Duvelisib: Difference between revisions
update ib |
Rescuing 1 sources and tagging 0 as dead.) #IABot (v2.0.8.8) (Whywhenwhohow - 10054 |
||
| Line 127: | Line 127: | ||
Duvelisib is intended to be used in patients who have received at least two prior systemic therapies, and carries a [[black box warning]] due to the risk of fatal/serious toxicities: infections, diarrhea or colitis, cutaneous reactions and pneumonitis.<ref>{{cite news|last1=Carroll|first1=John| name-list-style = vanc |title=Unwanted by AbbVie and Infinity, battered Verastem gets an OK for duvelisib and a second shot at success |url=https://endpts.com/unwanted-by-abbvie-and-infinity-verastem-gets-an-ok-for-duvelisib-and-a-second-shot-at-success/ |work=Endpoints News|date=24 September 2018}}</ref> |
Duvelisib is intended to be used in patients who have received at least two prior systemic therapies, and carries a [[black box warning]] due to the risk of fatal/serious toxicities: infections, diarrhea or colitis, cutaneous reactions and pneumonitis.<ref>{{cite news|last1=Carroll|first1=John| name-list-style = vanc |title=Unwanted by AbbVie and Infinity, battered Verastem gets an OK for duvelisib and a second shot at success |url=https://endpts.com/unwanted-by-abbvie-and-infinity-verastem-gets-an-ok-for-duvelisib-and-a-second-shot-at-success/ |work=Endpoints News|date=24 September 2018}}</ref> |
||
On 25 March 2021, the [[Committee for Medicinal Products for Human Use]] (CHMP) of the [[European Medicines Agency]] (EMA) adopted a positive opinion, recommending the granting of a marketing authorization for the medicinal product Copiktra, intended for the treatment of adults with relapsed or refractory chronic lymphocytic leukaemia (CLL) and refractory follicular lymphoma (FL).<ref name="Copiktra: Pending EC decision" /> The applicant for this medicinal product is Verastem Europe GmbH.<ref name="Copiktra: Pending EC decision">{{cite web | title=Copiktra: Pending EC decision | website=[[European Medicines Agency]] (EMA) | date=26 March 2021 | url=https://www.ema.europa.eu/en/medicines/human/summaries-opinion/copiktra | access-date=26 March 2021}} Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.</ref> Duvelisib was approved for medical use in the European Union in May 2021.<ref name="Copiktra EPAR" /> |
On 25 March 2021, the [[Committee for Medicinal Products for Human Use]] (CHMP) of the [[European Medicines Agency]] (EMA) adopted a positive opinion, recommending the granting of a marketing authorization for the medicinal product Copiktra, intended for the treatment of adults with relapsed or refractory chronic lymphocytic leukaemia (CLL) and refractory follicular lymphoma (FL).<ref name="Copiktra: Pending EC decision" /> The applicant for this medicinal product is Verastem Europe GmbH.<ref name="Copiktra: Pending EC decision">{{cite web | title=Copiktra: Pending EC decision | website=[[European Medicines Agency]] (EMA) | date=26 March 2021 | url=https://www.ema.europa.eu/en/medicines/human/summaries-opinion/copiktra | access-date=26 March 2021 | archive-date=26 March 2021 | archive-url=https://web.archive.org/web/20210326111756/https://www.ema.europa.eu/en/medicines/human/summaries-opinion/copiktra | url-status=dead }} Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.</ref> Duvelisib was approved for medical use in the European Union in May 2021.<ref name="Copiktra EPAR" /> |
||
== References == |
== References == |
||
Revision as of 16:43, 2 June 2022
 | |
| Clinical data | |
|---|---|
| Pronunciation | doo-VE-li-SIB |
| Trade names | Copiktra |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a618056 |
| License data | |
| Routes of administration | By mouth (capsules) |
| Drug class | PI3-Kinase inhibitor |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Metabolism | mainly metabolized by CYP3A4[2] |
| Onset of action | 1-2 hours after initial administration |
| Elimination half-life | 5.2 to 10.9 hours |
| Excretion | Feces (79%), urine (14%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.245.560 |
| Chemical and physical data | |
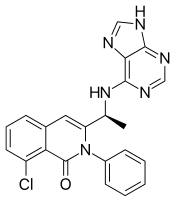
| Formula | C22H17ClN6O |
| Molar mass | 416.87 g·mol−1 |
| 3D model (JSmol) | |
| |
Duvelisib, sold under the brand name Copiktra, is a medication used to treat chronic lymphocytic leukemia (CLL), small lymphocytic lymphoma (SLL), and follicular lymphoma after other treatments have failed.[4] It is taken by mouth.[4]
Common side effects include diarrhea, low white blood cells, rash, feeling tired, fever, and muscle pains.[4] Other serious side effects include inflammation of the lungs and infections.[4] It is a dual inhibitor of PI3Kδ and PI3Kγ.[5] Duvelisib is marketed by Secura Bio.[2][6]
Medical uses
It is used to treat chronic lymphocytic leukemia (CLL), small lymphocytic lymphoma (SLL), and follicular lymphoma after other treatments have failed.[4] Further trials are ongoing to confirm benefits as of 2019.[4]
Mechanism of action
Duvelisib is a Phosphoinositide 3-kinase inhibitor, specifically of the delta and gamma isoforms of PI3K.[7] This class of compounds works by preventing PI3K from playing its role in transducing signals from outside of cells into various intracellular pathways involved in cell cycle regulation, apoptosis, DNA repair, senescence, angiogenesis and cell metabolism, including the PI3K/AKT/mTOR pathway.[7]
History
Duvelisib, also known as IPI-145, was discovered by Intellikine,[8] a company founded in September 2007 based on biochemistry research from the lab of Kevan Shokat at the University of California San Francisco.[9]
In mid-June 2016, Infinity announced results of Phase II clinical trial of duvelisib.[7]
In November 2016, Infinity exclusively licensed the worldwide rights to duvelisib to Verastem Oncology for little money compared to earlier deals; the deal included no upfront payment, a $6 million milestone for success in a Phase 3 trial in chronic lymphocytic leukemia, a $22 million payment for an FDA approval, and royalties.[10]
Duvelisib has received orphan drug designation in the United States for treatment of peripheral T-cell lymphoma (PTCL) in 2019.[11]
In September 2020, duvelisib was sold by Verastem to Secura Bio, Inc. for $70 million and additional payments based on milestones and royalties.[6]
Society and culture
Legal status
In April 2018, Verastem filed a New Drug Application (NDA) for duvelisib for the treatment of relapsed or refractory chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) and accelerated approval for relapsed or refractory follicular lymphoma (FL). The FDA approved the application in September 2018.[12][13] In April 2022, the FDA withdrew the approval of Duvelisib for relapsed or refractory FL on request of its then owner, Secura Bio.[14]
Duvelisib is intended to be used in patients who have received at least two prior systemic therapies, and carries a black box warning due to the risk of fatal/serious toxicities: infections, diarrhea or colitis, cutaneous reactions and pneumonitis.[15]
On 25 March 2021, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion, recommending the granting of a marketing authorization for the medicinal product Copiktra, intended for the treatment of adults with relapsed or refractory chronic lymphocytic leukaemia (CLL) and refractory follicular lymphoma (FL).[16] The applicant for this medicinal product is Verastem Europe GmbH.[16] Duvelisib was approved for medical use in the European Union in May 2021.[3]
References
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 Oct 2023.
- ^ a b c "Copiktra- duvelisib capsule". DailyMed. Retrieved 11 November 2021.
- ^ a b "Copiktra EPAR". European Medicines Agency. 24 March 2021. Retrieved 11 November 2021.
- ^ a b c d e f "Duvelisib Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Retrieved 28 February 2019.
- ^ "duvelisib (Rx)". Medscape. Retrieved 24 September 2018.
- ^ a b "Verastem Oncology Announces Closing of Copiktra (duvelisib) Sale to Secura Bio".
- ^ a b c Anastasia A, Rossi G (1 November 2016). "Novel Drugs in Follicular Lymphoma". Mediterranean Journal of Hematology and Infectious Diseases. 8 (1): e2016061. doi:10.4084/MJHID.2016.061. PMC 5111511. PMID 27872741.
- ^ "Duvelisib". AdisInsight. Retrieved 11 January 2017.
- ^ Timmerman L (20 December 2011). "Millennium: Takeda Acquires San Diego's Intellikine for $190M Upfront". Xconomy.
- ^ Fidler B (2 November 2016). "Verastem Takes a Low-Cost Flier on Infinity's Blood Cancer Drug". Xconomy.
- ^ "Copiktra Receives FDA's Orphan Drug Status for T-cell Lymphoma Treatment". Lymphoma News Today. Retrieved 5 November 2019.
- ^ "Duvelisib (Copiktra, Verastem, Inc.) for adult patients with relapsed or refractory chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL)". FDA. 24 September 2018.
- ^ "FDA Accepts New Drug Application for Duvelisib and Grants Priority Review". 2018-07-07.
- ^ "Secura Bio, Inc.; Withdrawal of Approval of Relapsed or Refractory Follicular Lymphoma Indication for COPIKTRA". 2022-04-13. Retrieved 2022-05-03.
- ^ Carroll J (24 September 2018). "Unwanted by AbbVie and Infinity, battered Verastem gets an OK for duvelisib and a second shot at success". Endpoints News.
- ^ a b "Copiktra: Pending EC decision". European Medicines Agency (EMA). 26 March 2021. Archived from the original on 26 March 2021. Retrieved 26 March 2021. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
External links
- "Duvelisib". Drug Information Portal. U.S. National Library of Medicine.
- "Duvelisib". National Cancer Institute. 17 October 2018.
- "Duvelisib". NCI Drug Dictionary. National Cancer Institute.
