Bisabolol: Difference between revisions
Just wanted to remove the word 'perceived' but ended up doing some research on this terpene and added some more info/cleaned up some other parts |
Didn't read well, fixed (this was actually a previous sentence I wrote some months ago) |
||
| Line 29: | Line 29: | ||
'''Bisabolol''', or more formally α-(−)-bisabolol or also known as levomenol,<ref>[http://www.omikron-online.de/naturhaus/angebote/info/bisabolo.htm Rohstoff-Lexikon Bisabolol<!-- Bot generated title -->] {{webarchive |url=https://web.archive.org/web/20080220222428/http://www.omikron-online.de/naturhaus/angebote/info/bisabolo.htm |date=February 20, 2008 }}</ref> is a natural monocyclic [[sesquiterpene]] alcohol. It is a colorless viscous oil that is the primary constituent of the [[essential oil]] from [[German chamomile]] (''[[Matricaria recutita]]'') and ''[[Myoporum crassifolium]]''.<ref>[http://www.omikron-online.de/naturhaus/angebote/info/bisab.htm Bisabolol (in english)<!-- Bot generated title -->] {{webarchive |url=https://web.archive.org/web/20071010110347/http://www.omikron-online.de/naturhaus/angebote/info/bisab.htm |date=October 10, 2007 }}</ref> High concentrations of bisabolol can also be found in certain [[medicinal cannabis]] cultivars. It is poorly soluble in water and [[glycerin]], but soluble in [[ethanol]].<ref name=UllmannEgg>{{cite encyclopedia|authors=M. Eggersdorfer|title=Terpenes|encyclopedia=Ullmann's Encyclopedia of Industrial Chemistry|year=2005|publisher=Wiley-VCH|place=Weinheim|doi=10.1002/14356007.a26_205|isbn=3527306730}}</ref> The [[enantiomer]], α-(+)-bisabolol, is also found naturally but is rare. Synthetic bisabolol is usually a [[racemic]] mixture of the two, α-(±)-bisabolol. It is the [[terpenoid]] responsible for distinctive aroma of [[chamomile]] flowers, and when isolated, its scent has also has been likened to apples, sugar and honey. |
'''Bisabolol''', or more formally α-(−)-bisabolol or also known as levomenol,<ref>[http://www.omikron-online.de/naturhaus/angebote/info/bisabolo.htm Rohstoff-Lexikon Bisabolol<!-- Bot generated title -->] {{webarchive |url=https://web.archive.org/web/20080220222428/http://www.omikron-online.de/naturhaus/angebote/info/bisabolo.htm |date=February 20, 2008 }}</ref> is a natural monocyclic [[sesquiterpene]] alcohol. It is a colorless viscous oil that is the primary constituent of the [[essential oil]] from [[German chamomile]] (''[[Matricaria recutita]]'') and ''[[Myoporum crassifolium]]''.<ref>[http://www.omikron-online.de/naturhaus/angebote/info/bisab.htm Bisabolol (in english)<!-- Bot generated title -->] {{webarchive |url=https://web.archive.org/web/20071010110347/http://www.omikron-online.de/naturhaus/angebote/info/bisab.htm |date=October 10, 2007 }}</ref> High concentrations of bisabolol can also be found in certain [[medicinal cannabis]] cultivars. It is poorly soluble in water and [[glycerin]], but soluble in [[ethanol]].<ref name=UllmannEgg>{{cite encyclopedia|authors=M. Eggersdorfer|title=Terpenes|encyclopedia=Ullmann's Encyclopedia of Industrial Chemistry|year=2005|publisher=Wiley-VCH|place=Weinheim|doi=10.1002/14356007.a26_205|isbn=3527306730}}</ref> The [[enantiomer]], α-(+)-bisabolol, is also found naturally but is rare. Synthetic bisabolol is usually a [[racemic]] mixture of the two, α-(±)-bisabolol. It is the [[terpenoid]] responsible for distinctive aroma of [[chamomile]] flowers, and when isolated, its scent has also has been likened to apples, sugar and honey. |
||
Bisabolol has a weak sweet floral aroma and is used in various fragrances. It has also been used for hundreds of years in cosmetics because of its skin healing properties including reducing wrinkles, skin toughness and repairing sun-damaged skin, and more recently it has been compounded with [[tretinoin]] as a topical treatment for [[acne]].<ref name=USPharmDOTcom>{{cite encyclopedia|authors=Loyd V. Allen, Jr.|title=Tretinoin 0.5 mg/g and α-Bisabolol 1 mg/g Gel and discussion on its use|year=2013|url=https://www.uspharmacist.com/article/tretinoin-05-mg-g-and-bisabolol-1-mg-g-gel-42388}}</ref> Bisabolol is known to have anti-irritant, anti-inflammatory, and anti-microbial properties.<ref>{{Cite journal|last1=Rocha|first1=Nayrton Flávio Moura|last2=Rios|first2=Emiliano Ricardo Vasconcelos|last3=Carvalho|first3=Alyne Mara Rodrigues|last4=Cerqueira|first4=Gilberto Santos|last5=Lopes|first5=Amanda de Araújo|last6=Leal|first6=Luzia Kalyne Almeida Moreira|last7=Dias|first7=Marília Leite|last8=de Sousa|first8=Damião Pergentino|last9=de Sousa|first9=Francisca Cléa Florenço|date=December 2011|title=Anti-nociceptive and anti-inflammatory activities of (-)-α-bisabolol in rodents|url=https://pubmed.ncbi.nlm.nih.gov/21870032/|journal=Naunyn-Schmiedeberg's Archives of Pharmacology|volume=384|issue=6|pages=525–533|doi=10.1007/s00210-011-0679-x|issn=1432-1912|pmid=21870032|s2cid=12654188 }}</ref><ref>{{Cite journal|last1=Rodrigues|first1=Fabíola Fernandes Galvão|last2=Colares|first2=Aracélio Viana|last3=Nonato|first3=Carla de Fatima Alves|last4=Galvão-Rodrigues|first4=Fabío Fernandes|last5=Mota|first5=Magaly Lima|last6=Moraes Braga|first6=Maria Flaviana Bezerra|last7=Costa|first7=José Galberto Martins da|date=December 2018|title=In vitro antimicrobial activity of the essential oil from Vanillosmopsis arborea Barker (Asteraceae) and its major constituent, α-bisabolol|url=https://linkinghub.elsevier.com/retrieve/pii/S0882401018308696|journal=Microbial Pathogenesis|language=en|volume=125|pages=144–149|doi=10.1016/j.micpath.2018.09.024|pmid=30219391 |s2cid=52282324 }}</ref> Bisabolol is also demonstrated to enhance the percutaneous absorption of certain molecules and has found use as a [[penetration enhancer]]: an agent used in topical formulations |
Bisabolol has a weak sweet floral aroma and is used in various fragrances. It has also been used for hundreds of years in cosmetics because of its skin healing properties including reducing wrinkles, skin toughness and repairing sun-damaged skin, and more recently it has been compounded with [[tretinoin]] as a topical treatment for [[acne]].<ref name=USPharmDOTcom>{{cite encyclopedia|authors=Loyd V. Allen, Jr.|title=Tretinoin 0.5 mg/g and α-Bisabolol 1 mg/g Gel and discussion on its use|year=2013|url=https://www.uspharmacist.com/article/tretinoin-05-mg-g-and-bisabolol-1-mg-g-gel-42388}}</ref> Bisabolol is known to have anti-irritant, anti-inflammatory, and anti-microbial properties.<ref>{{Cite journal|last1=Rocha|first1=Nayrton Flávio Moura|last2=Rios|first2=Emiliano Ricardo Vasconcelos|last3=Carvalho|first3=Alyne Mara Rodrigues|last4=Cerqueira|first4=Gilberto Santos|last5=Lopes|first5=Amanda de Araújo|last6=Leal|first6=Luzia Kalyne Almeida Moreira|last7=Dias|first7=Marília Leite|last8=de Sousa|first8=Damião Pergentino|last9=de Sousa|first9=Francisca Cléa Florenço|date=December 2011|title=Anti-nociceptive and anti-inflammatory activities of (-)-α-bisabolol in rodents|url=https://pubmed.ncbi.nlm.nih.gov/21870032/|journal=Naunyn-Schmiedeberg's Archives of Pharmacology|volume=384|issue=6|pages=525–533|doi=10.1007/s00210-011-0679-x|issn=1432-1912|pmid=21870032|s2cid=12654188 }}</ref><ref>{{Cite journal|last1=Rodrigues|first1=Fabíola Fernandes Galvão|last2=Colares|first2=Aracélio Viana|last3=Nonato|first3=Carla de Fatima Alves|last4=Galvão-Rodrigues|first4=Fabío Fernandes|last5=Mota|first5=Magaly Lima|last6=Moraes Braga|first6=Maria Flaviana Bezerra|last7=Costa|first7=José Galberto Martins da|date=December 2018|title=In vitro antimicrobial activity of the essential oil from Vanillosmopsis arborea Barker (Asteraceae) and its major constituent, α-bisabolol|url=https://linkinghub.elsevier.com/retrieve/pii/S0882401018308696|journal=Microbial Pathogenesis|language=en|volume=125|pages=144–149|doi=10.1016/j.micpath.2018.09.024|pmid=30219391 |s2cid=52282324 }}</ref> Bisabolol is also demonstrated to enhance the percutaneous absorption of certain molecules and has found use as a [[penetration enhancer]]: an agent used in topical formulations, increasing the substances propensity for absorption into the [[skin]].<ref name=USPharmDOTcom>{{cite encyclopedia|authors=Loyd V. Allen, Jr.|title=Tretinoin 0.5 mg/g and α-Bisabolol 1 mg/g Gel and discussion on its use|year=2013|url=https://www.uspharmacist.com/article/tretinoin-05-mg-g-and-bisabolol-1-mg-g-gel-42388}}</ref><ref>{{cite journal|last1=Kamatou|first1=Guy P. P.|last2=Viljoen|first2=Alvaro M.|title=A Review of the Application and Pharmacological Properties of α-Bisabolol and α-Bisabolol-Rich Oils|journal=Journal of the American Oil Chemists' Society|date=2010|volume=87|issue=1|pages=1–7|doi=10.1007/s11746-009-1483-3|s2cid=95169851|url=https://www.researchgate.net/publication/225389970}}</ref> |
||
A structurally related compound known as β-bisabolol ([[CAS registry number]] [15352-77-9]) differs only in the position of the tertiary alcohol functional group. |
A structurally related compound known as β-bisabolol ([[CAS registry number]] [15352-77-9]) differs only in the position of the tertiary alcohol functional group. |
||
Revision as of 15:34, 18 September 2022
| |
| Names | |
|---|---|
| Preferred IUPAC name
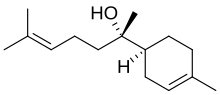
(2S)-6-Methyl-2-[(1S)-4-methylcyclohex-3-en-1-yl]hept-5-en-2-ol | |
| Other names
Levomenol
| |
| Identifiers | |
3D model (JSmol)
|
|
| 5733954 | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.041.279 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C15H26O | |
| Molar mass | 222.372 g·mol−1 |
| Density | 0.92 g cm−3 |
| Boiling point | 153 °C (307 °F; 426 K) at 12 mmHg |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Bisabolol, or more formally α-(−)-bisabolol or also known as levomenol,[1] is a natural monocyclic sesquiterpene alcohol. It is a colorless viscous oil that is the primary constituent of the essential oil from German chamomile (Matricaria recutita) and Myoporum crassifolium.[2] High concentrations of bisabolol can also be found in certain medicinal cannabis cultivars. It is poorly soluble in water and glycerin, but soluble in ethanol.[3] The enantiomer, α-(+)-bisabolol, is also found naturally but is rare. Synthetic bisabolol is usually a racemic mixture of the two, α-(±)-bisabolol. It is the terpenoid responsible for distinctive aroma of chamomile flowers, and when isolated, its scent has also has been likened to apples, sugar and honey.
Bisabolol has a weak sweet floral aroma and is used in various fragrances. It has also been used for hundreds of years in cosmetics because of its skin healing properties including reducing wrinkles, skin toughness and repairing sun-damaged skin, and more recently it has been compounded with tretinoin as a topical treatment for acne.[4] Bisabolol is known to have anti-irritant, anti-inflammatory, and anti-microbial properties.[5][6] Bisabolol is also demonstrated to enhance the percutaneous absorption of certain molecules and has found use as a penetration enhancer: an agent used in topical formulations, increasing the substances propensity for absorption into the skin.[4][7]
A structurally related compound known as β-bisabolol (CAS registry number [15352-77-9]) differs only in the position of the tertiary alcohol functional group.

References
- ^ Rohstoff-Lexikon Bisabolol Archived February 20, 2008, at the Wayback Machine
- ^ Bisabolol (in english) Archived October 10, 2007, at the Wayback Machine
- ^ "Terpenes". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. 2005. doi:10.1002/14356007.a26_205. ISBN 3527306730.
{{cite encyclopedia}}: Unknown parameter|authors=ignored (help) - ^ a b Tretinoin 0.5 mg/g and α-Bisabolol 1 mg/g Gel and discussion on its use. 2013.
{{cite encyclopedia}}: Unknown parameter|authors=ignored (help) - ^ Rocha, Nayrton Flávio Moura; Rios, Emiliano Ricardo Vasconcelos; Carvalho, Alyne Mara Rodrigues; Cerqueira, Gilberto Santos; Lopes, Amanda de Araújo; Leal, Luzia Kalyne Almeida Moreira; Dias, Marília Leite; de Sousa, Damião Pergentino; de Sousa, Francisca Cléa Florenço (December 2011). "Anti-nociceptive and anti-inflammatory activities of (-)-α-bisabolol in rodents". Naunyn-Schmiedeberg's Archives of Pharmacology. 384 (6): 525–533. doi:10.1007/s00210-011-0679-x. ISSN 1432-1912. PMID 21870032. S2CID 12654188.
- ^ Rodrigues, Fabíola Fernandes Galvão; Colares, Aracélio Viana; Nonato, Carla de Fatima Alves; Galvão-Rodrigues, Fabío Fernandes; Mota, Magaly Lima; Moraes Braga, Maria Flaviana Bezerra; Costa, José Galberto Martins da (December 2018). "In vitro antimicrobial activity of the essential oil from Vanillosmopsis arborea Barker (Asteraceae) and its major constituent, α-bisabolol". Microbial Pathogenesis. 125: 144–149. doi:10.1016/j.micpath.2018.09.024. PMID 30219391. S2CID 52282324.
- ^ Kamatou, Guy P. P.; Viljoen, Alvaro M. (2010). "A Review of the Application and Pharmacological Properties of α-Bisabolol and α-Bisabolol-Rich Oils". Journal of the American Oil Chemists' Society. 87 (1): 1–7. doi:10.1007/s11746-009-1483-3. S2CID 95169851.
