Jinzhu|last2=Dou|first2=Pan|last3=Gao|first3=Meng|last4=Kong|first4=Xuejun|last5=Li|first5=Changwei|last6=Liu|first6=Zhonghua|last7=Huang|first7=Tao|date=2019-09-01|title=Assessment of Causal Direction Between Gut Microbiota–Dependent Metabolites and Cardiometabolic Health: A Bidirectional Mendelian Randomization Analysis|url=https://diabetes.diabetesjournals.org/content/68/9/1747|journal=Diabetes|language=en|volume=68|issue=9|pages=1747–1755|doi=10.2337/db19-0153|issn=0012-1797|pmid=31167879|doi-access=free|access-date=2020-05-14|archive-date=2021-03-20|archive-url=https://web.archive.org/web/20210320222409/https://diabetes.diabetesjournals.org/content/68/9/1747|url-status=live}}</ref> |
||
Revision as of 16:25, 29 August 2021
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
N,N-Dimethylmethanamine N-oxide | |
| Other names
Trimethylamine oxide, TMAO, TMANO
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.013.341 |
| KEGG | |
PubChem CID
|
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C3H9NO | |
| Molar mass | 75.11 |
| Appearance | colorless solid |
| Melting point | 220 to 222 °C (428 to 432 °F; 493 to 495 K) (dihydrate: 96 °C) |
| good | |
| 5.4 D | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
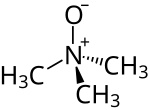
Trimethylamine N-oxide (TMAO) is an organic compound with the formula (CH3)3NO. It is in the class of amine oxides. Although the anhydrous compound is known, trimethylamine N-oxide is usually encountered as the dihydrate. Both the anhydrous and hydrated materials are white, water-soluble solids.
TMAO is found in the tissues of marine crustaceans and marine fish, where it prevents water pressure from distorting proteins and thus killing the animal. The concentration of TMAO increases with the depth at which the animal lives; TMAO is found in high concentrations in the deepest-living described species, Pseudoliparis swirei, which was found in the Mariana Trench, at a recorded depth of 8,076 m (26,496 ft).[1][2]
TMAO is a product of the oxidation of trimethylamine, a common metabolite of choline in animals.[3]
Marine animals
Trimethylamine N-oxide is an osmolyte found in molluscs, crustaceans, and all marine fishes and bony fishes. It is a protein stabilizer that serves to counteract the protein-destabilizing effects of pressure. In general, the bodies of animals living at great depths are adapted to high pressure environments by having pressure-resistant biomolecules and small organic molecules present in their cells, known as piezolytes, of which TMAO is the most abundant. These piezolytes give the proteins the flexibility they need to function properly under great pressure.[1][2][4][5][6]
TMAO decomposes to trimethylamine (TMA), which is the main odorant that is characteristic of degrading seafood.
Chemistry
TMAO can be synthesized from trimethylamine by treatment with hydrogen peroxide:[7]
- H2O2 + (CH3)3N → H2O + (CH3)3NO
The dihydrate is dehydrated by azeotropic distillation from dimethylformamide.[8]
Laboratory applications
Trimethylamine oxide is used in protein folding experiments to counteract the unfolding effects of urea.[9]
In the organometallic chemistry reaction of nucleophilic abstraction, Me3NO is employed as a decarbonylation agent according to the following stoichiometry:
- M(CO)n + Me3NO + L → M(CO)n-1L + Me3N + CO2
This reaction is used to decomplex organic ligands from metals, e.g. from (diene)Fe(CO)3.[7]
It is used in certain oxidation reactions, e.g. the conversion of alkyl iodides to the corresponding aldehyde.[10]
Effects on protein stability
The effects of TMAO on the backbone and charged residues of peptides are found to stabilize compact conformations,[11] whereas effects of TMAO on nonpolar residues lead to peptide swelling. This suggests competing mechanisms of TMAO on proteins, which accounts for hydrophobic swelling, backbone collapse, and stabilization of charge-charge interactions. These mechanisms are observed in Trp cage.[12]
Microbiotic associations
The order Clostridiales, the genus Ruminococcus, and the taxon Lachnospiraceae are positively associated with TMA and TMAO levels.[13] In contrast, proportions of S24-7, an abundant family from Bacteroidetes, are inversely associated with TMA and TMAO levels.[13]
Disorders
Trimethylaminuria
Trimethylaminuria is a rare defect in the production of the enzyme flavin-containing monooxygenase 3 (FMO3).[14][15] Those suffering from trimethylaminuria are unable to convert choline-derived trimethylamine into trimethylamine oxide. Trimethylamine then accumulates and is released in the person's sweat, urine, and breath, giving off a strong fishy odor.
Cardiovascular disease
A study published in 2013, assessing 513 adults with a history of major adverse cardiovascular events, an average age of 68, and 69% of whom previously or currently smoke, may indicate that high levels of TMAO in the blood are associated with an increased risk of additional cardiovascular events.[16]
Background
The concentration of TMAO in the blood increases after consuming foods containing carnitine[17] or lecithin[16] if the bacteria that convert those substances to TMAO are present in the gut.[18] High concentrations of carnitine are found in red meat, some energy drinks, and some dietary supplements. Some types of normal gut bacteria (e.g. species of Acinetobacter) in the human microbiome convert dietary carnitine to TMAO. TMAO alters cholesterol metabolism in the intestines, in the liver, and in artery walls. In the presence of TMAO, there is increased deposition of cholesterol in, and decreased removal of cholesterol from peripheral cells such as those in artery walls.[19] Lecithin is found in soy, eggs,[18] as an ingredient in processed food, is sold as a dietary supplement, is used as an emulsifier, and is used to prevent sticking (for example in non-stick cooking spray).
Controversy
Clouatre et al. argue that choline sources and dietary L-carnitine do not contribute to a significant elevation of blood TMAO. However, the study used to raise this dispute was sponsored by Lonza, Inc., who manufactures and sells an L-Carnitine supplement, so their dispute may be considered biased. Instead the main source of TMAO in the diet is fish.[20] And the link between cardiovascular diseases and TMAO is disputed in a mouse study.[21]
Another source of TMAO is dietary phosphatidylcholine, again by way of bacterial action in the gut. Phosphatidyl choline is present at high concentration in egg yolks and some meats. The strongest evidence to contradict the apparent causal relationship between TMAO and cardiovascular disease comes from a Mendelian randomization study that failed to detect a significant association between circulating TMAO levels and myocardial infarction or coronary artery disease.[22]
Hypertension and thrombosis
It has been suggested that TMAO may be involved in the regulation of arterial blood pressure and etiology of hypertension[23] and thrombosis (blood clots) in atherosclerotic disease.[24] A 2017 meta-analysis found higher circulating TMAO was associated with 23% higher risk of cardiovascular events and a 55% higher risk of mortality.[25]
Notably, toxic effects of TMA were described in several clinical and experimental papers in the mid 20th century[26] and very recent studies show deleterious effect of TMA on the circulatory system.[27][28][29] Furthermore, due to the obvious toxicity and, at the same time, widespread use in industry, various exposure limit guidelines with a detailed description of toxicity are available such as “Recommendation from the Scientific Committee on Occupational Exposure Limits” by the European Union Commission.[30] Therefore, it seems that it is TMA but not TMAO that may be a marker and mediator of cardiovascular risk.
Management of elevated levels
- Vegan and vegetarian diets appear to select against gut flora that metabolize carnitine (in favor of other gut flora more coordinated with their food supply). This apparent difference in their microbiome is associated with substantially reduced gut bacteria capable of converting carnitine to trimethylamine, which is later metabolized in the liver to TMAO.[17]
- Molybdenum containing enzymes exist in mammals. The so-called mitochondrial amidoxime reducing component (mARC) has been found to exist in two isoforms, mARC1 and mARC2, both being capable of reducing a variety of N-oxygenated compounds, including nonphysiological N-oxides.[31] Green peas and black beans are believed[by whom?] to be among the richest food sources of dietary molybdenum.
- 3,3-Dimethyl-1-butanol (DMB), a structural analog of choline, inhibits microbial TMA formation in mice and in human feces, thereby reducing plasma TMAO levels after choline or carnitine supplementation.[13] It is found in some balsamic vinegars, red wines, and some cold-pressed extra virgin olive oils and grape seed oils.[13]
- Resveratrol has been shown to reduce TMAO in mice by remodeling gut microbiota.[32]
References
- ^ a b Linley, T.D.; M.E. Gerringer; P.H. Yancey; J.C. Drazen; C.L. Weinstock; A.J. Jamieson (2016). "Fishes of the hadal zone including new species, in situ observations and depth records of Liparidae". Deep Sea Research Part I: Oceanographic Research Papers. 114: 99–110. Bibcode:2016DSRI..114...99L. doi:10.1016/j.dsr.2016.05.003.
- ^ a b Gerringer, M.E.; T.D. Linley; P.H. Yancey; A.J. Jamieson; E. Goetze; J.C. Drazen (2016). "Pseudoliparis swirei sp. nov.: A newly-discovered hadal snailfish (Scorpaeniformes: Liparidae) from the Mariana Trench". Zootaxa. 4358 (1): 161–177. doi:10.11646/zootaxa.4358.1.7. PMID 29245485.
- ^ Baker, J.R.; Chaykin, S. (1 April 1962). "The biosynthesis of trimethylamine-N-oxide". J. Biol. Chem. 237 (4): 1309–13. doi:10.1016/S0021-9258(18)60325-4. PMID 13864146.
- ^ Yancey, P. (2005). "Organic osmolytes as compatible, metabolic, and counteracting cytoprotectants in high osmolarity and other stresses". J. Exp. Biol. 208 (15): 2819–2830. doi:10.1242/jeb.01730. PMID 16043587.
- ^ Velasquez, M.T.; Ramezani, A.; Manal, A.; Raj, D.S. (8 November 2016). "Trimethylamine N-Oxide: The good, the bad and the unknown". Toxins. 8 (11): 326. doi:10.3390/toxins8110326. PMC 5127123. PMID 27834801.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ "What does it take to live at the bottom of the ocean?". BBC Earth. 2016. Archived from the original on 13 May 2016. Retrieved 19 May 2016.
- ^ a b A. J. Pearson "Trimethylamine N-Oxide" in Encyclopedia of Reagents for Organic Synthesis, John Wiley & Sons, 2001: New York. doi:10.1002/047084289X.rt268
- ^ "Crystalline anhydrous trimethylamine N-oxide". Tetrahedron Lett. 27 (34): 3961–3962. 1986. doi:10.1016/S0040-4039(00)84884-4.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ Zou, Q.; et al. (2002). "The Molecular Mechanism of Stabilization of Proteins by TMAO and Its Ability to Counteract the Effects of Urea". J. Am. Chem. Soc. 124 (7): 1192–1202. doi:10.1021/ja004206b. PMID 11841287.
- ^ Volker Franzen (1973). "Octanal". Organic Syntheses; Collected Volumes, vol. 5, p. 872.
- ^ Shea, Joan-Emma; Feinstein, Stuart C.; Lapointe, Nichole E.; Larini, Luca; Levine, Zachary A. (2015-03-03). "Regulation and aggregation of intrinsically disordered peptides". Proceedings of the National Academy of Sciences of the United States of America. 112 (9): 2758–2763. Bibcode:2015PNAS..112.2758L. doi:10.1073/pnas.1418155112. PMC 4352815. PMID 25691742.
- ^ Su, Zhaoqian; Mahmoudinobar, Farbod; Dias, Cristiano L. (2017). "Effects of Trimethylamine-N-oxide on the Conformation of Peptides and its Implications for Proteins". Physical Review Letters. 119 (10): 108102. Bibcode:2017PhRvL.119j8102S. doi:10.1103/physrevlett.119.108102. PMID 28949191.
- ^ a b c d Wang, Zeneng; Roberts, Adam B.; Buffa, Jennifer A.; Levison, Bruce S.; Zhu, Weifei; Org, Elin; Gu, Xiaodong; Huang, Ying; Zamanian-Daryoush, Maryam; Culley, Miranda K.; DiDonato, Anthony J.; Fu, Xiaoming; Hazen, Jennie E.; Krajcik, Daniel; DiDonato, Joseph A.; Lusis, Aldons J.; Hazen, Stanley L. (December 2015). "Non-lethal Inhibition of Gut Microbial Trimethylamine Production for the Treatment of Atherosclerosis". Cell. 163 (7): 1585–1595. doi:10.1016/j.cell.2015.11.055. PMC 4871610. PMID 26687352.
• A structural analog of choline, 3,3-dimethyl-1-butanol (DMB), is shown to non-lethally inhibit TMA formation from cultured microbes, to inhibit distinct microbial TMA lyases, and to both inhibit TMA production from physiologic polymicrobial cultures (e.g., intestinal contents, human feces) and reduce TMAO levels in mice fed a high-choline or L-carnitine diet.
• DMB was detected in some balsamic vinegars, in red wines, and in some cold-pressed extra virgin olive oils and grape seed oils{{cite journal}}:|archive-date=requires|archive-url=(help);|archive-url=requires|url=(help)CS1 maint: url-status (link) - ^ Treacy, E.P.; Akerman, BR; et al. (1998). "Mutations of the flavin-containing monooxygenase gene (FMO3) cause trimethylaminuria, a defect in detoxication". Human Molecular Genetics. 7 (5): 839–45. doi:10.1093/hmg/7.5.839. PMID 9536088.
- ^ Zschocke J, Kohlmueller D, Quak E, Meissner T, Hoffmann GF, Mayatepek E (1999). "Mild trimethylaminuria caused by common variants in FMO3 gene". Lancet. 354 (9181): 834–5. doi:10.1016/S0140-6736(99)80019-1. PMID 10485731. S2CID 9555588.
- ^ a b Tang, W.H. Wilson; Zeneng Wang; Bruce S. Levison; Robert A. Koeth; Earl B. Britt; Xiaoming Fu; Yuping Wu; Stanley L. Hazen (April 25, 2013). "Intestinal Microbial Metabolism of Phosphatidylcholine and Cardiovascular Risk". The New England Journal of Medicine. 368 (17): 1575–1584. doi:10.1056/NEJMoa1109400. PMC 3701945. PMID 23614584.
The production of TMAO from dietary phosphatidylcholine is dependent on metabolism by the intestinal microbiota. Increased TMAO levels are associated with an increased risk of incident major adverse cardiovascular events
- ^ a b Koeth, Robert A; Wang, Zeneng; Levison, Bruce S; Buffa, Jennifer A; Org, Elin; Sheehy, Brendan T; Britt, Earl B; Fu, Xiaoming; Wu, Yuping; Li, Lin; Smith, Jonathan D; DiDonato, Joseph A; Chen, Jun; Li, Hongzhe; Wu, Gary D; Lewis, James D; Warrier, Manya; Brown, J Mark; Krauss, Ronald M; Tang, W H Wilson; Bushman, Frederic D; Lusis, Aldons J; Hazen, Stanley L (April 7, 2013). "Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis". Nature Medicine. 19 (5): 576–85. doi:10.1038/nm.3145. PMC 3650111. PMID 23563705.
- ^ a b Gina Kolata (April 24, 2013). "Eggs, Too, May Provoke Bacteria to Raise Heart Risk". The New York Times. Archived from the original on March 20, 2021. Retrieved April 25, 2013.
- ^ Hazen, Stanley. "New Research On Red Meat And Heart Disease". The Diane Rehm Show (Transcript). WAMU 88.5 American University Radio. Archived from the original on 2021-03-20. Retrieved 10 April 2013.
- ^ Johri, A.M.; Heyland, D.K.; Hétu, M.-F.; Crawford, B.; Spence, J.D. (2014). "Carnitine therapy for the treatment of metabolic syndrome and cardiovascular disease: Evidence and controversies". Nutrition, Metabolism and Cardiovascular Diseases. 24 (8): 808–814. doi:10.1016/j.numecd.2014.03.007. ISSN 0939-4753. PMID 24837277.
- ^ Collins, Heidi L.; Drazul-Schrader, Denise; Sulpizio, Anthony C.; Koster, Paul D.; Williamson, Yuping; Adelman, Steven J.; Owen, Kevin; Sanli, Toran; Bellamine, Aouatef (2016). "L-Carnitine intake and high trimethylamine N-oxide plasma levels correlate with low aortic lesions in ApoE−/− transgenic mice expressing CETP". Atherosclerosis. 244: 29–37. doi:10.1016/j.atherosclerosis.2015.10.108. ISSN 0021-9150. PMID 26584136.
- ^ Jia, Jinzhu; Dou, Pan; Gao, Meng; Kong, Xuejun; Li, Changwei; Liu, Zhonghua; Huang, Tao (2019-09-01). "Assessment of Causal Direction Between Gut Microbiota–Dependent Metabolites and Cardiometabolic Health: A Bidirectional Mendelian Randomization Analysis". Diabetes. 68 (9): 1747–1755. doi:10.2337/db19-0153. ISSN 0012-1797. PMID 31167879. Archived from the original on 2021-03-20. Retrieved 2020-05-14.
- ^ Ufnal, Marcin; Jazwiec, Radoslaw; Dadlez, Michal; Drapala, Adrian; Sikora, Mariusz; Skrzypecki, Janusz (2014). "Trimethylamine-N-Oxide: A Carnitine-Derived Metabolite That Prolongs the Hypertensive Effect of Angiotensin II in Rats". Canadian Journal of Cardiology. 30 (12): 1700–1705. doi:10.1016/j.cjca.2014.09.010. PMID 25475471. Archived from the original on 2021-03-20. Retrieved 2014-09-23.
- ^ Tilg, Herbert (2016-06-22). "A Gut Feeling about Thrombosis". New England Journal of Medicine. 374 (25): 2494–2496. doi:10.1056/nejmcibr1604458. PMID 27332910.
- ^ Qi, Jiaqian; You, Tao; Li, Jing; Pan, Tingting; Xiang, Li; Han, Yue; Zhu, Li (2018). "Circulating trimethylamine N-oxide and the risk of cardiovascular diseases: a systematic review and meta-analysis of 11 prospective cohort studies". Journal of Cellular and Molecular Medicine. 22 (1): 185–194. doi:10.1111/jcmm.13307. ISSN 1582-4934. PMC 5742728. PMID 28782886.
- ^ Brieger, H.; Hodes, W.A. Toxic effects of exposure to vapors of aliphatic amines. Arch. Ind. Hyg. Occup. Med. 1951, 3, 287–291
- ^ TMA (trimethylamine), but not its oxide TMAO (trimethylamine-oxide), exerts hemodynamic effects-implications for interpretation of cardiovascular actions of gut microbiome. K Jaworska, K Bielinska, M Gawrys-Kopczynska, M Ufnal Cardiovascular Research, https://doi.org/10.1093/cvr/cvz231 Archived 2021-03-20 at the Wayback Machine
- ^ Kinga Jaworska, Marek Konop, Tomasz Hutsch, Karol Perlejewski, Marek Radkowski, Marta Grochowska, Anna Bielak-Zmijewska, Grażyna Mosieniak, Ewa Sikora, Marcin Ufnal, TMA but not TMAO increases with age in rat plasma and affects smooth muscle cells viability, The Journals of Gerontology: Series A, , glz181, https://doi.org/10.1093/gerona/glz181 Archived 2021-03-20 at the Wayback Machine
- ^ Toxins 2019, 11(9), 490; https://doi.org/10.3390/toxins11090490 Archived 2021-03-20 at the Wayback Machine
- ^ Pospischil, E.; Johanson, G.; Nielsen, G.; Papameletiou, D.; Klein, C. SCOEL/REC/179 Trimethylamine. Publ. Sci. Comm. Occup. Expo. Lim. Eur. Union 2017.
- ^ "Archived copy". Archived from the original on 2021-03-20. Retrieved 2020-01-02.
{{cite web}}: CS1 maint: archived copy as title (link) - ^ Dimitrov D, Thiele I, Ferguson LR (2016). "Editorial: The Human Gutome: Nutrigenomics of Host-Microbiome Interactions". Frontiers in Genetics. 7: 158. doi:10.3389/fgene.2016.00158. PMC 5012120. PMID 27656194.
Recent findings showed that resveratrol reduces levels of trimethylamine-N-oxide (TMAO), known to be a contributory factor in the development of atherosclerosis (Chen et al., 2016). This was partially mediated through down-regulating the enterohepatic farnesoid X receptor-fibroblast growth factor (FXR) axis, and indicates that gut microbiota may become an interesting target for pharmacological and nutritional precision medicine interventions to decrease the risk of developing metabolic diseases.
{{cite journal}}: CS1 maint: unflagged free DOI (link)
