Halogenation: Difference between revisions
No edit summary |
m Reverted 3 edits by 141.217.174.200 identified as vandalism to last revision by Thijs!bot. |
||
| Line 1: | Line 1: | ||
'''Halogenation''' is a [[chemical reaction]] that replaces a [[hydrogen]] atom with a [[halogen]] atom. More specific descriptions exist that specify the type of halogen: fluorination, chlorination, bromination, and iodination. |
|||
Dani loves mechanisms. Especially SN1. |
|||
In a [[Markovnikov's rule|Markovnikov addition]] reaction, a halogen like [[bromine]] is reacted with an [[alkene]] which causes the [[pi bond|π-bond]] to break forming an [[haloalkane]]. This makes the [[hydrocarbon]] more reactive and [[bromine]] as it turns out, is a good [[leaving group]] in further chemical reactions such as [[nucleophilic aliphatic substitution]] reactions and [[elimination reaction]]s |
|||
Several types of halogenation exist, including: |
|||
* [[Free radical halogenation]] |
|||
* [[Ketone halogenation]] |
|||
* [[Electrophilic halogenation]] |
|||
* [[Halogen addition reaction]] |
|||
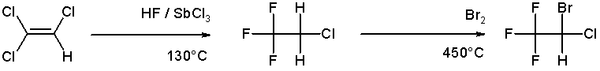
An example of halogenation can be found in the [[organic synthesis]] of the anesthetic [[halothane]] from [[trichloroethylene]] which involves a high temperature bromination in the second step <ref>''Synthesis of essential drugs'', Ruben Vardanyan, Victor Hruby; Elsevier 2005 ISBN 0-444-52166-6</ref>: |
|||
[[Image:Halothane synthesis.png|center|600px|Halothane synthesis]] |
|||
== Halogenation of Hydrocarbons == |
|||
It has been shown that the reaction of bromine water, for instance, in ethene caused the formation of 2-bromoethanol. This formation of alcohol which has the same trends for other aliphatic hydrocarbons yielded the colorless mixture formed. The case was different in benzene and cyclohexane. When bromine water reacted with toluene it yielded a bromophenol which caused the faint yellow color of the upper layer. |
|||
==See also== |
|||
*[[Haloalkane]] (Alkyl halide) |
|||
*[[Halogenoarene]] (Aryl halide) |
|||
*[[Haloketone]] |
|||
*[[Electrophilic substitution]] |
|||
==References== |
|||
<references /> |
|||
[[Category:Organic reactions]] |
|||
[[Category:Inorganic reactions]] |
|||
[[he:הלוגנציה]] |
|||
[[no:Halogenering]] |
|||
[[pt:Halogenação]] |
|||
[[sk:Chlorácia]] |
|||
[[sv:Halogenering]] |
|||
[[zh:卤化]] |
|||
Revision as of 17:29, 12 February 2007
Halogenation is a chemical reaction that replaces a hydrogen atom with a halogen atom. More specific descriptions exist that specify the type of halogen: fluorination, chlorination, bromination, and iodination.
In a Markovnikov addition reaction, a halogen like bromine is reacted with an alkene which causes the π-bond to break forming an haloalkane. This makes the hydrocarbon more reactive and bromine as it turns out, is a good leaving group in further chemical reactions such as nucleophilic aliphatic substitution reactions and elimination reactions
Several types of halogenation exist, including:
An example of halogenation can be found in the organic synthesis of the anesthetic halothane from trichloroethylene which involves a high temperature bromination in the second step [1]:
Halogenation of Hydrocarbons
It has been shown that the reaction of bromine water, for instance, in ethene caused the formation of 2-bromoethanol. This formation of alcohol which has the same trends for other aliphatic hydrocarbons yielded the colorless mixture formed. The case was different in benzene and cyclohexane. When bromine water reacted with toluene it yielded a bromophenol which caused the faint yellow color of the upper layer.
See also
- Haloalkane (Alkyl halide)
- Halogenoarene (Aryl halide)
- Haloketone
- Electrophilic substitution
References
- ^ Synthesis of essential drugs, Ruben Vardanyan, Victor Hruby; Elsevier 2005 ISBN 0-444-52166-6
