Bis(pinacolato)diboron: Difference between revisions
Use as reagent |
Sorry, added to "reactions". |
||
| Line 29: | Line 29: | ||
}} |
}} |
||
}} |
}} |
||
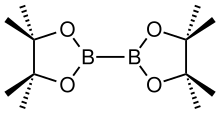
'''Bis(pinacolato)diboron''' is a [[covalent compound]] containing two [[boron]] atoms and two [[pinacol]]ato ligands. It has the formula [(CH<sub>3</sub>)<sub>4</sub>C<sub>2</sub>O<sub>2</sub>B]<sub>2</sub>; the pinacol groups are sometimes abbreviated as "pin", so the structure is sometimes represented as B<sub>2</sub>pin<sub>2</sub>. It is a colourless solid that is soluble in organic solvents. It is a commercially available reagent for making [[pinacol boronic ester]]s for [[organic synthesis]]. Unlike some other diboron compounds, B<sub>2</sub>pin<sub>2</sub> is not moisture-sensitive and can be handled in air.<ref name=OS/> |
'''Bis(pinacolato)diboron''' is a [[covalent compound]] containing two [[boron]] atoms and two [[pinacol]]ato ligands. It has the formula [(CH<sub>3</sub>)<sub>4</sub>C<sub>2</sub>O<sub>2</sub>B]<sub>2</sub>; the pinacol groups are sometimes abbreviated as "pin", so the structure is sometimes represented as B<sub>2</sub>pin<sub>2</sub>. It is a colourless solid that is soluble in organic solvents. It is a commercially available reagent for making [[pinacol boronic ester]]s for [[organic synthesis]]. Unlike some other diboron compounds, B<sub>2</sub>pin<sub>2</sub> is not moisture-sensitive and can be handled in air.<ref name=OS/> |
||
==Preparation and structure== |
==Preparation and structure== |
||
| Line 43: | Line 43: | ||
:CH<sub>3</sub>(CH<sub>2</sub>)<sub>6</sub>CH<sub>3</sub> + [pinB]<sub>2</sub> → pinBH + CH<sub>3</sub>(CH<sub>2</sub>)<sub>7</sub>Bpin |
:CH<sub>3</sub>(CH<sub>2</sub>)<sub>6</sub>CH<sub>3</sub> + [pinB]<sub>2</sub> → pinBH + CH<sub>3</sub>(CH<sub>2</sub>)<sub>7</sub>Bpin |
||
These reactions proceed via [[Transition metal boryl complex|boryl complexes]]. |
These reactions proceed via [[Transition metal boryl complex|boryl complexes]]. |
||
Bis(pinacolato)diboron can also be used as reducing agent for example in transition metal catalyzed hydrogenations of alkenes and alkynes. <ref>https://www.organic-chemistry.org/chemicals/reductions/bis(pinacolato)diboron-B2pin2.shtm</ref> |
|||
==References== |
==References== |
||
Revision as of 07:59, 18 December 2021
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
Octamethyl-2,2′-bi-1,3,2-dioxaborolane | |
| Identifiers | |
3D model (JSmol)
|
|
| Abbreviations | B2pin2 |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.111.245 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C12H24B2O4 | |
| Molar mass | 253.94 g·mol−1 |
| Melting point | 137 to 140 °C (279 to 284 °F; 410 to 413 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Bis(pinacolato)diboron is a covalent compound containing two boron atoms and two pinacolato ligands. It has the formula [(CH3)4C2O2B]2; the pinacol groups are sometimes abbreviated as "pin", so the structure is sometimes represented as B2pin2. It is a colourless solid that is soluble in organic solvents. It is a commercially available reagent for making pinacol boronic esters for organic synthesis. Unlike some other diboron compounds, B2pin2 is not moisture-sensitive and can be handled in air.[1]
Preparation and structure
This compound may be prepared by treating tetrakis(dimethylamino)diboron with pinacol in acidic conditions.[1] The B-B bond length is 1.711(6) Å.
Dehydrogenation of pinacolborane provides an alternative route:[2]
- 2 (CH3)4C2O2BH → (CH3)4C2O2B-BO2C2(CH3)4 + H2
Reactions

The B-B bond adds across alkenes and alkynes to give the 1,2-diborylated alkanes and alkenes. Using various organorhodium or organoiridium catalysts, it can also be installed onto saturated hydrocarbons:[3]
- CH3(CH2)6CH3 + [pinB]2 → pinBH + CH3(CH2)7Bpin
These reactions proceed via boryl complexes. Bis(pinacolato)diboron can also be used as reducing agent for example in transition metal catalyzed hydrogenations of alkenes and alkynes. [4]
References
- ^ a b Tatsuo Ishiyama; Miki Murata; Taka-aki Ahiko & Norio Miyaura (2004). "Bis(pinacolato)diboron". Organic Syntheses; Collected Volumes, vol. 10, p. 115.
- ^ Neeve, Emily C.; Geier, Stephen J.; Mkhalid, Ibraheem A. I.; Westcott, Stephen A.; Marder, Todd B. (2016). "Diboron(4) Compounds: From Structural Curiosity to Synthetic Workhorse". Chemical Reviews. 116 (16): 9091–9161. doi:10.1021/acs.chemrev.6b00193. PMID 27434758.
- ^ Xinyu Liu "Bis(pinacolato)diboron" Synlett 2003, pp 2442–2443. doi:10.1055/s-2003-43344
- ^ https://www.organic-chemistry.org/chemicals/reductions/bis(pinacolato)diboron-B2pin2.shtm
