Sulfurous acid: Difference between revisions
Bernardirfan (talk | contribs) Clarification |
No edit summary Tags: Reverted Visual edit Mobile edit Mobile web edit |
||
| Line 1: | Line 1: | ||
{{short description|Chemical compound}} |
{{short description|Chemical compound}} |
||
{{distinguish|Sulfuric acid}} |
{{distinguish|Sulfuric acid}}Jindgi ma pahali bar google par Eidit kiya ha{{chembox |
||
{{chembox |
|||
| verifiedrevid = 470482579 |
| verifiedrevid = 470482579 |
||
| Name = Sulfurous acid |
| Name = Sulfurous acid |
||
Revision as of 15:52, 21 August 2022
Jindgi ma pahali bar google par Eidit kiya ha
| |
| |
| Names | |
|---|---|
| IUPAC name
Sulfurous acid
| |
| Other names
Sulfuric(IV) acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.029.066 |
| EC Number |
|
| 1458 | |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
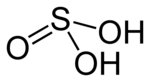
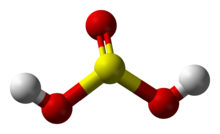
| H2SO3 | |
| Molar mass | 82.07 g/mol |
| Acidity (pKa) | 1.857, 7.172 |
| Conjugate base | Bisulfite |
| Hazards | |
| GHS labelling: | |
 
| |
| Danger | |
| H314, H332 | |
| P260, P261, P264, P271, P280, P301+P330+P331, P303+P361+P353, P304+P312, P304+P340, P305+P351+P338, P310, P312, P321, P363, P405, P501 | |
| Flash point | Non-flammable |
| Safety data sheet (SDS) | ICSC 0074 |
| Related compounds | |
Related compounds
|
Sulfur dioxide Sulfuric acid Selenous acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Sulfurous acid (also Sulfuric(IV) acid, Sulphurous acid (UK), Sulphuric(IV) acid (UK)) is the chemical compound with the formula H2SO3. There is no evidence that sulfurous acid exists in solution, but the molecule has been detected in the gas phase.[1] The conjugate bases of this elusive acid are, however, common anions, bisulfite (or hydrogen sulfite) and sulfite. Sulfurous acid is an intermediate species in the formation of acid rain from sulfur dioxide.[2]
Raman spectra of solutions of sulfur dioxide in water show only signals due to the SO2 molecule and the bisulfite ion, HSO−3.[3] The intensities of the signals are consistent with the following equilibrium:
- SO2 + H2O ⇌ HSO−3 + H+ Ka = 1.54×10−2; pKa = 1.81.
17O NMR spectroscopy provided evidence that solutions of sulfurous acid and protonated sulfites contain a mixture of isomers, which is in equilibrium:[4]
- [H−OSO2]− ⇌ [H−SO3]−
When trying to concentrate the solution by evaporation to produce waterless sulfurous acid it will decompose (reversing the forming reaction). In cooling down a clathrate 4SO2·23H2O will crystallise which decomposes again at 7 °C. Thus sulfurous acid H2SO3 cannot be isolated.
Sulfurous acid can be obtained by dissolving sulfur dioxide in water.
Uses
Aqueous solutions of sulfur dioxide, which sometimes are referred to as sulfurous acid, are used as reducing agents and as disinfectants, as are solutions of bisulfite and sulfite salts. They are oxidised to sulfuric acid or sulfate by accepting another oxygen atom.[5]
See also
References
- ^ D. Sülzle; M. Verhoeven; J. K. Terlouw; H. Schwarz (1988). "Generation and Characterization of Sulfurous Acid (H2SO3) and of Its Radical Cation as Stable Species in the Gas Phase". Angew. Chem. Int. Ed. Engl. 27 (11): 1533–4. doi:10.1002/anie.198815331.
- ^ McQuarrie; Rock (1987). General Chemistry (2nd ed.). New York: W.H. Freeman and Company. p. 243. ISBN 0-7167-1806-5.
- ^ Jolly, William L. (1991). Modern Inorganic Chemistry (2nd ed.). New York: McGraw-Hill. ISBN 0-07-032768-8.
- ^ Catherine E. Housecroft; Alan G. Sharpe (2008). "Chapter 16: The group 16 elements". Inorganic Chemistry, 3rd Edition. Pearson. p. 520. ISBN 978-0-13-175553-6.
- ^ L. Kolditz, Anorganische Chemie, VEB Deutscher Verlag der Wissenschaften, Berlin 1983, S. 476.
