Theacrine: Difference between revisions
Added content Tags: Mobile edit Mobile web edit |
contract slightly tortured comparison to caffeine |
||
| Line 35: | Line 35: | ||
}} |
}} |
||
'''Theacrine''', also known as '''1,3,7,9-tetramethyluric acid''', is a [[purine alkaloid]] found in [[Cupuaçu]] (''Theobroma grandiflorum'') and in a Chinese tea known as kucha ({{zh|c={{linktext|苦|茶}}|p=kǔ chá|l=bitter tea}}) (''[[Camellia assamica]] var. kucha'').<ref>{{Cite journal|pmid=12009315|year=2002|last1=Zheng|first1=XQ|last2=Ye|first2=CX|last3=Kato|first3=M|last4=Crozier|first4=A|last5=Ashihara|first5=H|title=Theacrine (1,3,7,9-tetramethyluric acid) synthesis in leaves of a Chinese tea, [[kucha]] (Camellia assamica var. Kucha)|volume=60|issue=2|pages=129–34|journal=Phytochemistry|doi=10.1016/s0031-9422(02)00086-9}}</ref><ref name=":0">{{Cite journal|doi = 10.1016/j.pbb.2012.04.014|title = Locomotor activation by theacrine, a purine alkaloid structurally similar to caffeine: Involvement of adenosine and dopamine receptors|year = 2012|last1 = Feduccia|first1 = Allison A.|last2 = Wang|first2 = Yuanyuan|last3 = Simms|first3 = Jeffrey A.|last4 = Yi|first4 = Henry Y.|last5 = Li|first5 = Rui|last6 = Bjeldanes|first6 = Leonard|last7 = Ye|first7 = Chuangxing|last8 = Bartlett|first8 = Selena E.|journal = Pharmacology Biochemistry and Behavior|volume = 102|issue = 2|pages = 241–8|pmid = 22579816|s2cid = 31549989}}</ref> It shows [[anti-inflammatory]] and [[analgesic]] effects and appears to affect [[adenosine]] signalling in a manner similar to [[caffeine]].<ref name=":0" /><ref>{{Cite journal|doi=10.1016/j.fitote.2010.03.008|title=Theacrine, a purine alkaloid with anti-inflammatory and analgesic activities|year=2010|last1=Wang|first1=Yuanyuan|last2=Yang|first2=Xiaorong|last3=Zheng|first3=Xinqiang|last4=Li|first4=Jing|last5=Ye|first5=Chuangxing|last6=Song|first6=Xiaohong|journal=Fitoterapia|volume=81|issue=6|pages=627–31|pmid=20227468}}</ref> In kucha leaves, theacrine is synthesized from caffeine in what is thought to be a three-step pathway.<ref name=":0" /> |
'''Theacrine''', also known as '''1,3,7,9-tetramethyluric acid''', is a [[purine alkaloid]] found in [[Cupuaçu]] (''Theobroma grandiflorum'') and in a Chinese tea known as kucha ({{zh|c={{linktext|苦|茶}}|p=kǔ chá|l=bitter tea}}) (''[[Camellia assamica]] var. kucha'').<ref>{{Cite journal|pmid=12009315|year=2002|last1=Zheng|first1=XQ|last2=Ye|first2=CX|last3=Kato|first3=M|last4=Crozier|first4=A|last5=Ashihara|first5=H|title=Theacrine (1,3,7,9-tetramethyluric acid) synthesis in leaves of a Chinese tea, [[kucha]] (Camellia assamica var. Kucha)|volume=60|issue=2|pages=129–34|journal=Phytochemistry|doi=10.1016/s0031-9422(02)00086-9}}</ref><ref name=":0">{{Cite journal|doi = 10.1016/j.pbb.2012.04.014|title = Locomotor activation by theacrine, a purine alkaloid structurally similar to caffeine: Involvement of adenosine and dopamine receptors|year = 2012|last1 = Feduccia|first1 = Allison A.|last2 = Wang|first2 = Yuanyuan|last3 = Simms|first3 = Jeffrey A.|last4 = Yi|first4 = Henry Y.|last5 = Li|first5 = Rui|last6 = Bjeldanes|first6 = Leonard|last7 = Ye|first7 = Chuangxing|last8 = Bartlett|first8 = Selena E.|journal = Pharmacology Biochemistry and Behavior|volume = 102|issue = 2|pages = 241–8|pmid = 22579816|s2cid = 31549989}}</ref> It shows [[anti-inflammatory]] and [[analgesic]] effects and appears to affect [[adenosine]] signalling in a manner similar to [[caffeine]].<ref name=":0" /><ref>{{Cite journal|doi=10.1016/j.fitote.2010.03.008|title=Theacrine, a purine alkaloid with anti-inflammatory and analgesic activities|year=2010|last1=Wang|first1=Yuanyuan|last2=Yang|first2=Xiaorong|last3=Zheng|first3=Xinqiang|last4=Li|first4=Jing|last5=Ye|first5=Chuangxing|last6=Song|first6=Xiaohong|journal=Fitoterapia|volume=81|issue=6|pages=627–31|pmid=20227468}}</ref> In kucha leaves, theacrine is synthesized from caffeine in what is thought to be a three-step pathway.<ref name=":0" /> Theacrine and [caffeine]] are structurally similar. |
||
==Similarity to caffeine== |
|||
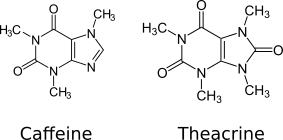
Theacrine is similar to caffeine with an additional [[methyl group]] in the 9-position, and a [[carbamide]] as a result of an additional [[carbonyl group]] at the 8-position. |
|||
[[File:Caffeine vs Theacrine.png|Caffeine vs theacrine]] |
[[File:Caffeine vs Theacrine.png|Caffeine vs theacrine]] |
||
Revision as of 20:52, 5 January 2023
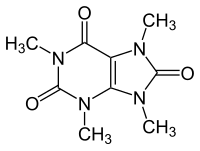
| |

| |
| Names | |
|---|---|
| IUPAC name
1,3,7,9-Tetramethylpurine-2,6,8-trione
| |
| Other names
1,3,7,9-Tetramethyluric acid; Temurin; Temorine; Tetramethyluric acid; Tetramethyl uric acid; TeaCrine (trade name)
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.017.268 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C9H12N4O3 | |
| Molar mass | 224.220 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Theacrine, also known as 1,3,7,9-tetramethyluric acid, is a purine alkaloid found in Cupuaçu (Theobroma grandiflorum) and in a Chinese tea known as kucha (Chinese: 苦茶; pinyin: kǔ chá; lit. 'bitter tea') (Camellia assamica var. kucha).[1][2] It shows anti-inflammatory and analgesic effects and appears to affect adenosine signalling in a manner similar to caffeine.[2][3] In kucha leaves, theacrine is synthesized from caffeine in what is thought to be a three-step pathway.[2] Theacrine and [caffeine]] are structurally similar.
Safety
Theacrine has demonstrated clinical safety and non-habituating effects in healthy humans over 8 weeks of daily use at up to 300 mg/day.[4] Moreover, there was no evidence of tachyphylaxis that is typical of neuroactive agents such as caffeine and other stimulants.[4]
In animal studies, theacrine has an LD50 of 810 mg/kg,[5][4] compared to 265 mg/kg for caffeine.[6]
See also
References
- ^ Zheng, XQ; Ye, CX; Kato, M; Crozier, A; Ashihara, H (2002). "Theacrine (1,3,7,9-tetramethyluric acid) synthesis in leaves of a Chinese tea, kucha (Camellia assamica var. Kucha)". Phytochemistry. 60 (2): 129–34. doi:10.1016/s0031-9422(02)00086-9. PMID 12009315.
- ^ a b c Feduccia, Allison A.; Wang, Yuanyuan; Simms, Jeffrey A.; Yi, Henry Y.; Li, Rui; Bjeldanes, Leonard; Ye, Chuangxing; Bartlett, Selena E. (2012). "Locomotor activation by theacrine, a purine alkaloid structurally similar to caffeine: Involvement of adenosine and dopamine receptors". Pharmacology Biochemistry and Behavior. 102 (2): 241–8. doi:10.1016/j.pbb.2012.04.014. PMID 22579816. S2CID 31549989.
- ^ Wang, Yuanyuan; Yang, Xiaorong; Zheng, Xinqiang; Li, Jing; Ye, Chuangxing; Song, Xiaohong (2010). "Theacrine, a purine alkaloid with anti-inflammatory and analgesic activities". Fitoterapia. 81 (6): 627–31. doi:10.1016/j.fitote.2010.03.008. PMID 20227468.
- ^ a b c Taylor L, Mumford P, Roberts M, Hayward S, Mullins J, Urbina S, Wilborn C (2016). "Safety of TeaCrine®, a non-habituating, naturally-occurring purine alkaloid over eight weeks of continuous use". Journal of the International Society of Sports Nutrition. 13: 2. doi:10.1186/s12970-016-0113-3. PMC 4711067. PMID 26766930.
• These findings support the clinical safety and non-habituating neuro-energetic effects of TeaCrine® supplementation over 8 weeks of daily use (up to 300 mg/day). Moreover, there was no evidence of a tachyphylactic response that is typical of neuroactive agents such as caffeine and other stimulants.
• Specifically, the acute toxicity for theacrine ingestion in mice has been previously reported [4] to be an LD50 of 810.6 mg/kg, which would equate to roughly 4.0 g for an individual weighing 76 kg.{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Wang Y, Yang X, Zheng X, Li J, Ye C, Song X (2010). "Theacrine, a purine alkaloid with anti-inflammatory and analgesic activities". Fitoterapia. 81 (6): 627–31. doi:10.1016/j.fitote.2010.03.008. PMID 20227468. Retrieved 2016-05-15.
the result of the acute toxicity test showed that the LD(50) of theacrine was 810.6 mg/kg (769.5-858.0mg/kg).
- ^ Warszawski D, Gorodischer R, Kaplanski J (1978). "Comparative toxicity of caffeine and aminophylline (theophylline ethylenediamine) in young and adult rats". Biology of the Neonate. 34 (1–2): 68–71. doi:10.1159/000241107. PMID 698326.
In adult rats, the LD50 of caffeine and aminophylline was the same after 24 h and after 1 week of observation: caffeine 265 mg/kg