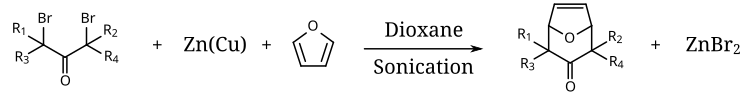Zinc–copper couple: Difference between revisions
See also links |
Citation bot (talk | contribs) Alter: pages, title. Formatted dashes. | Use this bot. Report bugs. | Suggested by Whoop whoop pull up | Category:Reducing agents | #UCB_Category 87/100 |
||
| Line 4: | Line 4: | ||
Zinc–copper couple has been prepared by numerous methods, which vary mainly with respect to the source of copper, but also by the ratio of copper to zinc, the physical state of the zinc (e.g. powder or granules), the use of protic acids and other additives, and temperature of the preparation. Most often the couple is generated and isolated prior to use, but routes have been described to storable forms of the alloy. Most methods involve reduction of an oxidized copper species with zinc, which is used in excess. |
Zinc–copper couple has been prepared by numerous methods, which vary mainly with respect to the source of copper, but also by the ratio of copper to zinc, the physical state of the zinc (e.g. powder or granules), the use of protic acids and other additives, and temperature of the preparation. Most often the couple is generated and isolated prior to use, but routes have been described to storable forms of the alloy. Most methods involve reduction of an oxidized copper species with zinc, which is used in excess. |
||
An early method for the synthesis of zinc–copper couple entailed treatment of a mixture of zinc dust and [[copper(II) oxide]] with [[hydrogen]] gas at 500 °C.<ref name=Simmons/> A more convenient and cheaper method proceeds by treatment of zinc powder with [[hydrochloric acid]] and [[copper(II) sulfate]].<ref>{{OrgSynth| title = Norcarane | author = Howard H. Simmons, Ronald D. Smith | year = 1973 | collvol = 5 | collvolpages = 855 | prep = cv5p0855}}</ref> Treatment of zinc powder with [[copper(II) acetate monohydrate]] in hot [[acetic acid]] is reportedly highly reproducible.<ref>{{cite journal | title = Cyclopropanes from an Easily Prepared, Highly Active Zinc-Copper Couple, Dibromomethane, and Olefins | author = Eugene LeGoff | journal = [[J. Org. Chem.]] | year = 1964 | volume = 29 | pages = |
An early method for the synthesis of zinc–copper couple entailed treatment of a mixture of zinc dust and [[copper(II) oxide]] with [[hydrogen]] gas at 500 °C.<ref name=Simmons/> A more convenient and cheaper method proceeds by treatment of zinc powder with [[hydrochloric acid]] and [[copper(II) sulfate]].<ref>{{OrgSynth| title = Norcarane | author = Howard H. Simmons, Ronald D. Smith | year = 1973 | collvol = 5 | collvolpages = 855 | prep = cv5p0855}}</ref> Treatment of zinc powder with [[copper(II) acetate monohydrate]] in hot [[acetic acid]] is reportedly highly reproducible.<ref>{{cite journal | title = Cyclopropanes from an Easily Prepared, Highly Active Zinc-Copper Couple, Dibromomethane, and Olefins | author = Eugene LeGoff | journal = [[J. Org. Chem.]] | year = 1964 | volume = 29 | pages = 2048–2050 | doi = 10.1021/jo01030a529 | issue = 7}}</ref> The couple may also be generated in situ by reaction of one equivalent of zinc dust with one equivalent of [[copper(I) chloride]] (or copper powder) in refluxing [[diethyl ether|ether]].<ref>{{cite journal | title = A Convenient Procedure for the Methylenation of Olefins to Cyclopropanes | author = Robert J. Rawson, Ian T. Harrison | journal = [[J. Org. Chem.]] | year = 1970 | volume = 35 | issue = 6 | pages = 2057–2058 | doi = 10.1021/jo00831a091}}</ref> |
||
The choice of method is dictated primarily by the application. The development of newer methods was motivated by the need for zinc–copper couple with reproducible behavior. |
The choice of method is dictated primarily by the application. The development of newer methods was motivated by the need for zinc–copper couple with reproducible behavior. |
||
| Line 15: | Line 15: | ||
:[[File:Cyclopropanation of Alkene.svg]] |
:[[File:Cyclopropanation of Alkene.svg]] |
||
The couple has also been employed to generate alkyl zinc reagents for [[conjugate addition]], as a dehalogenating reagent, as a promoter of reductive coupling of [[carbonyl compounds]], and to [[redox|reduce]] electron-deficient [[alkenes]] and [[alkynes]]. [[Sonication]] has been employed to enhance the rate of the zinc–copper couple-mediated [[cycloaddition]] of α,α’-dibromo [[ketones]] to [[1,3-dienes]].<ref>{{cite journal | title = Ultrasonics in the Metal Promoted Cycloaddition of α, |
The couple has also been employed to generate alkyl zinc reagents for [[conjugate addition]], as a dehalogenating reagent, as a promoter of reductive coupling of [[carbonyl compounds]], and to [[redox|reduce]] electron-deficient [[alkenes]] and [[alkynes]]. [[Sonication]] has been employed to enhance the rate of the zinc–copper couple-mediated [[cycloaddition]] of α,α’-dibromo [[ketones]] to [[1,3-dienes]].<ref>{{cite journal | title = Ultrasonics in the Metal Promoted Cycloaddition of α,α'-dibromo ketones to 1,3-dienes | author = Navalkishore N. Joshi, H. Martin R. Hoffmann | journal = [[Tetrahedron Letters]] | year = 1986 | volume = 27 | issue = 6 | pages = 687–690 | doi = 10.1016/S0040-4039(00)84073-3}}</ref> |
||
:[[File:Cycloaddition with Dibromoketone.svg]] |
:[[File:Cycloaddition with Dibromoketone.svg]] |
||
Revision as of 06:08, 17 December 2021
Zinc–copper couple is an alloy of zinc and copper that is employed as a reagent in organic synthesis. The “couple” was popularized after the report by Simmons and Smith in 1959 of its application as an activated source of zinc required for formation of an organozinc reagent in the Simmons–Smith cyclopropanation of alkenes.[1] The couple has been widely applied as a reagent in other reactions requiring activated zinc metal. Zinc–copper couple does not refer to a rigorously defined chemical structure or alloy composition. The couple may contain varying proportions of copper and zinc; the zinc content is typically greater than 90%, although an alloy containing similar proportions of zinc and copper is used in some cases. The couple is frequently prepared as a darkly-colored powder and is slurried in an ethereal solvent prior to being used in slight excess relative to the substrate. Activation of zinc by copper is essential to the couple’s utility, but the origin of this effect is poorly documented. It is speculated that copper enhances reactivity of zinc at the surface of the alloy.[2]
Synthesis
Zinc–copper couple has been prepared by numerous methods, which vary mainly with respect to the source of copper, but also by the ratio of copper to zinc, the physical state of the zinc (e.g. powder or granules), the use of protic acids and other additives, and temperature of the preparation. Most often the couple is generated and isolated prior to use, but routes have been described to storable forms of the alloy. Most methods involve reduction of an oxidized copper species with zinc, which is used in excess.
An early method for the synthesis of zinc–copper couple entailed treatment of a mixture of zinc dust and copper(II) oxide with hydrogen gas at 500 °C.[1] A more convenient and cheaper method proceeds by treatment of zinc powder with hydrochloric acid and copper(II) sulfate.[3] Treatment of zinc powder with copper(II) acetate monohydrate in hot acetic acid is reportedly highly reproducible.[4] The couple may also be generated in situ by reaction of one equivalent of zinc dust with one equivalent of copper(I) chloride (or copper powder) in refluxing ether.[5]
The choice of method is dictated primarily by the application. The development of newer methods was motivated by the need for zinc–copper couple with reproducible behavior.
Application
Zinc–copper couple has found widespread use in organic synthesis, especially in the Simmons–Smith cyclopropanation of alkenes. In this process, the couple (typically a slurry in an ethereal solvent) reacts with methylene iodide to generate iodomethylzinc iodide, which is the intermediate responsible for cyclopropanation.
The couple has also been employed to generate alkyl zinc reagents for conjugate addition, as a dehalogenating reagent, as a promoter of reductive coupling of carbonyl compounds, and to reduce electron-deficient alkenes and alkynes. Sonication has been employed to enhance the rate of the zinc–copper couple-mediated cycloaddition of α,α’-dibromo ketones to 1,3-dienes.[6]
See also
References
- ^ a b Howard H. Simmons, Ronald D. Smith (1959). "A New Synthesis of Cyclopropanes". J. Am. Chem. Soc. 81 (16): 4256–4264. doi:10.1021/ja01525a036.
- ^ Scott D. Rychnovsky, Jay P. Powers (2001). "Zinc/Copper Couple". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rz011. ISBN 0-471-93623-5.
- ^ Howard H. Simmons, Ronald D. Smith (1973). "Norcarane". Organic Syntheses; Collected Volumes, vol. 5, p. 855.
- ^ Eugene LeGoff (1964). "Cyclopropanes from an Easily Prepared, Highly Active Zinc-Copper Couple, Dibromomethane, and Olefins". J. Org. Chem. 29 (7): 2048–2050. doi:10.1021/jo01030a529.
- ^ Robert J. Rawson, Ian T. Harrison (1970). "A Convenient Procedure for the Methylenation of Olefins to Cyclopropanes". J. Org. Chem. 35 (6): 2057–2058. doi:10.1021/jo00831a091.
- ^ Navalkishore N. Joshi, H. Martin R. Hoffmann (1986). "Ultrasonics in the Metal Promoted Cycloaddition of α,α'-dibromo ketones to 1,3-dienes". Tetrahedron Letters. 27 (6): 687–690. doi:10.1016/S0040-4039(00)84073-3.