Pyrrhotite: Difference between revisions
Added new section "Mineral Abbreviations" with table format, citations, and references. |
Added new section "Formation of Pyrrhotite in Rocks" with information, citations, and references. |
||
| Line 68: | Line 68: | ||
==Occurrence== |
==Occurrence== |
||
Pyrrhotite is a rather common trace constituent of [[mafic]] [[igneous rocks]] especially [[norite]]s. It occurs as segregation deposits in [[layered intrusion]]s associated with [[pentlandite]], [[chalcopyrite]] and other sulfides. It is an important constituent of the [[Sudbury Basin|Sudbury intrusion]] (1.85 Ga old [[impact event|meteorite impact crater]] in [[Ontario]], Canada) where it occurs in masses associated with copper and nickel mineralisation.<ref name=Klein/> It also occurs in [[pegmatite]]s and in contact [[metamorphic rock|metamorphic]] zones. Pyrrhotite is often accompanied by pyrite, [[marcasite]] and magnetite. |
Pyrrhotite is a rather common trace constituent of [[mafic]] [[igneous rocks]] especially [[norite]]s. It occurs as segregation deposits in [[layered intrusion]]s associated with [[pentlandite]], [[chalcopyrite]] and other sulfides. It is an important constituent of the [[Sudbury Basin|Sudbury intrusion]] (1.85 Ga old [[impact event|meteorite impact crater]] in [[Ontario]], Canada) where it occurs in masses associated with copper and nickel mineralisation.<ref name=Klein/> It also occurs in [[pegmatite]]s and in contact [[metamorphic rock|metamorphic]] zones. Pyrrhotite is often accompanied by pyrite, [[marcasite]] and magnetite. |
||
== Formation of Pyrrhotite in Rocks == |
|||
Pyrrhotite requires both iron and sulfur to form.<ref name=":5" /> Iron is the fourth most [[Abundance of elements in Earth's crust|abundant element]] in the Earth's [[continental crust]] (average abundance of 5.63 % or 56,300 mg/kg in the crust)<ref name=":6">"Abundance of Elements in the Earth’s Crust and in the Sea," in CRC Handbook of Chemistry and Physics, 103rd Edition (Internet Version 2022), John R. Rumble, ed., CRC Press/Taylor & Francis, Boca Raton, FL.</ref>, and so the majority of rocks have sufficient iron abundance to form pyrrhotite.<ref name=":5" /> However, because sulfur is less abundant (average abundance of 0.035 % or 350 mg/kg in the crust)<ref name=":6" />, the formation of pyrrhotite is generally controlled by sulfur abundance.<ref name=":5" /> Also, the mineral [[pyrite]] is both the most common and most abundant sulfide mineral in the Earth's crust.<ref name=":5" /> If rocks containing pyrite undergo [[metamorphism]], there is a gradual release of [[Volatiles|volatile]] components like water and sulfur from pyrite.<ref name=":5" /> The loss of sulfur causes pyrite to [[Recrystallization (geology)|recrystallize]] into pyrrhotite.<ref name=":5" /> |
|||
Pyrrhotite can also form near [[Hydrothermal vent|black smoker hydrothermal vents]].<ref name=":5" /> Black smokers release high sulfur concentrations onto the sea floor, and when the surrounding rocks are metamorphosed, pyrrhotite can crystallize.<ref name=":5" /> Later [[Plate tectonics|tectonic processes]] [[Tectonic uplift|uplift]] the metamorphic rocks and expose pyrrhotite to the Earth's surface.<ref name=":5" /> |
|||
== Distribution == |
== Distribution == |
||
Revision as of 21:30, 11 April 2023
| Pyrrhotite | |
|---|---|
 Brassy, tabular crystals of pyrrhotite, with sphalerite and quartz, from Nikolaevskiy Mine, Primorskiy Kray, Russia. Specimen size: 5.3 x 4.1 x 3.8 cm | |
| General | |
| Category | Mineral |
| Formula (repeating unit) | Fe1−xS (x = 0 to 0.2) |
| IMA symbol | Pyh[1] |
| Strunz classification | 2.CC.10 |
| Crystal system | Monoclinic, with hexagonal polytypes |
| Crystal class | Prismatic (2/m) (same H-M symbol) |
| Space group | A2/a |
| Unit cell | a = 11.88 Å, b = 6.87 Å, c = 22.79 Å; β = 90.47°; Z = 26 |
| Identification | |
| Color | Bronze, dark brown |
| Crystal habit | Tabular or prismatic in hexagonal prisms; massive to granular |
| Cleavage | Absent |
| Fracture | Uneven |
| Mohs scale hardness | 3.5 – 4.5 |
| Luster | Metallic |
| Streak | Dark grey – black |
| Specific gravity | 4.58 – 4.65, average = 4.61 |
| Refractive index | Opaque |
| Fusibility | 3 |
| Solubility | Soluble in hydrochloric acid |
| Other characteristics | Weakly magnetic, strongly magnetic on heating; non-luminescent, non-radioactive |
| References | [2][3][4] |
Pyrrhotite (pyrrhos in Greek meaning "flame-coloured") is an iron sulfide mineral with the formula Fe(1-x)S (x = 0 to 0.2). It is a nonstoichiometric variant of FeS, the mineral known as troilite. Pyrrhotite is also called magnetic pyrite, because the color is similar to pyrite and it is weakly magnetic. The magnetism decreases as the iron content decreases, and troilite is non-magnetic.[5] Pyrrhotite is generally tabular and brassy/bronze in color with a metallic luster. The mineral occurs with mafic igneous rocks like norites. Pyrrhottie is associated and mined with other sulfide minerals like pentlandite, pyrite, chalcopyrite, and magnetite.
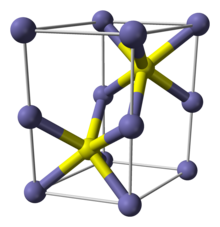


Structure
Pyrrhotite exists as a number of polytypes of hexagonal or monoclinic crystal symmetry; several polytypes often occur within the same specimen. Their structure is based on the NiAs unit cell. As such, Fe occupies an octahedral site and the sulfide centers occupy trigonal prismatic sites.[6][page needed]
Materials with the NiAs structure often are non-stoichiometric because they lack up to 1/8th fraction of the metal ions, creating vacancies. One of such structures is pyrrhotite-4C (Fe7S8). Here "4" indicates that iron vacancies define a superlattice that is 4 times larger than the unit cell in the "C" direction. The C direction is conventionally chosen parallel to the main symmetry axis of the crystal; this direction usually corresponds to the largest lattice spacing. Other polytypes include: pyrrhotite-5C (Fe9S10), 6C (Fe11S12), 7C (Fe9S10) and 11C (Fe10S11). Every polytype can have monoclinic (M) or hexagonal (H) symmetry, and therefore some sources label them, for example, not as 6C, but 6H or 6M depending on the symmetry.[2][7] The monoclinic forms are stable at temperatures below 254 °C, whereas the hexagonal forms are stable above that temperature. The exception is for those with high iron content, close to the troilite composition (47 to 50% atomic percent iron) which exhibit hexagonal symmetry.[8]
Magnetic properties
The ideal FeS lattice, such as that of troilite, is non-magnetic. Magnetic properties vary with Fe content. More Fe-rich, hexagonal pyrrhotites are antiferromagnetic. However, the Fe-deficient, monoclinic Fe7S8 is ferrimagnetic.[9] The ferromagnetism which is widely observed in pyrrhotite is therefore attributed to the presence of relatively large concentrations of iron vacancies (up to 20%) in the crystal structure. Vacancies lower the crystal symmetry. Therefore, monoclinic forms of pyrrhotite are in general more defect-rich than the more symmetrical hexagonal forms, and thus are more magnetic.[10] Monoclinic pyrrhotite undergoes a magnetic transition known as the Besnus transition at 30 K that leads to a loss of magnetic remanence.[11] The saturation magnetization of pyrrhotite is 0.12 tesla.[12]
Identification
Physical Properties
Pyrrhotite is brassy, bronze, or dark brown in color with a metallic luster and uneven or subconchoidal fracture.[13] Pyrrhotite may be confused with other brassy sulfide minerals like pyrite, chalcopyrite, or pentlandite. Certain diagnostic characteristics can be used for identification in hand samples. Unlike other common brassy-colored sulfide minerals, pyrrhotite is typically magnetic (varies inversely with iron content).[13] On the Mohs hardness scale, pyrrhotite ranges from 3.5 to 4,[14] compared to 6 to 6.5 for pyrite.[15] Streak can be used when properties between pyrrhotite and other sulfide minerals are similar. Pyrrhotite displays a dark grey to black streak.[14] Pyrite will display a greenish black to brownish black streak,[15] chalcopyrite will display a greenish black streak,[16] and pentlandite leaves a pale bronze-brown streak.[17] Pyrrhotite generally displays massive to granular crystal habit, and may show tabular/prismatic or hexagonal crystals which are sometimes iridescent.[13]
Diagnostic characteristics in hand sample include: brassy/bronze color with a grey/black streak, tabular or hexagonal crystals which show iridescence, subconchoidal fracture, metallic luster, and magnetic.
Optical Properties
Pyrrhotite is an opaque mineral and will therefore not transmit light. As a result, pyrrhotite will display extinction when viewed under plane polarized light and cross polarized light, making identification with petrographic polarizing light microscopes difficult. Pyrrhotite, and other opaque minerals can be identified optically using a reflected light ore microscope. The following optical properties[18] are representative of polished/puck sections using ore microscopy:

Pyrrhotite typically appears as anhedral, granular aggregates and is cream-pink to brownish in color.[18] Weak to strong reflection pleochroism which may be seen along grain boundaries.[18] Pyrrhotite has similar polishing hardness to pentlandite (medium), is softer than pyrite, and harder than chalcopyrite.[18] Pyrrhotite will not display twinning or internal reflections, and its strong anisotropy from yellow to greenish-gray or grayish-blue is characteristic.[18]
Diagnostic characteristics in polished section include: anhedral aggregates, cream-pink to brown in color and strong anisotropy.
Occurrence
Pyrrhotite is a rather common trace constituent of mafic igneous rocks especially norites. It occurs as segregation deposits in layered intrusions associated with pentlandite, chalcopyrite and other sulfides. It is an important constituent of the Sudbury intrusion (1.85 Ga old meteorite impact crater in Ontario, Canada) where it occurs in masses associated with copper and nickel mineralisation.[8] It also occurs in pegmatites and in contact metamorphic zones. Pyrrhotite is often accompanied by pyrite, marcasite and magnetite.
Formation of Pyrrhotite in Rocks
Pyrrhotite requires both iron and sulfur to form.[19] Iron is the fourth most abundant element in the Earth's continental crust (average abundance of 5.63 % or 56,300 mg/kg in the crust)[20], and so the majority of rocks have sufficient iron abundance to form pyrrhotite.[19] However, because sulfur is less abundant (average abundance of 0.035 % or 350 mg/kg in the crust)[20], the formation of pyrrhotite is generally controlled by sulfur abundance.[19] Also, the mineral pyrite is both the most common and most abundant sulfide mineral in the Earth's crust.[19] If rocks containing pyrite undergo metamorphism, there is a gradual release of volatile components like water and sulfur from pyrite.[19] The loss of sulfur causes pyrite to recrystallize into pyrrhotite.[19]
Pyrrhotite can also form near black smoker hydrothermal vents.[19] Black smokers release high sulfur concentrations onto the sea floor, and when the surrounding rocks are metamorphosed, pyrrhotite can crystallize.[19] Later tectonic processes uplift the metamorphic rocks and expose pyrrhotite to the Earth's surface.[19]
Distribution
The United States

Pyrrhotite occurs in a variety of locations in the United States.[19][21][22][23] In the eastern United States, pyrrhotite occurs in highly metamorphosed rock that forms a belt along the Appalachian Mountains.[19] Pyrrhotite-bearing rocks are generally unseen in the central United States as the area is unmetamorphosed and underlain by sedimentary rocks which do not contain pyrrhotite.[19] Discontinuous belts that contain pyrrhotite are present in the western United States along the Sierra Nevada mountain range and Cascade Range extending into the northwestern United States.[19] Pyrrhotite may also be found west and south of Lake Superior.[19]
Etymology and history
Named in 1847 by Ours-Pierre-Armand Petit-Dufrénoy.[24] "Pyrrhotite" is derived from the Greek word πνρρό, "pyrrhos", meaning flame-colored.[2]
Issues
If pyrrhotite-containing rocks are crushed and added to concrete filler, pyrrhotite creates a problem in the production of concrete.[25] Pyrrhotite has been linked to crumbling concrete basements in Quebec, Massachusetts and Connecticut when local quarries included it in their concrete mixtures.[26][27][28] The iron sulfide it contains can naturally react with oxygen and water, and over time pyrrhotite breaks down into sulfuric acid and secondary minerals like gypsum.[25][19] These secondary products occupy a larger volume than pyrrhotite, which expands and cracks the concrete leading to home foundation failure.[26][27][28][25][19]
Uses of Pyrrhotite
Other than a source of sulfur, pyrrhotite does not have specific applications.[29] It is generally not a valuable mineral unless significant nickel, copper, or other metals are present.[29][30] Iron is seldom extracted from pyrrhotite due to a complicated metallurgical process[29] It is mined primarily because it is associated with pentlandite, a sulfide mineral that can contain significant amounts of nickel and cobalt.[2] When found in mafic and ultramafic rocks, pyrrhotite can be a good indicator of economic nickel deposits.[29]
Mineral Abbreviations
| Abbreviation | Source |
|---|---|
| Pyh | IMA-CNMNC[31] |
| Po | Whitney and Evans, 2010[32]; The Canadian Mineralogist, 2019[33]. |
References
- ^ Warr, L.N. (2021). "IMA–CNMNC approved mineral symbols". Mineralogical Magazine. 85 (3): 291–320. Bibcode:2021MinM...85..291W. doi:10.1180/mgm.2021.43. S2CID 235729616.
- ^ a b c d "Pyrrhotite". Mindat.org. Retrieved 2009-07-07.
- ^ "Pyrrhotite" (PDF). Rruff.geo.arizona.edu. Retrieved 2015-07-10.
- ^ "Pyrrhotite Mineral Data". Webmineral.com. Retrieved 2015-07-10.
- ^ Haldar, S. K. (2017). Platinum-nickel-chromium deposits : geology, exploration and reserve base. Elsevier. p.12 ISBN 978-0-12-802041-8.
- ^ Shriver, D. F.; Atkins, P. W.; Overton, T. L.; Rourke, J. P.; Weller, M. T.; Armstrong, F. A. "Inorganic Chemistry" W. H. Freeman, New York, 2006. ISBN 0-7167-4878-9.[page needed]
- ^ Barnes, Hubert Lloyd (1997). Geochemistry of hydrothermal ore deposits. John Wiley and Sons. pp. 382–390. ISBN 0-471-57144-X.
- ^ a b Klein, Cornelis and Cornelius S. Hurlbut, Jr., Manual of Mineralogy, Wiley, 20th ed, 1985, pp. 278-9 ISBN 0-471-80580-7
- ^ Sagnotti, L., 2007, Iron Sulfides; in: Encyclopedia of Geomagnetism and Paleomagnetism; (Editors David Gubbins and Emilio Herrero-Bervera), Springer, 1054 pp., p. 454-459.
- ^ Atak, Suna; Önal, Güven; Çelik, Mehmet Sabri (1998). Innovations in Mineral and Coal Processing. Taylor & Francis. p. 131. ISBN 90-5809-013-2.
- ^ Volk, Michael W.R.; Gilder, Stuart A.; Feinberg, Joshua M. (1 December 2016). "Low-temperature magnetic properties of monoclinic pyrrhotite with particular relevance to the Besnus transition". Geophysical Journal International. 207 (3): 1783–1795. doi:10.1093/gji/ggw376.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Svoboda, Jan (2004). Magnetic techniques for the treatment of materials. Springer. p. 33. ISBN 1-4020-2038-4.
- ^ a b c "Pyrrhotite: Physical properties, uses, composition". geology.com. Retrieved 2023-02-20.
- ^ a b "Pyrrhotite". Mindat.org. Retrieved 2009-07-07.
- ^ a b "Pyrite" (PDF). rruff.info. Retrieved 2023-02-20.
- ^ "Chalcopyrite" (PDF). handbookofmineralogy. Retrieved 2023-02-20.
- ^ "Pentlandite" (PDF). handbookofmineralogy. Retrieved 2023-02-20.
- ^ a b c d e Spry, P. G., & Gedlinske, B. (1987). Tables for the determination of common opaque minerals. Economic Geology Pub.
- ^ a b c d e f g h i j k l m n o p Mauk, J.L., Crafford, T.C., Horton, J.D., San Juan, C.A., and Robinson, G.R., Jr., 2020, Pyrrhotite distribution in the conterminous United States, 2020: U.S. Geological Survey Fact Sheet 2020-3017, 4 p., https://doi.org/10.3133/fs20203017.
- ^ a b "Abundance of Elements in the Earth’s Crust and in the Sea," in CRC Handbook of Chemistry and Physics, 103rd Edition (Internet Version 2022), John R. Rumble, ed., CRC Press/Taylor & Francis, Boca Raton, FL.
- ^ Mauk, J. L., & Horton, J. D. (2020). Data to accompany U.S. Geological Survey Fact Sheet 2020-3017: Pyrrhotite distribution in the conterminous United States [Data set]. U.S. Geological Survey. https://doi.org/10.5066/P9QSWBU6.
- ^ U.S. Geological Survey, 2019, Mineral Resource Data System: accessed April 11, 2023, at http://mrdata.usgs.gov/mrds/.
- ^ Mindat.org, 2019, Mines, minerals and more: accessed April 11, 2023, at https://mindat.org/.
- ^ "Pyrrhotite". mindat.org. Retrieved March 24, 2023.
- ^ a b c "USGS Publishes Map of Potential Pyrrhotite Occurrences". USGS.gov. April 29, 2020. Retrieved April 11, 2023.
{{cite web}}: CS1 maint: url-status (link) - ^ a b Hussey, Kristin; Foderaro, Lisa W. (7 June 2016). "With Connecticut Foundations Crumbling, Your Home Is Now Worthless". The New York Times. Retrieved 2016-06-08.
- ^ a b "Crumbling Foundations". nbcconnecticut.com. Retrieved 2016-06-08.
- ^ a b "U.S. GAO - Crumbling Foundations: Extent of Homes with Defective Concrete Is Not Fully Known and Federal Options to Aid Homeowners Are Limited". gao.gov. Retrieved 2021-02-22.
- ^ a b c d Haldar, S. K. (2017). Platinum-nickel-chromium deposits : geology, exploration and reserve base. Elsevier. p.24. ISBN 978-0-12-802041-8.
- ^ Kolahdoozan, M. & Yen, W.T.. (2002). Pyrrhotite - An Important Gangue and a Source for Environmental Pollution. Green Processing 2002 - Proceedings: International Conference on the Sustainable Proceesing of Minerals. 245-249.
- ^ Warr, L.N. (2021). IMA–CNMNC approved mineral symbols. Mineralogical Magazine, 85(3), 291-320. https://doi.org/10.1180/mgm.2021.43.
- ^ Whitney, D.L. and Evans, B.W. (2010) Abbreviations for names of rock-forming minerals. American Mineralogist, 95, 185–187 https://doi.org/10.2138/am.2010.3371.
- ^ The Canadian Mineralogist (2019) The Canadian Mineralogist list of symbols for rock- and ore-forming minerals (December 30, 2019). https://www.mineralogicalassociation.ca/wordpress/wp-content/uploads/2020/01/symbols.pdf.
External links
- Spencer, Leonard James (1911). . Encyclopædia Britannica (11th ed.).
