Antibody-oligonucleotide conjugate: Difference between revisions
JeffreyMeth (talk | contribs) Antibody Analogues added |
JeffreyMeth (talk | contribs) m Reference adding |
||
| Line 9: | Line 9: | ||
The first AOC was reported in 1995 where the [[lysine]]s of a [[transferrin]]-antibody were connected using a SMCC bifunctional linker ([[Active ester|NHS ester]] and [[maleimide]] moiety) to radiolabelled and cys-bearing [[Oligonucleotide|ASOs]] targeting [[HIV]] [[Messenger RNA|mRNA]].<ref>{{Cite journal |last=Walker |first=Ian |last2=Irwin |first2=William J. |last3=Akhtar |first3=Saghir |date=1995 |url=http://dx.doi.org/10.1023/a:1016260110049 |journal=Pharmaceutical Research |volume=12 |issue=10 |pages=1548–1553 |doi=10.1023/a:1016260110049 |issn=0724-8741|title=Improved Cellular Delivery of Antisense Oligonucleotides Using Transferrin Receptor Antibody-Oligonucleotide Conjugates}}</ref> Another heterogenous construct based on the same chemistry but with a siRNA instead of an ASO was developed in 2011.<ref>{{Cite journal |last=Ma |first=Yuelong |last2=Kowolik |first2=Claudia M. |last3=Swiderski |first3=Piotr M. |last4=Kortylewski |first4=Marcin |last5=Yu |first5=Hua |last6=Horne |first6=David A. |last7=Jove |first7=Richard |last8=Caballero |first8=Otavia L. |last9=Simpson |first9=Andrew J. G. |last10=Lee |first10=Fook-Thean |last11=Pillay |first11=Vinochani |last12=Scott |first12=Andrew M. |date=2011-07-26 |title=Humanized Lewis-Y Specific Antibody Based Delivery of ''STAT3'' siRNA |url=http://dx.doi.org/10.1021/cb200176v |journal=ACS Chemical Biology |volume=6 |issue=9 |pages=962–970 |doi=10.1021/cb200176v |issn=1554-8929}}</ref> In 2013, MYERS and coworkers then unspecifically labelled an anti-CD19 antibody with N-succinimidyl 3-(2-pyridyl-dithio) propionate to form disulphide bonds with cys-modified ASO targeting the mRNA of oncoprotein E2A–PBX1.<ref>{{Cite journal |last=Zhang |first=Ke |last2=Hao |first2=Liangliang |last3=Hurst |first3=Sarah J. |last4=Mirkin |first4=Chad A. |date=2012-09-28 |title=Antibody-Linked Spherical Nucleic Acids for Cellular Targeting |url=http://dx.doi.org/10.1021/ja306854d |journal=Journal of the American Chemical Society |volume=134 |issue=40 |pages=16488–16491 |doi=10.1021/ja306854d |issn=0002-7863}}</ref> Ultimately, they could prove in-vivo antitumour effects which in contrast were not obtained with the single entities.<ref>{{Cite journal |last=Uckun |first=Fatih M. |last2=Qazi |first2=Sanjive |last3=Dibirdik |first3=Ilker |last4=Myers |first4=Dorothea E. |date=2012-09-18 |title=Rational design of an immunoconjugate for selective knock-down of leukemia-specific E2A–PBX1 fusion gene expression in human Pre-B leukemia |url=http://dx.doi.org/10.1039/c2ib20114c |journal=Integrative Biology |volume=5 |issue=1 |pages=122–132 |doi=10.1039/c2ib20114c |issn=1757-9708}}</ref> In the same timeframe, several antibodies were exploited for ON delivery in combination with [[nanoparticle]]s and in non-covalent strategies.<ref>{{Cite journal |last=Bäumer |first=Nicole |last2=Appel |first2=Neele |last3=Terheyden |first3=Lisa |last4=Buchholz |first4=Frank |last5=Rossig |first5=Claudia |last6=Müller-Tidow |first6=Carsten |last7=Berdel |first7=Wolfgang E |last8=Bäumer |first8=Sebastian |date=2015-12-03 |title=Antibody-coupled siRNA as an efficient method for in vivo mRNA knockdown |url=http://dx.doi.org/10.1038/nprot.2015.137 |journal=Nature Protocols |volume=11 |issue=1 |pages=22–36 |doi=10.1038/nprot.2015.137 |issn=1754-2189}}</ref><ref>{{Cite journal |last=Bäumer |first=Sebastian |last2=Bäumer |first2=Nicole |last3=Appel |first3=Neele |last4=Terheyden |first4=Lisa |last5=Fremerey |first5=Julia |last6=Schelhaas |first6=Sonja |last7=Wardelmann |first7=Eva |last8=Buchholz |first8=Frank |last9=Berdel |first9=Wolfgang E. |last10=Müller-Tidow |first10=Carsten |date=2015-03-12 |title=Antibody-Mediated Delivery of Anti–''KRAS''-siRNA ''In Vivo'' Overcomes Therapy Resistance in Colon Cancer |url=http://dx.doi.org/10.1158/1078-0432.ccr-13-2017 |journal=Clinical Cancer Research |volume=21 |issue=6 |pages=1383–1394 |doi=10.1158/1078-0432.ccr-13-2017 |issn=1078-0432}}</ref><ref>{{Cite journal |last=Song |first=Erwei |last2=Zhu |first2=Pengcheng |last3=Lee |first3=Sang-Kyung |last4=Chowdhury |first4=Dipanjan |last5=Kussman |first5=Steven |last6=Dykxhoorn |first6=Derek M |last7=Feng |first7=Yi |last8=Palliser |first8=Deborah |last9=Weiner |first9=David B |last10=Shankar |first10=Premlata |last11=Marasco |first11=Wayne A |last12=Lieberman |first12=Judy |date=2005-05-22 |title=Antibody mediated in vivo delivery of small interfering RNAs via cell-surface receptors |url=http://dx.doi.org/10.1038/nbt1101 |journal=Nature Biotechnology |volume=23 |issue=6 |pages=709–717 |doi=10.1038/nbt1101 |issn=1087-0156}}</ref> |
The first AOC was reported in 1995 where the [[lysine]]s of a [[transferrin]]-antibody were connected using a SMCC bifunctional linker ([[Active ester|NHS ester]] and [[maleimide]] moiety) to radiolabelled and cys-bearing [[Oligonucleotide|ASOs]] targeting [[HIV]] [[Messenger RNA|mRNA]].<ref>{{Cite journal |last=Walker |first=Ian |last2=Irwin |first2=William J. |last3=Akhtar |first3=Saghir |date=1995 |url=http://dx.doi.org/10.1023/a:1016260110049 |journal=Pharmaceutical Research |volume=12 |issue=10 |pages=1548–1553 |doi=10.1023/a:1016260110049 |issn=0724-8741|title=Improved Cellular Delivery of Antisense Oligonucleotides Using Transferrin Receptor Antibody-Oligonucleotide Conjugates}}</ref> Another heterogenous construct based on the same chemistry but with a siRNA instead of an ASO was developed in 2011.<ref>{{Cite journal |last=Ma |first=Yuelong |last2=Kowolik |first2=Claudia M. |last3=Swiderski |first3=Piotr M. |last4=Kortylewski |first4=Marcin |last5=Yu |first5=Hua |last6=Horne |first6=David A. |last7=Jove |first7=Richard |last8=Caballero |first8=Otavia L. |last9=Simpson |first9=Andrew J. G. |last10=Lee |first10=Fook-Thean |last11=Pillay |first11=Vinochani |last12=Scott |first12=Andrew M. |date=2011-07-26 |title=Humanized Lewis-Y Specific Antibody Based Delivery of ''STAT3'' siRNA |url=http://dx.doi.org/10.1021/cb200176v |journal=ACS Chemical Biology |volume=6 |issue=9 |pages=962–970 |doi=10.1021/cb200176v |issn=1554-8929}}</ref> In 2013, MYERS and coworkers then unspecifically labelled an anti-CD19 antibody with N-succinimidyl 3-(2-pyridyl-dithio) propionate to form disulphide bonds with cys-modified ASO targeting the mRNA of oncoprotein E2A–PBX1.<ref>{{Cite journal |last=Zhang |first=Ke |last2=Hao |first2=Liangliang |last3=Hurst |first3=Sarah J. |last4=Mirkin |first4=Chad A. |date=2012-09-28 |title=Antibody-Linked Spherical Nucleic Acids for Cellular Targeting |url=http://dx.doi.org/10.1021/ja306854d |journal=Journal of the American Chemical Society |volume=134 |issue=40 |pages=16488–16491 |doi=10.1021/ja306854d |issn=0002-7863}}</ref> Ultimately, they could prove in-vivo antitumour effects which in contrast were not obtained with the single entities.<ref>{{Cite journal |last=Uckun |first=Fatih M. |last2=Qazi |first2=Sanjive |last3=Dibirdik |first3=Ilker |last4=Myers |first4=Dorothea E. |date=2012-09-18 |title=Rational design of an immunoconjugate for selective knock-down of leukemia-specific E2A–PBX1 fusion gene expression in human Pre-B leukemia |url=http://dx.doi.org/10.1039/c2ib20114c |journal=Integrative Biology |volume=5 |issue=1 |pages=122–132 |doi=10.1039/c2ib20114c |issn=1757-9708}}</ref> In the same timeframe, several antibodies were exploited for ON delivery in combination with [[nanoparticle]]s and in non-covalent strategies.<ref>{{Cite journal |last=Bäumer |first=Nicole |last2=Appel |first2=Neele |last3=Terheyden |first3=Lisa |last4=Buchholz |first4=Frank |last5=Rossig |first5=Claudia |last6=Müller-Tidow |first6=Carsten |last7=Berdel |first7=Wolfgang E |last8=Bäumer |first8=Sebastian |date=2015-12-03 |title=Antibody-coupled siRNA as an efficient method for in vivo mRNA knockdown |url=http://dx.doi.org/10.1038/nprot.2015.137 |journal=Nature Protocols |volume=11 |issue=1 |pages=22–36 |doi=10.1038/nprot.2015.137 |issn=1754-2189}}</ref><ref>{{Cite journal |last=Bäumer |first=Sebastian |last2=Bäumer |first2=Nicole |last3=Appel |first3=Neele |last4=Terheyden |first4=Lisa |last5=Fremerey |first5=Julia |last6=Schelhaas |first6=Sonja |last7=Wardelmann |first7=Eva |last8=Buchholz |first8=Frank |last9=Berdel |first9=Wolfgang E. |last10=Müller-Tidow |first10=Carsten |date=2015-03-12 |title=Antibody-Mediated Delivery of Anti–''KRAS''-siRNA ''In Vivo'' Overcomes Therapy Resistance in Colon Cancer |url=http://dx.doi.org/10.1158/1078-0432.ccr-13-2017 |journal=Clinical Cancer Research |volume=21 |issue=6 |pages=1383–1394 |doi=10.1158/1078-0432.ccr-13-2017 |issn=1078-0432}}</ref><ref>{{Cite journal |last=Song |first=Erwei |last2=Zhu |first2=Pengcheng |last3=Lee |first3=Sang-Kyung |last4=Chowdhury |first4=Dipanjan |last5=Kussman |first5=Steven |last6=Dykxhoorn |first6=Derek M |last7=Feng |first7=Yi |last8=Palliser |first8=Deborah |last9=Weiner |first9=David B |last10=Shankar |first10=Premlata |last11=Marasco |first11=Wayne A |last12=Lieberman |first12=Judy |date=2005-05-22 |title=Antibody mediated in vivo delivery of small interfering RNAs via cell-surface receptors |url=http://dx.doi.org/10.1038/nbt1101 |journal=Nature Biotechnology |volume=23 |issue=6 |pages=709–717 |doi=10.1038/nbt1101 |issn=1087-0156}}</ref> |
||
Only recently the first examples for a site-selective conjugation between an ON therapeutic and a mAb was published: in 2015 [[Genentech]] exploited the SMCC linker to conjugate siRNA to several engineered mAb based on their proprietary Thiomab technology, which allows site-specific introduction of a cysteine into the antibody sequence[32].<ref name=":1">{{Cite journal |last=Cuellar |first=Trinna L. |last2=Barnes |first2=Dwight |last3=Nelson |first3=Christopher |last4=Tanguay |first4=Joshua |last5=Yu |first5=Shang-Fan |last6=Wen |first6=Xiaohui |last7=Scales |first7=Suzie J. |last8=Gesch |first8=Julie |last9=Davis |first9=David |last10=van Brabant Smith |first10=Anja |last11=Leake |first11=Devin |last12=Vandlen |first12=Richard |last13=Siebel |first13=Christian W. |date=2014-12-30 |title=Systematic evaluation of antibody-mediated siRNA delivery using an industrial platform of THIOMAB–siRNA conjugates |url=http://dx.doi.org/10.1093/nar/gku1362 |journal=Nucleic Acids Research |volume=43 |issue=2 |pages=1189–1203 |doi=10.1093/nar/gku1362 |issn=1362-4962}}</ref> They could prove the functionality of both entities in the construct and by screening different antibodies, they validated their importance for an effective antisense effect.<ref name=":1" /> The main obstacle encountered was a limited [[Endosome|endosomal escape]] but ultimately a functional construct which shows [[Antisense therapy|antisense effect]] in-vivo was reported.<ref name=":1" /> After development of the SMCC based conjugates, there were two constructs reported in literature based on [[Click chemistry|strain-promoted alkyne-azide cycloadditions]]: an [[MXD3]] mRNA targeting [[gapmer]] (cEt and PS modified) linked to an anti-[[CD22]] antibody targeting pre[[B cell]]s leads to in-vitro [[apoptosis]] of targeted cells and in-vivo increased length of mouse survival in [[Xenotransplantation|xenograft]] models. Notably, the dose required for the same therapeutic effect was 20 times lower for the developed conjugate (vs. naked mAb).<ref>{{Cite journal |last=Satake |first=Noriko |last2=Duong |first2=Connie |last3=Yoshida |first3=Sakiko |last4=Oestergaard |first4=Michael |last5=Chen |first5=Cathy |last6=Peralta |first6=Rachael |last7=Guo |first7=Shuling |last8=Seth |first8=Punit P |last9=Li |first9=Yueju |last10=Beckett |first10=Laurel |last11=Chung |first11=Jong |last12=Nolta |first12=Jan |last13=Nitin |first13=Nitin |last14=Tuscano |first14=Joseph M |date=January 2016 |title=Novel Targeted Therapy for Precursor B-Cell Acute Lymphoblastic Leukemia: Anti-CD22 Antibody-MXD3 Antisense Oligonucleotide Conjugate |url=http://dx.doi.org/10.2119/molmed.2015.00210 |journal=Molecular Medicine |volume=22 |issue=1 |pages=632–642 |doi=10.2119/molmed.2015.00210 |issn=1076-1551}}</ref> Another reported conjugate, exploiting the same unselective conjugation chemistry, employs an [[CD44]] respectively [[EPH receptor A2|EphA2]] targeting antibody which covalently carries a therapeutically irrelevant “sense-carrier” oligonucleotide |
Only recently the first examples for a site-selective conjugation between an ON therapeutic and a mAb was published: in 2015 [[Genentech]] exploited the SMCC linker to conjugate siRNA to several engineered mAb based on their proprietary Thiomab technology, which allows site-specific introduction of a cysteine into the antibody sequence[32].<ref name=":1">{{Cite journal |last=Cuellar |first=Trinna L. |last2=Barnes |first2=Dwight |last3=Nelson |first3=Christopher |last4=Tanguay |first4=Joshua |last5=Yu |first5=Shang-Fan |last6=Wen |first6=Xiaohui |last7=Scales |first7=Suzie J. |last8=Gesch |first8=Julie |last9=Davis |first9=David |last10=van Brabant Smith |first10=Anja |last11=Leake |first11=Devin |last12=Vandlen |first12=Richard |last13=Siebel |first13=Christian W. |date=2014-12-30 |title=Systematic evaluation of antibody-mediated siRNA delivery using an industrial platform of THIOMAB–siRNA conjugates |url=http://dx.doi.org/10.1093/nar/gku1362 |journal=Nucleic Acids Research |volume=43 |issue=2 |pages=1189–1203 |doi=10.1093/nar/gku1362 |issn=1362-4962}}</ref> They could prove the functionality of both entities in the construct and by screening different antibodies, they validated their importance for an effective antisense effect.<ref name=":1" /> The main obstacle encountered was a limited [[Endosome|endosomal escape]] but ultimately a functional construct which shows [[Antisense therapy|antisense effect]] in-vivo was reported.<ref name=":1" /> After development of the SMCC based conjugates, there were two constructs reported in literature based on [[Click chemistry|strain-promoted alkyne-azide cycloadditions]]: an [[MXD3]] mRNA targeting [[gapmer]] (cEt and PS modified) linked to an anti-[[CD22]] antibody targeting pre[[B cell]]s leads to in-vitro [[apoptosis]] of targeted cells and in-vivo increased length of mouse survival in [[Xenotransplantation|xenograft]] models. Notably, the dose required for the same therapeutic effect was 20 times lower for the developed conjugate (vs. naked mAb).<ref>{{Cite journal |last=Satake |first=Noriko |last2=Duong |first2=Connie |last3=Yoshida |first3=Sakiko |last4=Oestergaard |first4=Michael |last5=Chen |first5=Cathy |last6=Peralta |first6=Rachael |last7=Guo |first7=Shuling |last8=Seth |first8=Punit P |last9=Li |first9=Yueju |last10=Beckett |first10=Laurel |last11=Chung |first11=Jong |last12=Nolta |first12=Jan |last13=Nitin |first13=Nitin |last14=Tuscano |first14=Joseph M |date=January 2016 |title=Novel Targeted Therapy for Precursor B-Cell Acute Lymphoblastic Leukemia: Anti-CD22 Antibody-MXD3 Antisense Oligonucleotide Conjugate |url=http://dx.doi.org/10.2119/molmed.2015.00210 |journal=Molecular Medicine |volume=22 |issue=1 |pages=632–642 |doi=10.2119/molmed.2015.00210 |issn=1076-1551}}</ref> Another reported conjugate, exploiting the same unselective conjugation chemistry, employs an [[CD44]] respectively [[EPH receptor A2|EphA2]] targeting antibody which covalently carries a therapeutically irrelevant “sense-carrier” oligonucleotide<ref>{{Cite journal |last=Arnold |first=Amy E. |last2=Malek-Adamian |first2=Elise |last3=Le |first3=Phuong U. |last4=Meng |first4=Anika |last5=Martínez-Montero |first5=Saúl |last6=Petrecca |first6=Kevin |last7=Damha |first7=Masad J. |last8=Shoichet |first8=Molly S. |date=2018-06 |title=Antibody-Antisense Oligonucleotide Conjugate Downregulates a Key Gene in Glioblastoma Stem Cells |url=http://dx.doi.org/10.1016/j.omtn.2018.04.004 |journal=Molecular Therapy - Nucleic Acids |volume=11 |pages=518–527 |doi=10.1016/j.omtn.2018.04.004 |issn=2162-2531}}</ref>. This oligonucleotide base pairs with the actual antisense oligonucleotide (gapmer bearing phosphorothioate linkages and 2’-deoxy-2’-fluoro-beta-D-arabinonucleic acid modifications and a terminal fluorophor) aiming for an increased RNaseH activity.<ref>{{Cite journal |last=Arnold |first=Amy E. |last2=Malek-Adamian |first2=Elise |last3=Le |first3=Phuong U. |last4=Meng |first4=Anika |last5=Martínez-Montero |first5=Saúl |last6=Petrecca |first6=Kevin |last7=Damha |first7=Masad J. |last8=Shoichet |first8=Molly S. |date=June 2018 |title=Antibody-Antisense Oligonucleotide Conjugate Downregulates a Key Gene in Glioblastoma Stem Cells |url=http://dx.doi.org/10.1016/j.omtn.2018.04.004 |journal=Molecular Therapy - Nucleic Acids |volume=11 |pages=518–527 |doi=10.1016/j.omtn.2018.04.004 |issn=2162-2531}}</ref><ref>{{Cite journal |last=Craig |first=Kevin |last2=Abrams |first2=Marc |last3=Amiji |first3=Mansoor |date=2018-05-16 |title=Recent preclinical and clinical advances in oligonucleotide conjugates |url=http://dx.doi.org/10.1080/17425247.2018.1473375 |journal=Expert Opinion on Drug Delivery |volume=15 |issue=6 |pages=629–640 |doi=10.1080/17425247.2018.1473375 |issn=1742-5247}}</ref><ref>{{Cite journal |last=Dovgan |first=Igor |last2=Koniev |first2=Oleksandr |last3=Kolodych |first3=Sergii |last4=Wagner |first4=Alain |date=2019-07-24 |title=Antibody–Oligonucleotide Conjugates as Therapeutic, Imaging, and Detection Agents |url=http://dx.doi.org/10.1021/acs.bioconjchem.9b00306 |journal=Bioconjugate Chemistry |volume=30 |issue=10 |pages=2483–2501 |doi=10.1021/acs.bioconjchem.9b00306 |issn=1043-1802}}</ref> |
||
== Antibody Analogue-Oligonucleotide Conjugate == |
== Antibody Analogue-Oligonucleotide Conjugate == |
||
Revision as of 12:45, 2 March 2023
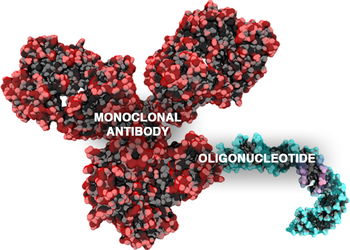
Antibody-oligonucleotide conjugates or AOCs belong to a class of chimeric molecules combining in their structure two important families of biomolecules: monoclonal antibodies and oligonucleotides.[1]
Combination of exceptional targeting capabilities of monoclonal antibodies with numerous functional modalities of oligonucleotides has been fruitful for a variety of applications with AOC including imaging, detection and targeted therapeutics.[1][2][3]
Cell uptake/internalisation still represents the biggest hurdle towards successful ON therapeutics. A straightforward uptake, like for most small-molecule drugs, is hindered by the polyanionic backbone and the molecular size of ONs. Being adapted from the broad and succesfull class of Antibody-Drug conjugates, antibodies and antibody analogues are more and more used in research in order to overcome hurdles related to delivery and internalisation of ON therapeutics. By exploiting bioconjugation methodology several conjugates have been obtained.
Development of therapeutic AOCs
The first AOC was reported in 1995 where the lysines of a transferrin-antibody were connected using a SMCC bifunctional linker (NHS ester and maleimide moiety) to radiolabelled and cys-bearing ASOs targeting HIV mRNA.[4] Another heterogenous construct based on the same chemistry but with a siRNA instead of an ASO was developed in 2011.[5] In 2013, MYERS and coworkers then unspecifically labelled an anti-CD19 antibody with N-succinimidyl 3-(2-pyridyl-dithio) propionate to form disulphide bonds with cys-modified ASO targeting the mRNA of oncoprotein E2A–PBX1.[6] Ultimately, they could prove in-vivo antitumour effects which in contrast were not obtained with the single entities.[7] In the same timeframe, several antibodies were exploited for ON delivery in combination with nanoparticles and in non-covalent strategies.[8][9][10]
Only recently the first examples for a site-selective conjugation between an ON therapeutic and a mAb was published: in 2015 Genentech exploited the SMCC linker to conjugate siRNA to several engineered mAb based on their proprietary Thiomab technology, which allows site-specific introduction of a cysteine into the antibody sequence[32].[11] They could prove the functionality of both entities in the construct and by screening different antibodies, they validated their importance for an effective antisense effect.[11] The main obstacle encountered was a limited endosomal escape but ultimately a functional construct which shows antisense effect in-vivo was reported.[11] After development of the SMCC based conjugates, there were two constructs reported in literature based on strain-promoted alkyne-azide cycloadditions: an MXD3 mRNA targeting gapmer (cEt and PS modified) linked to an anti-CD22 antibody targeting preB cells leads to in-vitro apoptosis of targeted cells and in-vivo increased length of mouse survival in xenograft models. Notably, the dose required for the same therapeutic effect was 20 times lower for the developed conjugate (vs. naked mAb).[12] Another reported conjugate, exploiting the same unselective conjugation chemistry, employs an CD44 respectively EphA2 targeting antibody which covalently carries a therapeutically irrelevant “sense-carrier” oligonucleotide[13]. This oligonucleotide base pairs with the actual antisense oligonucleotide (gapmer bearing phosphorothioate linkages and 2’-deoxy-2’-fluoro-beta-D-arabinonucleic acid modifications and a terminal fluorophor) aiming for an increased RNaseH activity.[14][15][16]
Antibody Analogue-Oligonucleotide Conjugate
Despite their tremendous potential, ADCs and AOCs suffer from the physical size of the antibody (mAb) entity (150 kDa) which limits solid tumour penetration (at least at low concentrations). Moreover, the site-selective modification of the antibody is hardly achievable: due to the difficult production of mAbs the selective introduction of an unnatural amino acid into the protein is not easily possible.[17]
Thats why there is intensive research to exploit antibody analogues and antibody fragments which retain a high target specificity but combined with a smaller size and a greater possibility of modification. Nanobodies for example are natural single-domain antibodies found in camelids with an average mass of 15kDa. They bear an increased stability, solubility and tissue penetration compared to mAbs.[18][19][20]
One conjugate, consisting out of an EGFR Nanobody and a siRNA being combined through maleimide bioconjugation, proofs the possibility of succesfull delivery of ONs by nanobodies. [21]
Another example consists out of an anti-CD71 Fab fragment which was conjugated to a maleimide bearing siRNA (itself having 2’OMe/2’F modifciations and phosphorothioate linkages). Several (cleavable and uncleavable) linkers between the maleimide moiety and the siRNA were screened revealing only a small influence on silencing efficacy (uncleavable linkers leading to the best results). To play out the small size of the Fab fragment, subcutaneous administration was investigated in mouse models leading to equivalent silencing results compared to intravenous administration. By comparison with other mAb-siRNA conjugates the authors even speculate that endosomal escape is largely facilitated by the smaller size of the Fab (vs. mAb).[22]
Moreover, Nanobody-ON conjugates are intensively used for imaging purposes exploiting the small nanobody size to reduce imaging displacement[23][24].
See also
References
- ^ a b Dovgan, Igor; Koniev, Oleksandr; Kolodych, Sergii; Wagner, Alain (2019). "Antibody–Oligonucleotide Conjugates as Therapeutic, Imaging, and Detection Agents". Bioconjugate Chemistry. 30 (10): 2483–2501. doi:10.1021/acs.bioconjchem.9b00306. ISSN 1043-1802. PMID 31339691. S2CID 198491258.
- ^ Levin, Arthur A. (2017). "Targeting Therapeutic Oligonucleotides". New England Journal of Medicine. 376 (1). Massachusetts Medical Society: 86–88. doi:10.1056/nejmcibr1613559. ISSN 0028-4793. PMID 28052219. S2CID 33970969.
- ^ Winkler, Johannes (2013). "Oligonucleotide conjugates for therapeutic applications". Therapeutic Delivery. 4 (7). Future Science Ltd: 791–809. doi:10.4155/tde.13.47. ISSN 2041-5990. PMC 3787477. PMID 23883124.
- ^ Walker, Ian; Irwin, William J.; Akhtar, Saghir (1995). "Improved Cellular Delivery of Antisense Oligonucleotides Using Transferrin Receptor Antibody-Oligonucleotide Conjugates". Pharmaceutical Research. 12 (10): 1548–1553. doi:10.1023/a:1016260110049. ISSN 0724-8741.
- ^ Ma, Yuelong; Kowolik, Claudia M.; Swiderski, Piotr M.; Kortylewski, Marcin; Yu, Hua; Horne, David A.; Jove, Richard; Caballero, Otavia L.; Simpson, Andrew J. G.; Lee, Fook-Thean; Pillay, Vinochani; Scott, Andrew M. (2011-07-26). "Humanized Lewis-Y Specific Antibody Based Delivery of STAT3 siRNA". ACS Chemical Biology. 6 (9): 962–970. doi:10.1021/cb200176v. ISSN 1554-8929.
- ^ Zhang, Ke; Hao, Liangliang; Hurst, Sarah J.; Mirkin, Chad A. (2012-09-28). "Antibody-Linked Spherical Nucleic Acids for Cellular Targeting". Journal of the American Chemical Society. 134 (40): 16488–16491. doi:10.1021/ja306854d. ISSN 0002-7863.
- ^ Uckun, Fatih M.; Qazi, Sanjive; Dibirdik, Ilker; Myers, Dorothea E. (2012-09-18). "Rational design of an immunoconjugate for selective knock-down of leukemia-specific E2A–PBX1 fusion gene expression in human Pre-B leukemia". Integrative Biology. 5 (1): 122–132. doi:10.1039/c2ib20114c. ISSN 1757-9708.
- ^ Bäumer, Nicole; Appel, Neele; Terheyden, Lisa; Buchholz, Frank; Rossig, Claudia; Müller-Tidow, Carsten; Berdel, Wolfgang E; Bäumer, Sebastian (2015-12-03). "Antibody-coupled siRNA as an efficient method for in vivo mRNA knockdown". Nature Protocols. 11 (1): 22–36. doi:10.1038/nprot.2015.137. ISSN 1754-2189.
- ^ Bäumer, Sebastian; Bäumer, Nicole; Appel, Neele; Terheyden, Lisa; Fremerey, Julia; Schelhaas, Sonja; Wardelmann, Eva; Buchholz, Frank; Berdel, Wolfgang E.; Müller-Tidow, Carsten (2015-03-12). "Antibody-Mediated Delivery of Anti–KRAS-siRNA In Vivo Overcomes Therapy Resistance in Colon Cancer". Clinical Cancer Research. 21 (6): 1383–1394. doi:10.1158/1078-0432.ccr-13-2017. ISSN 1078-0432.
- ^ Song, Erwei; Zhu, Pengcheng; Lee, Sang-Kyung; Chowdhury, Dipanjan; Kussman, Steven; Dykxhoorn, Derek M; Feng, Yi; Palliser, Deborah; Weiner, David B; Shankar, Premlata; Marasco, Wayne A; Lieberman, Judy (2005-05-22). "Antibody mediated in vivo delivery of small interfering RNAs via cell-surface receptors". Nature Biotechnology. 23 (6): 709–717. doi:10.1038/nbt1101. ISSN 1087-0156.
- ^ a b c Cuellar, Trinna L.; Barnes, Dwight; Nelson, Christopher; Tanguay, Joshua; Yu, Shang-Fan; Wen, Xiaohui; Scales, Suzie J.; Gesch, Julie; Davis, David; van Brabant Smith, Anja; Leake, Devin; Vandlen, Richard; Siebel, Christian W. (2014-12-30). "Systematic evaluation of antibody-mediated siRNA delivery using an industrial platform of THIOMAB–siRNA conjugates". Nucleic Acids Research. 43 (2): 1189–1203. doi:10.1093/nar/gku1362. ISSN 1362-4962.
- ^ Satake, Noriko; Duong, Connie; Yoshida, Sakiko; Oestergaard, Michael; Chen, Cathy; Peralta, Rachael; Guo, Shuling; Seth, Punit P; Li, Yueju; Beckett, Laurel; Chung, Jong; Nolta, Jan; Nitin, Nitin; Tuscano, Joseph M (January 2016). "Novel Targeted Therapy for Precursor B-Cell Acute Lymphoblastic Leukemia: Anti-CD22 Antibody-MXD3 Antisense Oligonucleotide Conjugate". Molecular Medicine. 22 (1): 632–642. doi:10.2119/molmed.2015.00210. ISSN 1076-1551.
- ^ Arnold, Amy E.; Malek-Adamian, Elise; Le, Phuong U.; Meng, Anika; Martínez-Montero, Saúl; Petrecca, Kevin; Damha, Masad J.; Shoichet, Molly S. (2018-06). "Antibody-Antisense Oligonucleotide Conjugate Downregulates a Key Gene in Glioblastoma Stem Cells". Molecular Therapy - Nucleic Acids. 11: 518–527. doi:10.1016/j.omtn.2018.04.004. ISSN 2162-2531.
{{cite journal}}: Check date values in:|date=(help) - ^ Arnold, Amy E.; Malek-Adamian, Elise; Le, Phuong U.; Meng, Anika; Martínez-Montero, Saúl; Petrecca, Kevin; Damha, Masad J.; Shoichet, Molly S. (June 2018). "Antibody-Antisense Oligonucleotide Conjugate Downregulates a Key Gene in Glioblastoma Stem Cells". Molecular Therapy - Nucleic Acids. 11: 518–527. doi:10.1016/j.omtn.2018.04.004. ISSN 2162-2531.
- ^ Craig, Kevin; Abrams, Marc; Amiji, Mansoor (2018-05-16). "Recent preclinical and clinical advances in oligonucleotide conjugates". Expert Opinion on Drug Delivery. 15 (6): 629–640. doi:10.1080/17425247.2018.1473375. ISSN 1742-5247.
- ^ Dovgan, Igor; Koniev, Oleksandr; Kolodych, Sergii; Wagner, Alain (2019-07-24). "Antibody–Oligonucleotide Conjugates as Therapeutic, Imaging, and Detection Agents". Bioconjugate Chemistry. 30 (10): 2483–2501. doi:10.1021/acs.bioconjchem.9b00306. ISSN 1043-1802.
- ^ Baah, Stephanie; Laws, Mark; Rahman, Khondaker Miraz (2021-05-15). "Antibody–Drug Conjugates—A Tutorial Review". Molecules. 26 (10): 2943. doi:10.3390/molecules26102943. ISSN 1420-3049.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Škrlec, Katja; Štrukelj, Borut; Berlec, Aleš (2015-07). "Non-immunoglobulin scaffolds: a focus on their targets". Trends in Biotechnology. 33 (7): 408–418. doi:10.1016/j.tibtech.2015.03.012. ISSN 0167-7799.
{{cite journal}}: Check date values in:|date=(help) - ^ Muyldermans, Serge (2021-02-16). "Applications of Nanobodies". Annual Review of Animal Biosciences. 9 (1): 401–421. doi:10.1146/annurev-animal-021419-083831. ISSN 2165-8102.
- ^ Vazquez-Lombardi, Rodrigo; Phan, Tri Giang; Zimmermann, Carsten; Lowe, David; Jermutus, Lutz; Christ, Daniel (2015-10). "Challenges and opportunities for non-antibody scaffold drugs". Drug Discovery Today. 20 (10): 1271–1283. doi:10.1016/j.drudis.2015.09.004. ISSN 1878-5832. PMID 26360055.
{{cite journal}}: Check date values in:|date=(help) - ^ Zavoiura, Oleksandr; Brunner, Bodo; Casteels, Peter; Zimmermann, Luciana; Ozog, Matthias; Boutton, Carlo; Helms, Mike W.; Wagenaar, Timothy; Adam, Volker; Peterka, Josefine; Metz-Weidmann, Christiane; Deschaght, Pieter; Scheidler, Sabine; Jahn-Hofmann, Kerstin (2021-01-14). "Nanobody–siRNA Conjugates for Targeted Delivery of siRNA to Cancer Cells". Molecular Pharmaceutics. 18 (3): 1048–1060. doi:10.1021/acs.molpharmaceut.0c01001. ISSN 1543-8384.
- ^ Sugo, Tsukasa; Terada, Michiko; Oikawa, Tatsuo; Miyata, Kenichi; Nishimura, Satoshi; Kenjo, Eriya; Ogasawara-Shimizu, Mari; Makita, Yukimasa; Imaichi, Sachiko; Murata, Shumpei; Otake, Kentaro; Kikuchi, Kuniko; Teratani, Mika; Masuda, Yasushi; Kamei, Takayuki (2016-09-10). "Development of antibody-siRNA conjugate targeted to cardiac and skeletal muscles". Journal of Controlled Release. 237: 1–13. doi:10.1016/j.jconrel.2016.06.036. ISSN 0168-3659.
- ^ Hebbrecht, Tim; Liu, Jing; Zwaenepoel, Olivier; Boddin, Gaëlle; Van Leene, Chloé; Decoene, Klaas; Madder, Annemieke; Braeckmans, Kevin; Gettemans, Jan (2020-11). "Nanobody click chemistry for convenient site-specific fluorescent labelling, single step immunocytochemistry and delivery into living cells by photoporation and live cell imaging". New Biotechnology. 59: 33–43. doi:10.1016/j.nbt.2020.05.004. ISSN 1871-6784.
{{cite journal}}: Check date values in:|date=(help) - ^ Sograte-Idrissi, Shama; Oleksiievets, Nazar; Isbaner, Sebastian; Eggert-Martinez, Mariana; Enderlein, Jörg; Tsukanov, Roman; Opazo, Felipe (2018-12-19). "Nanobody Detection of Standard Fluorescent Proteins Enables Multi-Target DNA-PAINT with High Resolution and Minimal Displacement Errors". dx.doi.org. Retrieved 2023-03-02.
