Ullmann reaction: Difference between revisions
Citation bot (talk | contribs) Alter: date, journal. Add: pmid, s2cid, authors 1-1. Removed parameters. Some additions/deletions were parameter name changes. | Use this bot. Report bugs. | Suggested by MaxP34 | Category:CS1 errors: dates | #UCB_Category 2/45 |
Added some more references and information in the Mechanisms section. |
||
| Line 15: | Line 15: | ||
==Mechanism== |
==Mechanism== |
||
The [[reaction mechanism]] of the Ullmann reaction has been extensively studied. [[Electron spin resonance]] rules out a [[radical (chemistry)|radical]] intermediate. This was confirmed in a set of experiments performed in 2008 by Hartwig and c-workers.<ref>{{Cite journal |last=Tye |first=Jesse W. |last2=Weng |first2=Zhiqiang |last3=Johns |first3=Adam M. |last4=Incarvito |first4=Christopher D. |last5=Hartwig |first5=John F. |date=2008-07-01 |title=Copper Complexes of Anionic Nitrogen Ligands in the Amidation and Imidation of Aryl Halides |url=http://dx.doi.org/10.1021/ja076668w |journal=Journal of the American Chemical Society |volume=130 |issue=30 |pages=9971–9983 |doi=10.1021/ja076668w |issn=0002-7863}}</ref> The [[oxidative addition]] / [[reductive elimination]] sequence observed with [[palladium]] catalysts is unlikely for [[copper]] because copper(III) is rarely observed. The reaction likely involves the formation of an [[organocopper compound]] (RCuX) which reacts with the other aryl reactant in a [[nucleophilic aromatic substitution]]. Alternative mechanisms have been proposed such as [[σ-bond metathesis]].<ref>Derek van Allen, PhD Thesis, [[University of Massachusetts at Amherst]] '''2004'''. [http://www.people.umass.edu/dv/group/pdf/dvathesis.pdf Electronic thesis]</ref><ref>{{Cite journal |last=Bacon |first=R. G. R. |last2=Hill |first2=H. A. O. |date=1964 |title=210. Metal ions and complexes in organic reactions. Part I. Substitution reactions between aryl halides and cuprous salts in organic solvents |url=http://dx.doi.org/10.1039/jr9640001097 |journal=Journal of the Chemical Society (Resumed) |pages=1097 |doi=10.1039/jr9640001097 |issn=0368-1769}}</ref><ref>{{Cite journal |last=Weingarten |first=Harold |date=1964-12 |title=Mechanism of the Ullmann Condensation 1 |url=https://pubs.acs.org/doi/abs/10.1021/jo01035a046 |journal=The Journal of Organic Chemistry |language=en |volume=29 |issue=12 |pages=3624–3626 |doi=10.1021/jo01035a046 |issn=0022-3263}}</ref> There is still debate on the true mechanism of the Ullmann reaction.<ref>{{Cite journal |last=Sambiagio |first=Carlo |last2=Marsden |first2=Stephen P. |last3=Blacker |first3=A. John |last4=McGowan |first4=Patrick C. |date=2014-04-22 |title=Copper catalysed Ullmann type chemistry: from mechanistic aspects to modern development |url=https://pubs.rsc.org/en/content/articlelanding/2014/cs/c3cs60289c |journal=Chemical Society Reviews |language=en |volume=43 |issue=10 |pages=3525–3550 |doi=10.1039/C3CS60289C |issn=1460-4744}}</ref> |
|||
The [[reaction mechanism|mechanism]] of the Ullmann reaction is extensively studied. Complications arise because the reactions are often heterogeneous. With copper as the halide acceptor, [[organocopper]] intermediates are invoked. |
|||
==Scope== |
==Scope== |
||
| Line 52: | Line 52: | ||
==Imidazole Ullmann reaction== |
==Imidazole Ullmann reaction== |
||
The [[reaction mechanism]] of the Ullmann reaction is extensively studied. [[Electron spin resonance]] rules out a [[radical (chemistry)|radical]] intermediate. The [[oxidative addition]] / reductive elimination sequence observed with [[palladium]] catalysts is unlikely for [[copper]] because copper(III) is rarely observed. The reaction probably involves the formation of an [[organocopper compound]] (RCuX) which reacts with the other aryl reactant in a [[nucleophilic aromatic substitution]]. Alternative mechanisms do exist such as [[σ-bond metathesis]].<ref>Derek van Allen, PhD Thesis, [[University of Massachusetts at Amherst]] '''2004'''. [http://www.people.umass.edu/dv/group/pdf/dvathesis.pdf Electronic thesis]</ref> |
|||
The Ullmann reaction is limited to electron-deficient aryl halides and requires harsh reaction conditions. In [[organic synthesis]] this reaction is often replaced by palladium coupling reactions such as the [[Heck reaction]], the [[Hiyama coupling]], and the [[Sonogashira coupling]] |
The Ullmann reaction is limited to electron-deficient aryl halides and requires harsh reaction conditions. In [[organic synthesis]] this reaction is often replaced by palladium coupling reactions such as the [[Heck reaction]], the [[Hiyama coupling]], and the [[Sonogashira coupling]] |
||
Revision as of 05:53, 8 December 2023
| Ullmann reaction | |
|---|---|
| Named after | Fritz Ullmann |
| Reaction type | Coupling reaction |
| Identifiers | |
| Organic Chemistry Portal | ullmann-reaction |
| RSC ontology ID | RXNO:0000040 |
The Ullmann reaction or Ullmann coupling, named after Fritz Ullmann, couples two aryl or alkyl groups with the help of copper. The reaction was first reported by Ullmann and his student Bielecki in 1901. It has been later shown that palladium and nickel can also be effectively used.[1][2]
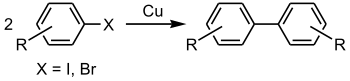
Aryl-Aryl bond formation is a fundamental tool in modern organic synthesis, with applications spanning natural product synthesis, pharmaceuticals, agrochemicals, and the development of commercial dyes and polyaromatics. With over a century of history, the Ullmann reaction has been one of the first to use a transition metal, primarily copper, in its higher oxidation states. Despite the significant implications of biaryl coupling in industries, the Ullmann reaction was plagued by a number of problems in its early development. However, in modern times the Ullmann reaction has revived interest due to several advantages of copper over other catalytic metals.
Mechanism
The reaction mechanism of the Ullmann reaction has been extensively studied. Electron spin resonance rules out a radical intermediate. This was confirmed in a set of experiments performed in 2008 by Hartwig and c-workers.[3] The oxidative addition / reductive elimination sequence observed with palladium catalysts is unlikely for copper because copper(III) is rarely observed. The reaction likely involves the formation of an organocopper compound (RCuX) which reacts with the other aryl reactant in a nucleophilic aromatic substitution. Alternative mechanisms have been proposed such as σ-bond metathesis.[4][5][6] There is still debate on the true mechanism of the Ullmann reaction.[7]
Scope
Fritz Ullmann and his student Bielecki were the first to report the reaction.[8] This groundbreaking result was the first to show that a transition metal could help perform an aryl carbon-carbon bond formation.
A typical example of classic Ullmann biaryl coupling is the conversion of ortho-chloronitrobenzene into 2,2'-dinitrobiphenyl with a copper - bronze alloy.[9][10]
- 2 C6H4(NO2)Cl + 2 Cu → (C6H4(NO2))2 + 2 CuCl
The reaction has been applied to fairly elaborate substrates.
The traditional version of the Ullmann reaction requires stoichimoetric equivalents of copper, harsh reaction conditions, and the reaction has a reputation for erratic yields. The traditional Ullmann reaction thus had poor atom economy and produced toxic CuI. Because of these problems many improvements and alternative procedures have been introduced.[11][12][13]
The classical Ullmann reaction is limited to electron deficient aryl halides (hence the example of 2-nitrophenyl chloride above) and requires harsh reaction conditions. Modern variants of the Ullman reaction employing palladium and nickel have widened the substrate scope of the reaction and rendered reaction conditions more mild. Yields are generally still moderate, however.[14] In organic synthesis this reaction is often replaced by palladium coupling reactions such as the Heck reaction, the Hiyama coupling, and the Sonogashira coupling.
Biphenylenes had been obtained before with reasonable yields using 2,2-diiodobiphenyl or 2,2-diiodobiphenylonium ion as starting material.
Closure of 5-membered rings is more facile, but larger rings have also been made using this approach.
Unsymmetric and asymmetric couplings
Ullmann synthesis of biaryl compounds can be used to generate chiral products from chiral reactants.[15] Nelson and collaborators worked on the synthesis of asymmetric biaryl compounds and obtained the thermodynamically controlled product.[15]
The diastereomeric ratio of the products is enhanced with bulkier R groups in the auxiliary oxazoline group.
Unsymmetrical Ullmann reactions are rarely pursued but have been achieved when one of the two coupling components is in excess.[12]
Imidazole Ullmann reaction
The Ullmann reaction is limited to electron-deficient aryl halides and requires harsh reaction conditions. In organic synthesis this reaction is often replaced by palladium coupling reactions such as the Heck reaction, the Hiyama coupling, and the Sonogashira coupling
In a variation of the Ullmann reaction, β-bromostyrene is reacted with imidazole in an ionic liquid such as 1-butyl-3-methylimidazolium tetrafluoroborate to give an N-styrylimidazole.[16] The reaction requires Lproline in addition to copper iodide as catalyst.

See also
- Ullmann condensation - copper-promoted conversion of aryl halides to ethers, also developed by Fritz Ullmann
- Copper(I) thiophene-2-carboxylate, a copper reagent used in the Ullmann reaction
- Wurtz–Fittig reaction, a similar reaction useful for alkylbenzenes synthesis
References
- ^ Yin; Liebscher, Jürgen (2007-01-01). "Carbon−Carbon Coupling Reactions Catalyzed by Heterogeneous Palladium Catalysts". Chemical Reviews. 107 (1): 133–173. doi:10.1021/cr0505674. ISSN 0009-2665.
- ^ Nelson, Todd D.; Crouch, R. David (2004-11-23). "Cu‐, Ni‐, and Pd‐Mediated Homocoupling Reactions in Biaryl Syntheses: The Ullmann Reaction". ChemInform. 35 (51). doi:10.1002/chin.200451250. ISSN 0931-7597.
- ^ Tye, Jesse W.; Weng, Zhiqiang; Johns, Adam M.; Incarvito, Christopher D.; Hartwig, John F. (2008-07-01). "Copper Complexes of Anionic Nitrogen Ligands in the Amidation and Imidation of Aryl Halides". Journal of the American Chemical Society. 130 (30): 9971–9983. doi:10.1021/ja076668w. ISSN 0002-7863.
- ^ Derek van Allen, PhD Thesis, University of Massachusetts at Amherst 2004. Electronic thesis
- ^ Bacon, R. G. R.; Hill, H. A. O. (1964). "210. Metal ions and complexes in organic reactions. Part I. Substitution reactions between aryl halides and cuprous salts in organic solvents". Journal of the Chemical Society (Resumed): 1097. doi:10.1039/jr9640001097. ISSN 0368-1769.
- ^ Weingarten, Harold (1964-12). "Mechanism of the Ullmann Condensation 1". The Journal of Organic Chemistry. 29 (12): 3624–3626. doi:10.1021/jo01035a046. ISSN 0022-3263.
{{cite journal}}: Check date values in:|date=(help) - ^ Sambiagio, Carlo; Marsden, Stephen P.; Blacker, A. John; McGowan, Patrick C. (2014-04-22). "Copper catalysed Ullmann type chemistry: from mechanistic aspects to modern development". Chemical Society Reviews. 43 (10): 3525–3550. doi:10.1039/C3CS60289C. ISSN 1460-4744.
- ^ Ullmann, F.; Bielecki, Jean (May 1901). "Ueber Synthesen in der Biphenylreihe". Berichte der Deutschen Chemischen Gesellschaft. 34 (2): 2174–2185. doi:10.1002/cber.190103402141. ISSN 0365-9496.
- ^ Reynold C. Fuson; E. A. Cleveland (1940). "2,2'-Dinitrobiphenyl". Org. Synth. 20: 45. doi:10.15227/orgsyn.020.0045.
- ^ Fanta, P.E. (1974). "The Ullmann Synthesis of Biaryls". Synthesis. 1974: 9–21. doi:10.1055/s-1974-23219. PMID 21016995. S2CID 30018391.
- ^ Beletkaya, I.P.; Cheprakov, A.V. (2004). "Copper in Cross Coupling Reactions: The Post Ullman Chemistry". Coord. Chem. Rev. 248: 2337–2364. doi:10.1016/j.ccr.2004.09.014.
- ^ a b J. Hassan; M. Sevignon; C. Gozzi; E. Schulz; M. Lemaire (2002). "Aryl–Aryl Bond Formation One Century after the Discovery of the Ullmann Reaction". Chemical Reviews. 102 (5): 1359–1470. doi:10.1021/cr000664r. PMID 11996540.
- ^ Sambiagio, Carlo; Marsden, Stephen P.; Blacker, A. John; McGowan, Patrick C. (2014-04-22). "Copper catalysed Ullmann type chemistry: from mechanistic aspects to modern development". Chemical Society Reviews. 43 (10): 3525–3550. doi:10.1039/C3CS60289C. ISSN 1460-4744. PMID 24585151.
- ^ Nelson, T. D.; Crouch, R. D. (2004). "Cu, Ni, and Pd Mediated Homocoupling Reactions in Biaryl Syntheses: The Ullmann Reaction". Org. React. 63: 265. doi:10.1002/0471264180.or063.03. ISBN 0-471-26418-0.
- ^ a b Nelson, T.D.; Meyers, A.I. (1994). "The asymmetric Ullman reaction, 2. The synthesis of enantiomerically pure C2-Symmetric Binaphtyls". J. Org. Chem. 59 (9): 2655–2658. doi:10.1021/jo00088a066.
- ^ Zhiming Wang, Weiliang Bao and Yong Jiang, "L-Proline promoted Ullmann-type reaction of vinyl bromides with imidazoles in ionic liquids", Chemical Communications, 2005, 2849-51. doi:10.1039/b501628b




