Phenolphthalein: Difference between revisions
m Reverted edits by 12.171.15.114 (talk) to last version by 129.97.58.55 |
No edit summary |
||
| Line 48: | Line 48: | ||
|- |
|- |
||
|} |
|} |
||
<font face="comic sans ms"> |
|||
'''Phenolphthalein''' is a sensitive chemical with the formula C<sub> |
'''Phenolphthalein''' is a sensitive chemical with the formula C<sub>10</sub>H<sub>7</sub>O<sub>2</sub> (often written as "'''HIn'''" in shorthand notation). Often used in [[titration]]s, it turns colorless in [[acid]]ic solutions and pink in [[base (chemistry)|basic]] solutions. If the concentration of indicator is particularly strong, it can appear purple. In solutions containing a pH below 0, phenolphthalein turns a bright orange color. |
||
In strongly basic solutions, phenolphthalein's pink color undergoes a rather slow fading reaction and becomes colorless again. In other words, the molecule has four forms: |
In strongly basic solutions, phenolphthalein's pink color undergoes a rather slow fading reaction and becomes colorless again. In other words, the molecule has four forms: |
||
| Line 123: | Line 123: | ||
[[tr:Fenolftalein]] |
[[tr:Fenolftalein]] |
||
[[zh:酚酞]] |
[[zh:酚酞]] |
||
</font> |
|||
Revision as of 16:53, 17 March 2007
| Phenolphthalein (pH indicator) | ||
| below pH 8.2 | above pH 10.0 | |
| colorless | ⇌ | reddish purple |
| Phenolphthalein | |
|---|---|
| |
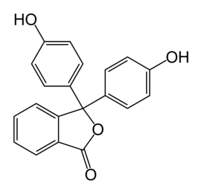
| Systematic name | Phenolphthalein |
| Chemical formula | C20H14O4 |
| Molecular mass | 318.33 g mol−1 |
| Density | 1.299 g cm−3, solid |
| Melting point | 263-265 °C |
| Boiling point | 258 °C |
| CAS number | [77-09-8] |
| SMILES | Oc1ccc(cc1)C3(OC(=O)c2ccccc23) c4ccc(O)cc4 |
| Disclaimer and references | |
Phenolphthalein is a sensitive chemical with the formula C10H7O2 (often written as "HIn" in shorthand notation). Often used in titrations, it turns colorless in acidic solutions and pink in basic solutions. If the concentration of indicator is particularly strong, it can appear purple. In solutions containing a pH below 0, phenolphthalein turns a bright orange color.
In strongly basic solutions, phenolphthalein's pink color undergoes a rather slow fading reaction and becomes colorless again. In other words, the molecule has four forms:
| species | ||||
|---|---|---|---|---|
| structure |  |
 |
 | |
| model |  |
 |

| |
| pH | ||||
| conditions | ||||
| color | ||||
| image |  |
The rather slow fading reaction that produces the colorless In−OH3− ion is sometimes used in classes for the study of reaction kinetics.
Phenolphthalein is insoluble in water, and is usually dissolved in alcohols for use in experiments. It is itself a weak acid, which can lose H+ ions in solution. The phenolphthalein molecule is colorless. However, the phenolphthalein ion is pink. When a base is added to the phenolphthalein, the molecule ⇌ ions equilibrium shifts to the right, leading to more ionization as H+ ions are removed. This is predicted by Le Chatelier's principle.
Phenolphthalein is synthesized by condensation of phthalic anhydride with two equivalents of phenol under acidic conditions (hence the name). It was discovered in 1871 by Adolf von Baeyer.
Uses
Phenolphthalein has been used for over a century as a laxative, but is now being removed from the market because of concerns over carcinogenicity. However, the small amounts usually used in experiments are harmless.[citation needed] Phenolphthalein is also commonly used in a mixture, primarily by forensic scientists, to test for the presence of blood.
Phenolphthalein is used to perform a presumptive blood test, and is commonly known as the Kastle-Meyer test. A dry sample is collected with a swab or filter paper. First a few drops of alcohol, then a few drops of phenolphthalein and finally a few drops of hydrogen peroxide are dripped onto the sample. If the sample turns pink then it is a positive test. This test is nondestructive to the sample; it can be kept and used in further tests at the lab. This test has the same reaction with blood from any animal, so further testing would be required to determine whether it originates from a human.
Phenolphthalein is used in toys, for example as a component of disappearing inks, or disappearing dye on the Hollywood Hair Barbie hair. In the ink it is mixed with sodium hydroxide, which reacts with carbon dioxide in the air. This reaction leads to the pH falling below the color change threshold as hydrogen ions are released via the reaction:
- OH− (aq) + CO2 → CO32− (aq) + H+ (aq)
To develop the hair and "magic" graphical patterns, the ink is sprayed with a solution of hydroxide, which leads to the appearance of the hidden graphics by the same mechanism described above for colour change in alkaline solution. The pattern will eventually disappear by the same reaction with carbon dioxide detailed above. Thymolphthalein is used for the same purpose and in the same way, when blue color is desired. [1]

