Arsenic acid: Difference between revisions
No edit summary |
|||
| Line 72: | Line 72: | ||
}} |
}} |
||
}} |
}} |
||
[[File:Arseniksyrlighet, prov.jpg|thumb|Three bottles of arsenic acid from the [[Great Exhibition]]: impure, pure and distilled.]] |
|||
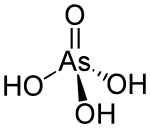
'''Arsenic acid''' is the [[chemical compound]] with the [[chemical formula|formula]] H<sub>3</sub>AsO<sub>4</sub>. More descriptively written as AsO(OH)<sub>3</sub>, this colorless [[acid]] is the [[arsenic]] analogue of [[phosphoric acid]]. [[Arsenate]] and [[phosphate]] salts behave very similarly. Arsenic acid as such has not been isolated, but is only found in solution, where it is largely ionized. Its [[hemihydrate]] form (H<sub>3</sub>AsO<sub>4</sub>·{{sfrac|1|2}}H<sub>2</sub>O) does form stable crystals. Crystalline samples dehydrate with condensation at 100 °C.<ref>{{ cite book |author1=Holleman, A. F. |author2=Wiberg, E. | title = Inorganic Chemistry | publisher = Academic Press | location = San Diego | year = 2001 | isbn = 0-12-352651-5 }}</ref> |
'''Arsenic acid''' is the [[chemical compound]] with the [[chemical formula|formula]] H<sub>3</sub>AsO<sub>4</sub>. More descriptively written as AsO(OH)<sub>3</sub>, this colorless [[acid]] is the [[arsenic]] analogue of [[phosphoric acid]]. [[Arsenate]] and [[phosphate]] salts behave very similarly. Arsenic acid as such has not been isolated, but is only found in solution, where it is largely ionized. Its [[hemihydrate]] form (H<sub>3</sub>AsO<sub>4</sub>·{{sfrac|1|2}}H<sub>2</sub>O) does form stable crystals. Crystalline samples dehydrate with condensation at 100 °C.<ref>{{ cite book |author1=Holleman, A. F. |author2=Wiberg, E. | title = Inorganic Chemistry | publisher = Academic Press | location = San Diego | year = 2001 | isbn = 0-12-352651-5 }}</ref> |
||
Revision as of 16:45, 5 October 2021
| |
| |
| Names | |
|---|---|
| IUPAC name
Arsenic acid, arsoric acid
| |
| Other names
Arsenic acid
Orthoarsenic acid Desiccant L-10 Zotox | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.029.001 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 1553, 1554 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| H3AsO4 | |
| Molar mass | 141.94 g/mol |
| Appearance | White translucent crystals, hygroscopic. |
| Density | 2.5 g/cm3 |
| Melting point | 35.5 °C (95.9 °F; 308.6 K) |
| Boiling point | 120 °C (248 °F; 393 K) decomposes |
| 16.7 g/100 mL | |
| Solubility | soluble in alcohol |
| Vapor pressure | 55 hPa (50 °C) |
| Acidity (pKa) | 2.19, 6.94, 11.5 |
| Conjugate base | Arsenate |
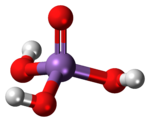
| Structure | |
| Tetrahedral | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Extremely toxic, carcinogenic, corrosive |
| GHS labelling: | |
    
| |
| Danger | |
| H301, H312, H314, H331, H350, H361, H410 | |
| P201, P202, P260, P261, P264, P270, P271, P273, P280, P281, P301+P310, P301+P330+P331, P302+P352, P303+P361+P353, P304+P340, P305+P351+P338, P308+P313, P310, P311, P312, P321, P322, P330, P363, P391, P403+P233, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
48 mg/kg (rat, oral)
6 mg/kg (rabbit, oral) |
| Related compounds | |
Other anions
|
Phosphoric acid |
Other cations
|
Sodium arsenate |
Related compounds
|
Arsenous acid Arsenic pentoxide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |

Arsenic acid is the chemical compound with the formula H3AsO4. More descriptively written as AsO(OH)3, this colorless acid is the arsenic analogue of phosphoric acid. Arsenate and phosphate salts behave very similarly. Arsenic acid as such has not been isolated, but is only found in solution, where it is largely ionized. Its hemihydrate form (H3AsO4·1/2H2O) does form stable crystals. Crystalline samples dehydrate with condensation at 100 °C.[1]
Properties
It is a tetrahedral species of idealized symmetry C3v with As–O bond lengths ranging from 1.66 to 1.71 Å.[2]
Being a triprotic acid, its acidity is described by three equilibria:
- H3AsO4 + H2O ⇌ H
2AsO−
4 + H3O+ (pKa1 = 2.19) - H
2AsO−
4 + H2O ⇌ HAsO2−
4 + H3O+ (pKa2 = 6.94) - HAsO2−
4 + H2O ⇌ AsO3−
4 + H3O+ (pKa3 = 11.5)
These pKa values are close to those for phosphoric acid. The highly basic arsenate ion (AsO3−
4) is the product of the third ionization. Unlike phosphoric acid, arsenic acid is an oxidizer, as illustrated by its ability to convert iodide to iodine.
Preparation
Arsenic acid is prepared by treating arsenic trioxide with concentrated nitric acid. Dinitrogen trioxide is produced as a by-product.[3]
- As2O3 + 2 HNO3 + 2 H2O → 2 H3AsO4 + N2O3
The resulting solution is cooled to give colourless crystals of the hemihydrate H3AsO4·1/2H2O, although the dihydrate H3AsO4·2H2O is produced when crystallisation occurs at lower temperatures.[3]
Other methods
Arsenic acid is slowly formed when arsenic pentoxide is dissolved in water, and when meta- or pyroarsenic acid is treated with cold water. Arsenic acid can also be prepared directly from elemental arsenic by moistening it and treating with ozone.
- 2 As + 3 H2O + 5 O3 → 2 H3AsO4 + 5 O2
Applications
Commercial applications of arsenic acid are limited by its toxicity. It is a precursor to a variety of pesticides. It has found occasional use as a wood preservative, a broad-spectrum biocide, a finishing agent for glass and metal, and a reagent in the synthesis of some dyestuffs and organic arsenic compounds.[4]
Safety
Arsenic acid is extremely toxic and carcinogenic, like all arsenic compounds. It is also corrosive. The LD50 in rabbits is 6 mg/kg (0.006 g/kg).[5]
References
- ^ Holleman, A. F.; Wiberg, E. (2001). Inorganic Chemistry. San Diego: Academic Press. ISBN 0-12-352651-5.
- ^ Lee, C.; Harrison, W. T. A. (2007). "Tetraethylammonium dihydrogenarsenate bis(arsenic acid) and 1,4-diazoniabicyclo[2.2.2]octane bis(dihydrogenarsenate) arsenic acid: hydrogen-bonded networks containing dihydrogenarsenate anions and neutral arsenic acid molecules". Acta Crystallographica C. 63 (Pt 7): m308 – m311. doi:10.1107/S0108270107023967. PMID 17609552.
- ^ a b G. Brauer, ed. (1963). "Arsenic Acid". Handbook of Preparative Inorganic Chemistry. Vol. 1 (2nd ed.). New York: Academic Press. p. 601.
- ^ Minerals Yearbook, 2008, V. 1, Metals and Minerals. Government Printing Office. 2010. pp. 6–. ISBN 978-1-4113-3015-3.
- ^ Grund, Sabina C.; Hanusch, Kunibert; Wolf, Hans Uwe (2008). "Arsenic and Arsenic Compounds". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a03_113.pub2. ISBN 978-3527306732.

