Disulfur monoxide: Difference between revisions
→As a ligand: Hapticity is same for all |
m v2.04b - Bot T20 CW#61 - Fix errors for CW project (Reference before punctuation) |
||
| Line 83: | Line 83: | ||
===As a ligand === |
===As a ligand === |
||
Disulfur monoxide occurs as a [[ligand]] bound to [[transition metal]]s, typically with [[hapticity]] 2.<ref name=":0">{{Cite journal|last=Urove|first=Greg A.|last2=Welker|first2=Mark E.|date=April 1988|title=Synthesis of a stable disulfur monoxide precursor and trapping of disulfur monoxide with transition-metal complexes|url=https://pubs.acs.org/doi/pdf/10.1021/om00094a037|journal=Organometallics|volume=7|issue=4|pages=1013–1014|doi=10.1021/om00094a037|issn=0276-7333}}</ref> Examples include {{Chem2|OsCl(NO)(PPh3)2(S2O)}};<ref>{{Cite book|last=Pandey|first=Krishna K.|url=https://books.google.com/books?id=-mtAYfs9B3YC&pg=PA492|title=Progress in Inorganic Chemistry|date=2009-09-17|publisher=John Wiley & Sons|isbn=978-0-470-16698-7|editor-last=Lippard|editor-first=Stephen J.|volume=80|pages=492|language=en|chapter=Coordination chemistry of thionitrosyl ({{chem|N|S}}), thiazate ({{chem|N|S|O|-}}), disulfidothionitrate ({{chem|S|3|N|-}}), sulfur monoxide ({{chem|S|O}}), and disulfur monoxide ({{chem|S|2|O}}) ligands}}</ref> {{Chem2|[Ir(PPh2)2(S2O)]+}}; and {{Chem2|MeCpMn(CO2)(S2O)}}<ref name=":0" /> |
Disulfur monoxide occurs as a [[ligand]] bound to [[transition metal]]s, typically with [[hapticity]] 2.<ref name=":0">{{Cite journal|last=Urove|first=Greg A.|last2=Welker|first2=Mark E.|date=April 1988|title=Synthesis of a stable disulfur monoxide precursor and trapping of disulfur monoxide with transition-metal complexes|url=https://pubs.acs.org/doi/pdf/10.1021/om00094a037|journal=Organometallics|volume=7|issue=4|pages=1013–1014|doi=10.1021/om00094a037|issn=0276-7333}}</ref> Examples include {{Chem2|OsCl(NO)(PPh3)2(S2O)}};<ref>{{Cite book|last=Pandey|first=Krishna K.|url=https://books.google.com/books?id=-mtAYfs9B3YC&pg=PA492|title=Progress in Inorganic Chemistry|date=2009-09-17|publisher=John Wiley & Sons|isbn=978-0-470-16698-7|editor-last=Lippard|editor-first=Stephen J.|volume=80|pages=492|language=en|chapter=Coordination chemistry of thionitrosyl ({{chem|N|S}}), thiazate ({{chem|N|S|O|-}}), disulfidothionitrate ({{chem|S|3|N|-}}), sulfur monoxide ({{chem|S|O}}), and disulfur monoxide ({{chem|S|2|O}}) ligands}}</ref> {{Chem2|[Ir(PPh2)2(S2O)]+}}; and {{Chem2|MeCpMn(CO2)(S2O)}}.<ref name=":0" /> These complexes are closely related to [[transition metal sulfur dioxide complex]]es. |
||
==Reactions== |
==Reactions== |
||
Revision as of 00:55, 20 January 2022
| |
| |
| Names | |
|---|---|
| Other names
sulfur suboxide; sulfuroxide;
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| Properties | |
| S2O | |
| Molar mass | 80.1294 g/mol[1] |
| Appearance | colourless gas or dark red solid[2] |
| Structure | |
| bent | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
toxic |
| Related compounds | |
Related compounds
|
Trisulfur SO Ozone SO2 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Disulfur monoxide or sulfur suboxide is an inorganic compound with formula S2O, one of the lower sulfur oxides. It is a colourless gas and condenses to give a roughly sea green-colored solid that is unstable at room temperature.
S
2O occurs rarely in natural atmospheres, but can be made by a variety of laboratory procedures. For this reason, its spectroscopic signature is very well understood.
Structure and spectrum
Condensed solid S2O absorbs at 420 nm (roughly indigo) and 530 nm (roughly lime). These bands have been assigned to decomposition products S3 and S4.[3]
In the ultraviolet, S2O has absorption band systems in the ranges 250–340 nm and 190–240 nm. There are bands at 323.5 and 327.8 nm.[4] The band in the 315–340 nm range is due to the C1A′–X1A′ (π* ← π) transition.[5]
Gaseous disulfur monoxide does not absorb in the visible spectrum.
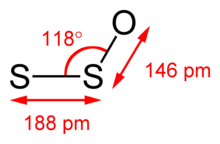
The microwave spectrum of S2O has the following rotational parameters: A = 41915.44 MHz, B = 5059.07 MHz, and C = 4507.19 MHz.[6] Moreover, the microwave spectrum suggests the S−S−O angle is 117.88° with S−S and S−O bond lengths of 188.4 and 146.5 pm, respectively.[7] In the 327.8 nm excited state, the central angle tightens to 109°.[4]
The harmonic frequency for S−S stretching is 415.2 cm−1.[5]
Synthesis
Historical
Disulfur monoxide was discovered by Peter W. Schenk in 1933[8] with a glow discharge though sulfur vapour and sulfur dioxide. He discovered that the gas could survive for hours at single digit pressures of mercury in clean glass, but it decomposed near 30 mmHg (4 kPa). Schenk assigned the formula as SO and called it sulfur monoxide. In 1956, D. J. Meschi and R. J. Myers established the formula as S2O.[9]
Preparation
Oxidizing sulfur with copper(II) oxide:[10][11]
- 3 S8 + 12 CuO → 12 CuS + 4 S2O + 4 SO2
A relatively pure generator is the reaction of thionyl chloride with silver(I) sulfide:[12]
- SOCl2 + Ag2S → 2 AgCl + S2O
Also 5,6-di-tert-butyl-2,3,7-trithiabicyclo[2.2.1]hept-5-ene 2-endo-7-endo-dioxide decomposes upon heating with release of S2O:[13]
Occurrence
Volcanism
Volcanoes on Io produce substantial quantities of S
2O. It can form between 1% and 6% when hot 100-bar S2 and SO2 gas erupts from volcanoes. It is believed that Pele on Io is surrounded by solid S2O.[14]
Terran atmosphere
Disulfur monoxide is too unstable to survive at standard conditions,[8] but transient sources include incomplete combustion of sulfur vapor[15] and thermal decomposition of sulfur dioxide in a glow discharge.[16]
As a ligand
Disulfur monoxide occurs as a ligand bound to transition metals, typically with hapticity 2.[17] Examples include OsCl(NO)(PPh3)2(S2O);[18] [Ir(PPh2)2(S2O)]+; and MeCpMn(CO2)(S2O).[17] These complexes are closely related to transition metal sulfur dioxide complexes.
Reactions
On decomposition at room temperature it forms SO2 via the formation of polysulfur oxides:[16]
- 2 S2O → "S3" + SO2
S
2O reacts with diazoalkanes to form dithiirane 1-oxides.[19]
Further reading
- Possible biological occurrence: Iverson, W. P. (26 May 1967). "Disulfur monoxide: production by Desulfovibrio". Science. 156 (3778): 1112–1114. Bibcode:1967Sci...156.1112I. doi:10.1126/science.156.3778.1112. PMID 6024190. S2CID 3058359.
- Cyclic disulfur monoxide: Lo, Wen-Jui; Wu, Yu-Jong; Lee, Yuan-Pern (September 2003). "Ultraviolet Absorption Spectrum of Cyclic S2O in Solid Ar". The Journal of Physical Chemistry A. 107 (36): 6944–6947. Bibcode:2003JPCA..107.6944L. doi:10.1021/jp034563j.
- Discovery of S2O: Schenk, Peter W. (18 March 1933). "Über das Schwefelmonoxyd" [On sulfur monoxide]. Zeitschrift für Anorganische und Allgemeine Chemie (in German). 211 (1–2): 150–160. doi:10.1002/zaac.19332110117.
References
- ^ a b c "Disulfur monoxide". NIST. 2008.
- ^ Hapke, B.; Graham, F. (May 1989). "Spectral properties of condensed phases of disulfur monoxide, polysulfur oxide, and irradiated sulfur". Icarus. 79 (1): 47. Bibcode:1989Icar...79...47H. doi:10.1016/0019-1035(89)90107-3.
- ^ a b Hallin, K-E. J.; Merer, A. J.; Milton, D. J. (November 1977). "Rotational analysis of bands of the 3400 Å system of disulphur monoxide (S2O)". Canadian Journal of Physics. 55 (21): 1858–1867. Bibcode:1977CaJPh..55.1858H. doi:10.1139/p77-226.
- ^ a b Zhang, Qingguo; Dupré, Patrick; Grzybowski, Bartosz; Vaccaro, Patrick H. (1995). "Laser-induced fluorescence studies of jet-cooled S2O: Axis-switching and predissociation effects". The Journal of Chemical Physics. 103 (1): 67. Bibcode:1995JChPh.103...67Z. doi:10.1063/1.469623.
- ^ Cook, Robert L; Winnewisser, Gisbert; Lindsey, D.C (May 1973). "The centrifugal distortion constants of disulfur monoxide". Journal of Molecular Spectroscopy. 46 (2): 276–284. Bibcode:1973JMoSp..46..276C. doi:10.1016/0022-2852(73)90042-8.
- ^ Meschi, D. J.; Myers, R. J. (1959). "The microwave spectrum, structure, and dipole moment of disulfur monoxide". Journal of Molecular Spectroscopy. 3 (1–6): 405–416. Bibcode:1959JMoSp...3..405M. doi:10.1016/0022-2852(59)90036-0.
- ^ a b Steudel, R. (2003). "Sulfur-Rich Oxides SnO and SnO2". In Steudel, R. (ed.). Elemental Sulfur and Sulfur-Rich Compounds II. Berlin/Heidelberg: Springer. ISBN 9783540449515.
- ^ Meschi, David J.; Myers, Rollie J. (30 July 1956). "Disulfur Monoxide. I. Its Identification as the Major Constituent in Schenk's "Sulfur Monoxide"". Journal of the American Chemical Society. 78 (24): 6220. doi:10.1021/ja01605a002.
- ^ Satyanarayana, S. R.; Vasudeva Murthy, A. R. (1964). "Reactions with Disulphur monoxide Solutions Obtained by the Reduction of Cupric Oxide by Elemental Sulphur" (PDF). Proceedings of the Indian Academy of Sciences, Section A. 59 (4): 263–267. doi:10.1007/BF03046440. S2CID 91428580.
- ^ Dodson, R. M.; Srinivasan, V.; Sharma, K. S.; Sauers, Richard F. (July 1972). "Disulfur monoxide. Reaction with dienes". The Journal of Organic Chemistry. 37 (15): 2367–2372. doi:10.1021/jo00980a001. ISSN 0022-3263.
- ^ Schenk, P. W.; Steudel, R. (1964). "Preparation of Pure Disulfur Monoxide". Angewandte Chemie International Edition in English. 3 (1): 61–61. doi:10.1002/anie.196400611. ISSN 1521-3773.
- ^ Nakayama, J.; Aoki, S.; Takayama, J.; Sakamoto, A.; Sugihara, Y.; Ishii, A. (28 July 2004). "Reversible disulfur monoxide (S2O)-forming retro-Diels–Alder reaction. disproportionation of S2O to trithio-ozone (S3) and sulfur dioxide (SO2) and reactivities of S2O and S3". Journal of the American Chemical Society. 126 (29): 9085–9093. doi:10.1021/ja047729i. PMID 15264842.
- ^ Zolotov, Mikhail Yu.; Fegley, Bruce (9 March 1998). "Volcanic Origin of Disulfur Monoxide (S2O) on Io" (PDF). Icarus. 133 (2): 293. Bibcode:1998Icar..133..293Z. doi:10.1006/icar.1998.5930.
- ^ Khan, Ashikur R. (August 1999). Experimental Studies of the Homogenous Conversion of Sulfur Di-Oxide to Sulfur Tri-Oxide via Natural Gas Reburning (Thesis). Ohio University. p. 8.
Other sulfur oxides are sulfur monoxide, SO, its dimer, (SO)
z,and disulfur monoxide, S
2O. These are too unstable or reactive to appear as products of combustion in the ordinary sense, but they are known to occur as intermediates in appropriate circumstances. - ^ a b Cotton and Wilkinson (1966). Advanced Inorganic Chemistry: A Comprehensive Treatise. p. 540.
- ^ a b Urove, Greg A.; Welker, Mark E. (April 1988). "Synthesis of a stable disulfur monoxide precursor and trapping of disulfur monoxide with transition-metal complexes". Organometallics. 7 (4): 1013–1014. doi:10.1021/om00094a037. ISSN 0276-7333.
- ^ Pandey, Krishna K. (2009-09-17). "Coordination chemistry of thionitrosyl (NS), thiazate (NSO−
), disulfidothionitrate (S
3N−
), sulfur monoxide (SO), and disulfur monoxide (S
2O) ligands". In Lippard, Stephen J. (ed.). Progress in Inorganic Chemistry. Vol. 80. John Wiley & Sons. p. 492. ISBN 978-0-470-16698-7. - ^ Ishii, A.; Kawai, T.; Tekura, K.; Oshida, H.; Nakayama, J. (18 May 2001). "A Convenient Method for the Generation of a Disulfur Monoxide Equivalent and Its Reaction with Diazoalkanes to Yield Dithiirane 1-Oxides". Angewandte Chemie International Edition. 40 (10): 1924–1926. doi:10.1002/1521-3773(20010518)40:10<1924::AID-ANIE1924>3.0.CO;2-F. PMID 11385674.

