Glyceollin I: Difference between revisions
Appearance
Content deleted Content added
m corrected IUPAC name |
added Category:Heterocyclic compounds with 5 rings using HotCat |
||
| Line 60: | Line 60: | ||
[[Category:Pterocarpans]] |
[[Category:Pterocarpans]] |
||
[[Category:Phytoalexins]] |
[[Category:Phytoalexins]] |
||
[[Category:Heterocyclic compounds with 5 rings]] |
|||
{{aromatic-stub}} |
{{aromatic-stub}} |
||
Revision as of 21:54, 1 June 2023
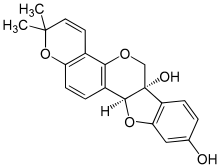
| |
| Names | |
|---|---|
| Preferred IUPAC name
(6aS,11aS)-2,2-Dimethyl-2H,6H-[1]benzofuro[3,2-c]pyrano[2,3-h][1]benzopyran-6a,9(11aH)-diol | |
| Other names
(−)-Glyceollin I
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.222.666 |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C20H18O5 | |
| Molar mass | 338 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Glyceollin I is a glyceollin, a type of prenylated pterocarpan. It is a phytoalexin found in the soybean.[1]
Glyceollin synthase is an enzyme responsible for the production of glyceollin.[2] The five substrates of this enzyme are 2-dimethylallyl-(6aS,11aS)-3,6a,9-trihydroxypterocarpan, 4-dimethylallyl-(6aS,11aS)-3,6a,9-trihydroxypterocarpan, NADPH, H+, and O2, whereas its three products are glyceollin, NADP+, and H2O.
In in vitro studies, this molecule has been shown to exhibit antiestrogenic properties.[3]
References
- ^ Zimmermann, M. C.; Tilghman, S. L.; Boué, S. M.; Salvo, V. A.; Elliott, S.; Williams, K. Y.; Skripnikova, E. V.; Ashe, H.; Payton-Stewart, F.; Vanhoy-Rhodes, L.; Fonseca, J. P.; Corbitt, C.; Collins-Burow, B. M.; Howell, M. H.; Lacey, M.; Shih, B. Y.; Carter-Wientjes, C.; Cleveland, T. E.; McLachlan, J. A.; Wiese, T. E.; Beckman, B. S.; Burow, M. E. (2009). "Glyceollin I, a Novel Antiestrogenic Phytoalexin Isolated from Activated Soy". Journal of Pharmacology and Experimental Therapeutics. 332 (1): 35–45. doi:10.1124/jpet.109.160382. PMC 2802480. PMID 19797619.
- ^ Welle, R.; Grisebach, H. (1988). "Induction of phytoalexin synthesis in soybean: Enzymatic cyclization of prenylated pterocarpans to glyceollin isomers". Archives of Biochemistry and Biophysics. 263 (1): 191–198. doi:10.1016/0003-9861(88)90627-3. PMID 3369863.
- ^ Payton-Stewart, F.; Khupse, R. S.; Boué, S. M.; Elliott, S.; Zimmermann, M. C.; Skripnikova, E. V.; Ashe, H.; Tilghman, S. L.; Beckman, B. S.; Cleveland, T. E.; McLachlan, J. A.; Bhatnagar, D.; Wiese, T. E.; Erhardt, P.; Burow, M. E. (2010). "Glyceollin I enantiomers distinctly regulate ER-mediated gene expression". Steroids. 75 (12): 870–878. doi:10.1016/j.steroids.2010.05.007. PMID 20493896. S2CID 14878980.
