Dimethyl sulfate: Difference between revisions
InakiXabier (talk | contribs) link to wiki article |
Added GHS information |
||
| Line 51: | Line 51: | ||
| NFPA-R = 1 |
| NFPA-R = 1 |
||
| NFPA-F = 2 |
| NFPA-F = 2 |
||
| GHSPictograms = {{GHS health hazard}} {{GHS corrosion}} {{GHS skull and crossbones}} |
|||
| RPhrases = {{R45}}, {{R25}}, {{R26}}, {{R34}},<br/>{{R43}}, {{R68}} |
|||
| GHSSignalWord = '''Danger''' |
|||
| SPhrases = {{S53}}, {{S45}}, {{S30}}, {{S60}}, {{S61}} |
|||
| HPhrases = {{H-phrases|301|314|317|330|335|341|350}} |
|||
| PEL = TWA 1 ppm (5 mg/m<sup>3</sup>) [skin]<ref name=PGCH>{{PGCH|0229}}</ref> |
| PEL = TWA 1 ppm (5 mg/m<sup>3</sup>) [skin]<ref name=PGCH>{{PGCH|0229}}</ref> |
||
| REL = Ca TWA 0.1 ppm (0.5 mg/m<sup>3</sup>) [skin]<ref name=PGCH/> |
| REL = Ca TWA 0.1 ppm (0.5 mg/m<sup>3</sup>) [skin]<ref name=PGCH/> |
||
Revision as of 06:57, 8 September 2021
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
Dimethyl sulfate | |
| Other names
Dimethyl sulphate; Sulfuric acid dimethyl ester; Me2SO4; DMSO4; Dimethyl ester of sulfuric acid; Methyl sulfate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.000.963 |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C2H6O4S | |
| Molar mass | 126.13 g/mol |
| Appearance | Colorless, oily liquid |
| Odor | faint, onion-like[1] |
| Density | 1.33 g/ml, liquid |
| Melting point | −32 °C (−26 °F; 241 K) |
| Boiling point | 188 °C (370 °F; 461 K) (decomposes) |
| Reacts | |
| Solubility | Methanol, dichloromethane, acetone |
| Vapor pressure | 0.1 mmHg (20°C)[1] |
| -62.2·10−6 cm3/mol | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Extremely toxic, contact hazard, inhalation hazard, corrosive, environmental hazard, carcinogenic, mutagenic |
| GHS labelling: | |
  
| |
| Danger | |
| H301, H314, H317, H330, H335, H341, H350 | |
| NFPA 704 (fire diamond) | |
| Flash point | 83 °C; 182 °F; 356 K [1] |
| Lethal dose or concentration (LD, LC): | |
LC50 (median concentration)
|
8.6 ppm (rat, 4 hr) 75 ppm (guinea pig, 20 min) 53 ppm (mouse) 32 ppm (guinea pig, 1 hr)[2] |
LCLo (lowest published)
|
97 ppm (human, 10 min)[2] |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 1 ppm (5 mg/m3) [skin][1] |
REL (Recommended)
|
Ca TWA 0.1 ppm (0.5 mg/m3) [skin][1] |
IDLH (Immediate danger)
|
Ca [7 ppm][1] |
| Related compounds | |
Related compounds
|
Diethyl sulfate, methyl triflate, dimethyl carbonate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
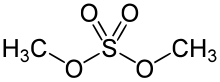
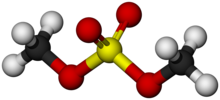
Dimethyl sulfate is a chemical compound with formula (CH3O)2SO2. As the diester of methanol and sulfuric acid, its formula is often written as (CH3)2SO4 or Me2SO4, where CH3 or Me is methyl. Me2SO4 is mainly used as a methylating agent in organic synthesis.
Me2SO4 is a colourless oily liquid with a slight onion-like odour (although smelling it would represent significant exposure). Like all strong alkylating agents, Me2SO4 is extremely toxic. Its use as a laboratory reagent has been superseded to some extent by methyl triflate, CF3SO3CH3, the methyl ester of trifluoromethanesulfonic acid.
History
Dimethyl sulfate was discovered in the early 19th century in an impure form.[3] J. P. Claesson later extensively studied its preparation.[4][5] It was used in chemical warfare in WWI.[6][7]
Production
Dimethyl sulfate can be synthesized in the laboratory by many different methods,[8] the simplest being the esterification of sulfuric acid with methanol:[clarification needed]
- 2 CH3OH + H2SO4 → (CH3)2SO4 + 2 H2O
Another possible synthesis involves distillation of methyl hydrogen sulfate:[5]
- 2 CH3HSO4 → H2SO4 + (CH3)2SO4
Methyl nitrite and methyl chlorosulfonate also result in dimethyl sulfate:[5]
- CH3ONO + (CH3)OSO2Cl → (CH3)2SO4 + NOCl
Me2SO4 has been produced commercially since the 1920s. A common process is the continuous reaction of dimethyl ether with sulfur trioxide.[9]
- (CH3)2O + SO3 → (CH3)2SO4
Uses
Dimethyl sulfate is best known as a reagent for the methylation of phenols, amines, and thiols. One methyl group is transferred more quickly than the second. Methyl transfer is assumed to occur via an SN2 reaction. Compared to other methylating agents, dimethyl sulfate is preferred by the industry because of its low cost and high reactivity.
Methylation at oxygen
Most commonly Me2SO4 is employed to methylate phenols. Some simple alcohols are also suitably methylated, as illustrated by the conversion of tert-butanol to t-butyl methyl ether:
- 2 (CH3)3COH + (CH3O)2SO2 → 2 (CH3)3COCH3 + H2SO4
Alkoxide salts are rapidly methylated:[10]
- RO− Na+ + (CH3O)2SO2 → ROCH3 + Na(CH3)SO4
The methylation of sugars is called Haworth methylation.[11]
Methylation at amine nitrogen
Me2SO4 is used to prepare both quaternary ammonium salts or tertiary amines:
- C6H5CH=NC4H9 + (CH3O)2SO2 → C6H5CH=N+(CH3)C4H9 + CH3OSO3−
Quaternized fatty ammonium compounds are used as a surfactant or fabric softeners. Methylation to create a tertiary amine is illustrated as:[10]
- CH3(C6H4)NH2 + (CH3O)2SO2 (in NaHCO3 aq.) → CH3(C6H4)N(CH3)2 + Na(CH3)SO4
Methylation at sulfur
Similar to the methylation of alcohols, mercaptide salts are easily methylated by Me2SO4:[10]
- RS−Na+ + (CH3O)2SO2 → RSCH3 + Na(CH3)SO4
An example is:[12]
- p-CH3C6H4SO2Na + (CH3O)2SO2 → p-CH3C6H4SO2CH3 + Na(CH3)SO4
This method has been used to prepare thioesters:
- RC(O)SH + (CH3O)2SO2 → RC(O)S(CH3) + HOSO3CH3
Reactions with nucleic acids
Dimethyl sulfate (DMS) is used to determine the secondary structure of RNA. At neutral pH, DMS methylates unpaired adenine and cytosine residues at their canonical Watson-Crick faces, but it cannot methylate base-paired nucleotides. Using the method known as DMS-MaPseq,[13] RNA is incubated with DMS to methylate unpaired bases. Then the RNA is reverse-transcribed; the reverse transcriptase frequently adds an incorrect DNA base when it encounters a methylated RNA base. These mutations can be detected via sequencing, and the RNA is inferred to be single-stranded at bases with above-background mutation rates.
Dimethyl sulfate can effect the base-specific cleavage of DNA by attacking the imidazole rings present in guanine.[14] Dimethyl sulfate also methylates adenine in single-stranded portions of DNA (e.g., those with proteins like RNA polymerase progressively melting and re-annealing the DNA). Upon re-annealing, these methyl groups interfere with adenine-guanine base-pairing. Nuclease S1 can then be used to cut the DNA in single-stranded regions (anywhere with a methylated adenine). This is an important technique for analyzing protein-DNA interactions.
Alternatives
Although dimethyl sulfate is highly effective and affordable, its toxicity has encouraged the use of other methylating reagents. Methyl iodide is a reagent used for O-methylation, like dimethyl sulfate, but is less hazardous and more expensive.[12] Dimethyl carbonate, which is less reactive, has far lower toxicity compared to both dimethyl sulfate and methyl iodide.[15] High pressure can be used to accelerate methylation by dimethyl carbonate. In general, the toxicity of methylating agents is correlated with their efficiency as methyl transfer reagents.
Safety
Dimethyl sulfate is carcinogenic[9] and mutagenic, highly poisonous, corrosive, and environmentally hazardous.[16] Dimethyl sulfate is absorbed through the skin, mucous membranes, and gastrointestinal tract, and can cause a fatal delayed respiratory tract reaction. An ocular reaction is also common. There is no strong odor or immediate irritation to warn of lethal concentration in the air. The LD50 (acute, oral) is 205 mg/kg (rat) and 140 mg/kg (mouse), and LC50 (acute) is 45 ppm / 4 hours (rat).[17] The vapor pressure of 65 Pa[18] is sufficiently large to produce a lethal concentration in air by evaporation at 20 °C. Delayed toxicity allows potentially fatal exposures to occur prior to development of any warning symptoms.[16] Symptoms may be delayed 6–24 hours. Concentrated solutions of bases (ammonia, alkalis) can be used to hydrolyze minor spills and residues on contaminated equipment, but the reaction may become violent with larger amounts of dimethyl sulfate (see ICSC). Although the compound hydrolyses, treatment with water cannot be assumed to decontaminate dimethyl sulfate.
References
- ^ a b c d e f NIOSH Pocket Guide to Chemical Hazards. "#0229". National Institute for Occupational Safety and Health (NIOSH).
- ^ a b "Dimethyl sulfate". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ Dumas, J.; Péligot, E. (1835). "Mémoire sur l'esprit de bois et sur les divers composés ethérés qui en proviennent" [Memoir on spirit of wood [i.e., methanol] and on various ethereal compounds that come from it]. Annales de Chimie et de Physique. 2nd series (in French). 58: 5–74.
- ^ Claesson, Peter (1879). "Ueber die neutralen und sauren Sulfate des Methyl- und Aethylalkohols" [On the neutral and acid sulfates of methyl and ethyl alcohol]. Journal für praktische Chemie. 2nd series (in German). 19: 231–265. doi:10.1002/prac.18790190123.
- ^ a b c Suter, C. M. (1944). The Organic Chemistry of Sulfur: Tetracovalent Sulfur Compounds. John Wiley & Sons. pp. 49–53. LCCN 44001248.
- ^ "Dimethyl Sulfate 77-78-1". EPA.
- ^ "Poison Facts: Low Chemicals: Dimethyl Sulfate". The University of Kansas Hospital.
- ^ Shirley, D. A. (1966). Organic Chemistry. Holt, Rinehart and Winston. p. 253. LCCN 64010030.
- ^ a b "Dimethyl Sulfate CAS No. 77-78-1" (PDF). 12th Report on Carcinogens (RoC). US Department of Health and Human Services. 2011.
- ^ a b c "Dupont product information". Archived from the original on 2008-11-19. Retrieved 2006-05-08.
- ^ W. N. Haworth (1915). "III. A New Method of Preparing Alkylated Sugars". Journal of the Chemical Society, Transactions. 107: 8–16. doi:10.1039/CT9150700008.
- ^ a b Fieser, L. F.; Fieser, M. (1967). Reagents for Organic Synthesis. John Wiley & Sons. p. 295. ISBN 9780471258759.
- ^ Zubradt, Meghan; Gupta, Paromita; Persad, Sitara; Lambowitz, Alan; Weissman, Jonathan; Rouskin, Silvi (2017). "DMS-MaPseq for genome-wide or targeted RNA structure probing in vivo". Nature Methods. 14 (1): 75–82. doi:10.1038/nmeth.4057. PMC 5508988. PMID 27819661.
- ^ Streitwieser, A.; Heathcock, C. H.; Kosower, E. M. (1992). Introduction to Organic Chemistry (4th ed.). Macmillan. p. 1169. ISBN 978-0024181701.
- ^ Shieh, W. C.; Dell, S.; Repic, O. (2001). "1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU) and Microwave-Accelerated Green Chemistry in Methylation of Phenols, Indoles, and Benzimidazoles with Dimethyl Carbonate". Organic Letters. 3 (26): 4279–4281. doi:10.1021/ol016949n. PMID 11784197.
- ^ a b Rippey, J. C. R.; Stallwood, M. I. (2005). "Nine Cases of Accidental Exposure to Dimethyl Sulphate — A Potential Chemical Weapon". Emergency Medicine Journal. 22 (12): 878–879. doi:10.1136/emj.2004.015800. PMC 1726642. PMID 16299199.
- ^ "Material Safety Data Sheet - Dimethyl sulfate MSDS". ScienceLab. Archived from the original on 2012-04-06. Retrieved 2011-10-02.
- ^ ICSC

