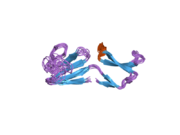MSMB: Difference between revisions
m Open access bot: doi updated in citation with #oabot. |
Citation bot (talk | contribs) Add: doi-access. | Use this bot. Report bugs. | #UCB_CommandLine |
||
| Line 9: | Line 9: | ||
== Evolution and structure == |
== Evolution and structure == |
||
MSMB is a rapidly evolving protein.<ref name="pmid2037304">{{cite journal |vauthors=Nolet S, St-Louis D, Mbikay M, Chrétien M | title = Rapid evolution of prostatic protein PSP94 suggested by sequence divergence between rhesus monkey and human cDNAs | journal = Genomics | volume = 9 | issue = 4 | pages = 775–7 |date=April 1991 | pmid = 2037304 | doi = 10.1016/0888-7543(91)90375-o}}</ref> Solution structures of human and porcine MSMB show remarkable similarity despite having only 51% of amino acids in common.<ref name="Ghasriani_2006">{{cite journal |vauthors=Ghasriani H, Teilum K, Johnsson Y, Fernlund P, Drakenberg T | title = Solution structures of human and porcine beta-microseminoprotein | journal = J. Mol. Biol. | volume = 362 | issue = 3 | pages = 502–15 |date=September 2006 | pmid = 16930619 | doi = 10.1016/j.jmb.2006.07.029 }}</ref> The C-terminus domain of MSMB contains two two-stranded β-sheets; these have no resemblance to other structural motifs.<ref name="Ghasriani_2006"/> The rapid evolution of MSMB can be attributed to either sexual selection or innate pathogen defense;<ref name="pmid16170411">{{cite journal |vauthors=Clark NL, Swanson WJ | title = Pervasive adaptive evolution in primate seminal proteins | journal = PLOS Genet. | volume = 1 | issue = 3 | pages = e35 |date=September 2005 | pmid = 16170411 | pmc = 1201370 | doi = 10.1371/journal.pgen.0010035 }} {{open access}}</ref> the wide distribution of MSMB in the body and the fungicidal properties of the C-terminus suggest that innate pathogen defense plays a role in driving this evolution.<ref name="pmid22496651">{{cite journal |vauthors=Edström Hägerwall AM, Rydengård V, Fernlund P, Mörgelin M, Baumgarten M, Cole AM, Malmsten M, Kragelund BB, Sørensen OE | title = β-Microseminoprotein endows post coital seminal plasma with potent candidacidal activity by a calcium- and pH-dependent mechanism | journal = PLOS Pathog. | volume = 8 | issue = 4 | pages = e1002625 | year = 2012 | pmid = 22496651 | pmc = 3320615 | doi = 10.1371/journal.ppat.1002625 }} {{open access}}</ref> |
MSMB is a rapidly evolving protein.<ref name="pmid2037304">{{cite journal |vauthors=Nolet S, St-Louis D, Mbikay M, Chrétien M | title = Rapid evolution of prostatic protein PSP94 suggested by sequence divergence between rhesus monkey and human cDNAs | journal = Genomics | volume = 9 | issue = 4 | pages = 775–7 |date=April 1991 | pmid = 2037304 | doi = 10.1016/0888-7543(91)90375-o}}</ref> Solution structures of human and porcine MSMB show remarkable similarity despite having only 51% of amino acids in common.<ref name="Ghasriani_2006">{{cite journal |vauthors=Ghasriani H, Teilum K, Johnsson Y, Fernlund P, Drakenberg T | title = Solution structures of human and porcine beta-microseminoprotein | journal = J. Mol. Biol. | volume = 362 | issue = 3 | pages = 502–15 |date=September 2006 | pmid = 16930619 | doi = 10.1016/j.jmb.2006.07.029 }}</ref> The C-terminus domain of MSMB contains two two-stranded β-sheets; these have no resemblance to other structural motifs.<ref name="Ghasriani_2006"/> The rapid evolution of MSMB can be attributed to either sexual selection or innate pathogen defense;<ref name="pmid16170411">{{cite journal |vauthors=Clark NL, Swanson WJ | title = Pervasive adaptive evolution in primate seminal proteins | journal = PLOS Genet. | volume = 1 | issue = 3 | pages = e35 |date=September 2005 | pmid = 16170411 | pmc = 1201370 | doi = 10.1371/journal.pgen.0010035 | doi-access = free }} {{open access}}</ref> the wide distribution of MSMB in the body and the fungicidal properties of the C-terminus suggest that innate pathogen defense plays a role in driving this evolution.<ref name="pmid22496651">{{cite journal |vauthors=Edström Hägerwall AM, Rydengård V, Fernlund P, Mörgelin M, Baumgarten M, Cole AM, Malmsten M, Kragelund BB, Sørensen OE | title = β-Microseminoprotein endows post coital seminal plasma with potent candidacidal activity by a calcium- and pH-dependent mechanism | journal = PLOS Pathog. | volume = 8 | issue = 4 | pages = e1002625 | year = 2012 | pmid = 22496651 | pmc = 3320615 | doi = 10.1371/journal.ppat.1002625 | doi-access = free }} {{open access}}</ref> |
||
== Function == |
== Function == |
||
Revision as of 02:53, 3 December 2023
| MSMB | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | MSMB, HPC13, IGBF, MSP, MSPB, PN44, PRPS, PSP, PSP-94, PSP57, PSP94, microseminoprotein, beta-, microseminoprotein beta | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 157145; MGI: 97166; HomoloGene: 1832; GeneCards: MSMB; OMA:MSMB - orthologs | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Beta-microseminoprotein is a protein that in humans is encoded by the MSMB gene.[5][6] For historical reasons, the scientific literature may also refer to this protein as Prostate secretory protein 94 (PSP94), microseminoprotein (MSP), microseminoprotein-beta (MSMB), beta-inhibitin, prostatic inhibin peptide (PIP), and inhibitin like material (ILM).
Distribution
MSMB is one of the three major proteins secreted by the epithelial cells of the prostate[7] and has a concentration in seminal plasma of 0.5 to 1 mg/mL[8] Two comprehensive studies of beta-microseminoprotein in tissue have shown that it is secreted by epithelial cells in many other organs: liver, lung, breast, kidney, colon, stomach, pancreas, esophagus, duodenum, salivary glands, fallopian tube, corpus uteri, bulbourethral glands and cervix.[9][10] This list corresponds closely to the sites from which all late onset cancers develop.[11]
Evolution and structure
MSMB is a rapidly evolving protein.[12] Solution structures of human and porcine MSMB show remarkable similarity despite having only 51% of amino acids in common.[13] The C-terminus domain of MSMB contains two two-stranded β-sheets; these have no resemblance to other structural motifs.[13] The rapid evolution of MSMB can be attributed to either sexual selection or innate pathogen defense;[14] the wide distribution of MSMB in the body and the fungicidal properties of the C-terminus suggest that innate pathogen defense plays a role in driving this evolution.[15]
Function
Beta-microseminoprotein is a member of the immunoglobulin binding factor family. This protein has been reported to have inhibin-like properties,[16] though this finding has been disputed.[17][18] It may have a role as an autocrine and/or paracrine factor in uterine, breast, and other female reproductive tissues.[citation needed] Two alternatively spliced transcript variants encoding different isoforms are described for this gene. Despite having only 4 out of 11 amino acids in common, both the porcine and human fungicidal peptide on MSMB's C-terminus are potently fungicidal in the absence of calcium ions.[15] The protein inhibits growth of cancer cells in an experimental model of prostate cancer,[19][20] though this property is cell line–specific.[21]
Clinical significance
Two large genome-wide association studies showed that decreased expression of the MSMB protein caused by the rs10993994 single nucleotide polymorphism is associated with an increased risk of developing prostate cancer (odds ratio for CT allele pair ~1.2x, and for TT allele pair ~1.6x, when compared to the low risk CC allele pair).[22] A 2003 study proposed using a truncated form of the MSMB protein called PSP61 as a biomarker for benign prostatic hyperplasia (BPH). This study found PSP61 in the expressed prostatic secretion of 10 out of 10 men suffering from BPH, while not finding it in 10 out of 10 age-matched BPH-free men.[23] This truncated form of the MSMB protein lacks the fungicidal peptide identified in 2012. The expression of MSMB is found to be decreased in prostate cancer, so it may be usable as a biomarker for prostate cancer.[24] Urinary MSMB has been found to be superior to urinary PSA at differentiating men with prostate cancer, at all Gleason grades.[25]
References
- ^ a b c GRCh38: Ensembl release 89: ENSG00000263639 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000021907 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
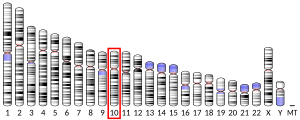
- ^ Ulvsback M, Spurr NK, Lundwall A (Mar 1992). "Assignment of the human gene for beta-microseminoprotein (MSMB) to chromosome 10 and demonstration of related genes in other vertebrates". Genomics. 11 (4): 920–4. doi:10.1016/0888-7543(91)90015-7. PMID 1783399.
- ^ "Entrez Gene: MSMB microseminoprotein, beta-".
- ^ Lilja H, Abrahamsson PA (1988). "Three predominant proteins secreted by the human prostate gland". Prostate. 12 (1): 29–38. doi:10.1002/pros.2990120105. PMID 3347596. S2CID 12843459.
- ^ Valtonen-André C, Sävblom C, Fernlund P, Lilja H, Giwercman A, Lundwall A (2008). "Beta-microseminoprotein in serum correlates with the levels in seminal plasma of young, healthy males". J. Androl. 29 (3): 330–7. doi:10.2164/jandrol.107.003616. PMID 18222915.
- ^ Weiber H, Andersson C, Murne A, Rannevik G, Lindström C, Lilja H, Fernlund P (September 1990). "Beta microseminoprotein is not a prostate-specific protein. Its identification in mucous glands and secretions". Am. J. Pathol. 137 (3): 593–603. PMC 1877516. PMID 2205099.
- ^ Ohkubo I, Tada T, Ochiai Y, Ueyama H, Eimoto T, Sasaki M (June 1995). "Human seminal plasma beta-microseminoprotein: its purification, characterization, and immunohistochemical localization". Int. J. Biochem. Cell Biol. 27 (6): 603–11. doi:10.1016/1357-2725(95)00021-G. PMID 7671139.
- ^ Laurence M (2018). PSP94, what is it good for? Sixth edition.
- ^ Nolet S, St-Louis D, Mbikay M, Chrétien M (April 1991). "Rapid evolution of prostatic protein PSP94 suggested by sequence divergence between rhesus monkey and human cDNAs". Genomics. 9 (4): 775–7. doi:10.1016/0888-7543(91)90375-o. PMID 2037304.
- ^ a b Ghasriani H, Teilum K, Johnsson Y, Fernlund P, Drakenberg T (September 2006). "Solution structures of human and porcine beta-microseminoprotein". J. Mol. Biol. 362 (3): 502–15. doi:10.1016/j.jmb.2006.07.029. PMID 16930619.
- ^ Clark NL, Swanson WJ (September 2005). "Pervasive adaptive evolution in primate seminal proteins". PLOS Genet. 1 (3): e35. doi:10.1371/journal.pgen.0010035. PMC 1201370. PMID 16170411.

- ^ a b Edström Hägerwall AM, Rydengård V, Fernlund P, Mörgelin M, Baumgarten M, Cole AM, Malmsten M, Kragelund BB, Sørensen OE (2012). "β-Microseminoprotein endows post coital seminal plasma with potent candidacidal activity by a calcium- and pH-dependent mechanism". PLOS Pathog. 8 (4): e1002625. doi:10.1371/journal.ppat.1002625. PMC 3320615. PMID 22496651.

- ^ Sheth AR, Arabatti N, Carlquist M, Jörnvall H (January 1984). "Characterization of a polypeptide from human seminal plasma with inhibin (inhibition of FSH secretion)-like activity". FEBS Lett. 165 (1): 11–5. doi:10.1016/0014-5793(84)80004-6. PMID 6692908. S2CID 40923254.
- ^ Kohan S, Fröysa B, Cederlund E, Fairwell T, Lerner R, Johansson J, Khan S, Ritzen M, Jörnvall H, Cekan S (April 1986). "Peptides of postulated inhibin activity. Lack of in vitro inhibin activity of a 94-residue peptide isolated from human seminal plasma, and of a synthetic replicate of its C-terminal 28-residue segment". FEBS Lett. 199 (2): 242–8. doi:10.1016/0014-5793(86)80488-4. PMID 3084296. S2CID 33940801.
- ^ Gordon WL, Liu WK, Akiyama K, Tsuda R, Hara M, Schmid K, Ward DN (May 1987). "Beta-microseminoprotein (beta-MSP) is not an inhibin". Biol. Reprod. 36 (4): 829–35. doi:10.1095/biolreprod36.4.829. PMID 3109514.
- ^ Garde S, Sheth A, Porter AT, Pienta KJ (1993). "Effect of prostatic inhibin peptide (PIP) on prostate cancer cell growth in vitro and in vivo". Prostate. 22 (3): 225–33. doi:10.1002/pros.2990220305. PMID 8488155. S2CID 20872869.
- ^ Shukeir N, Arakelian A, Kadhim S, Garde S, Rabbani SA (May 2003). "Prostate secretory protein PSP-94 decreases tumor growth and hypercalcemia of malignancy in a syngenic in vivo model of prostate cancer". Cancer Res. 63 (9): 2072–8. PMID 12727822.
- ^ Pathak BR, Breed AA, Nakhawa VH, Jagtap DD, Mahale SD (September 2010). "Growth inhibition mediated by PSP94 or CRISP-3 is prostate cancer cell line specific". Asian J. Androl. 12 (5): 677–89. doi:10.1038/aja.2010.56. PMC 3739318. PMID 20676114.
- ^ Eeles RA, Kote-Jarai Z, Giles GG, Olama AA, Guy M, Jugurnauth SK, Mulholland S, Leongamornlert DA, Edwards SM, Morrison J, et al. (March 2008). "Multiple newly identified loci associated with prostate cancer susceptibility". Nat. Genet. 40 (3): 316–21. doi:10.1038/ng.90. PMID 18264097. S2CID 30968525.
- ^ Xu K, Wang X, Ling MT, Lee DT, Fan T, Chan FL, Xuan JJ, Tsao SW, Wong YC (April 2003). "Identification of a specifically expressed modified form of novel PSP-94 protein in the secretion of benign prostatic hyperplasia". Electrophoresis. 24 (7–8): 1311–8. doi:10.1002/elps.200390167. PMID 12707925. S2CID 21348603.
- ^ Whitaker HC, Warren AY, Eeles R, Kote-Jarai Z, Neal DE (February 2010). "The potential value of microseminoprotein-beta as a prostate cancer biomarker and therapeutic target". Prostate. 70 (3): 333–40. doi:10.1002/pros.21059. PMID 19790236. S2CID 206397505.
- ^ Whitaker HC, Kote-Jarai Z, Ross-Adams H, Warren AY, Burge J, George A, Bancroft E, Jhavar S, Leongamornlert D, Tymrakiewicz M, et al. (2010). "The rs10993994 risk allele for prostate cancer results in clinically relevant changes in microseminoprotein-beta expression in tissue and urine". PLOS ONE. 5 (10): e13363. Bibcode:2010PLoSO...513363W. doi:10.1371/journal.pone.0013363. PMC 2954177. PMID 20967219.

Further reading
- Liang ZG, Kamada M, Koide SS (1991). "Structural identity of immunoglobulin binding factor and prostatic secretory protein of human seminal plasma". Biochem. Biophys. Res. Commun. 180 (1): 356–9. doi:10.1016/S0006-291X(05)81300-2. PMID 1930232.
- Nolet S, Mbikay M, Chrétien M (1991). "Prostatic secretory protein PSP94: gene organization and promoter sequence in rhesus monkey and human". Biochim. Biophys. Acta. 1089 (2): 247–9. doi:10.1016/0167-4781(91)90016-F. PMID 2054385.
- Green CB, Liu WY, Kwok SC (1990). "Cloning and nucleotide sequence analysis of the human beta-microseminoprotein gene". Biochem. Biophys. Res. Commun. 167 (3): 1184–90. doi:10.1016/0006-291X(90)90648-7. PMID 2322265.
- Ito Y, Tsuda R, Kimura H (1989). "Ultrastructural localizations of beta-microseminoprotein, a prostate-specific antigen, in human prostate and sperm: comparison with gamma-seminoprotein, another prostate-specific antigen". J. Lab. Clin. Med. 114 (3): 272–7. PMID 2475560.
- Ulvsbäck M, Lindström C, Weiber H, et al. (1989). "Molecular cloning of a small prostate protein, known as beta-microsemenoprotein, PSP94 or beta-inhibin, and demonstration of transcripts in non-genital tissues". Biochem. Biophys. Res. Commun. 164 (3): 1310–5. doi:10.1016/0006-291X(89)91812-3. PMID 2590204.
- Abrahamsson PA, Andersson C, Björk T, et al. (1989). "Radioimmunoassay of beta-microseminoprotein, a prostatic-secreted protein present in sera of both men and women". Clin. Chem. 35 (7): 1497–503. doi:10.1093/clinchem/35.7.1497. PMID 2758596.
- Mbikay M, Nolet S, Fournier S, et al. (1987). "Molecular cloning and sequence of the cDNA for a 94-amino-acid seminal plasma protein secreted by the human prostate". DNA. 6 (1): 23–9. doi:10.1089/dna.1987.6.23. PMID 3829888.
- Akiyama K, Yoshioka Y, Schmid K, et al. (1985). "The amino acid sequence of human beta-microseminoprotein". Biochim. Biophys. Acta. 829 (2): 288–94. doi:10.1016/0167-4838(85)90200-6. PMID 3995056.
- Seidah NG, Arbatti NJ, Rochemont J, et al. (1984). "Complete amino acid sequence of human seminal plasma beta-inhibin. Prediction of post Gln-Arg cleavage as a maturation site". FEBS Lett. 175 (2): 349–55. doi:10.1016/0014-5793(84)80766-8. PMID 6434350. S2CID 82405441.
- Liu AY, Bradner RC, Vessella RL (1994). "Decreased expression of prostatic secretory protein PSP94 in prostate cancer". Cancer Lett. 74 (1–2): 91–9. doi:10.1016/0304-3835(93)90049-F. PMID 7506990.
- Xuan JW, Chin JL, Guo Y, et al. (1995). "Alternative splicing of PSP94 (prostatic secretory protein of 94 amino acids) mRNA in prostate tissue". Oncogene. 11 (6): 1041–7. PMID 7566962.
- Ochiai Y, Inazawa J, Ueyama H, Ohkubo I (1995). "Human gene for beta-microseminoprotein: its promoter structure and chromosomal localization". J. Biochem. 117 (2): 346–52. doi:10.1093/jb/117.2.346. PMID 7608123.
- Ohkubo I, Tada T, Ochiai Y, et al. (1995). "Human seminal plasma beta-microseminoprotein: its purification, characterization, and immunohistochemical localization". Int. J. Biochem. Cell Biol. 27 (6): 603–11. doi:10.1016/1357-2725(95)00021-G. PMID 7671139.
- Fernlund P, Granberg LB, Roepstorff P (1994). "Amino acid sequence of beta-microseminoprotein from porcine seminal plasma". Arch. Biochem. Biophys. 309 (1): 70–6. doi:10.1006/abbi.1994.1086. PMID 8117114.
- Sasaki T, Matsumoto N, Jinno Y, et al. (1997). "Assignment of the human beta-microseminoprotein gene (MSMB) to chromosome 10q11.2". Cytogenet. Cell Genet. 72 (2–3): 177–8. doi:10.1159/000134180. PMID 8978767.
- Kamada M, Mori H, Maeda N, et al. (1998). "beta-Microseminoprotein/prostatic secretory protein is a member of immunoglobulin binding factor family". Biochim. Biophys. Acta. 1388 (1): 101–10. doi:10.1016/S0167-4838(98)00164-2. PMID 9774712.
- Mäkinen M, Valtonen-André C, Lundwall A (1999). "New world, but not Old World, monkeys carry several genes encoding beta-microseminoprotein". Eur. J. Biochem. 264 (2): 407–14. doi:10.1046/j.1432-1327.1999.00614.x. PMID 10491085.
- Baijal-Gupta M, Clarke MW, Finkelman MA, et al. (2000). "Prostatic secretory protein (PSP94) expression in human female reproductive tissues, breast and in endometrial cancer cell lines". J. Endocrinol. 165 (2): 425–33. doi:10.1677/joe.0.1650425. PMID 10810306.