Sodium cyanoborohydride: Difference between revisions
→Reductive amination (Borch reaction): rm image |
→Reductive deoxygenation of ketones: not mentioned in eEROS, un-notable |
||
| Line 62: | Line 62: | ||
=== Reductive amination (Borch reaction) === |
=== Reductive amination (Borch reaction) === |
||
[[Reductive amination]], sometimes called the ''Borch reaction'', is the conversion of a [[Carbonyl group|carbonyl]] into an [[amine]] through an intermediate [[imine]].<ref>{{OrgSynth|author=Richard F. Borch|year=1988|title=Reductive Amination with Sodium Cyanoborohydride: N,N-Dimethylcyclohexylamine|collvol=6|collvolpages=499|prep=CV6P0499}}</ref> The carbonyl is first treated with ammonia to promote imine formation by nucleophilic attack. The imine is then reduced to an amine by sodium cyanoborohydride. This reaction works on both aldehydes and ketones. The carbonyl can be treated with [[ammonia]], a [[Amine|primary amine]], or a secondary amine to produce, respectively, 1°, 2°, and 3° amines.<ref>{{cite journal |author=Richard F. Borch and Mark D. Bernstein and H. Dupont Durst |year=1971 |title=Cyanohydridoborate Anion as a Selective Reducing Agent |journal=[[J. Am. Chem. Soc.]] |volume=93 |issue=12 |pages=2897–2904 |doi=10.1021/ja00741a013}}</ref> |
[[Reductive amination]], sometimes called the ''Borch reaction'', is the conversion of a [[Carbonyl group|carbonyl]] into an [[amine]] through an intermediate [[imine]].<ref>{{OrgSynth|author=Richard F. Borch|year=1988|title=Reductive Amination with Sodium Cyanoborohydride: N,N-Dimethylcyclohexylamine|collvol=6|collvolpages=499|prep=CV6P0499}}</ref> The carbonyl is first treated with ammonia to promote imine formation by nucleophilic attack. The imine is then reduced to an amine by sodium cyanoborohydride. This reaction works on both aldehydes and ketones. The carbonyl can be treated with [[ammonia]], a [[Amine|primary amine]], or a secondary amine to produce, respectively, 1°, 2°, and 3° amines.<ref>{{cite journal |author=Richard F. Borch and Mark D. Bernstein and H. Dupont Durst |year=1971 |title=Cyanohydridoborate Anion as a Selective Reducing Agent |journal=[[J. Am. Chem. Soc.]] |volume=93 |issue=12 |pages=2897–2904 |doi=10.1021/ja00741a013}}</ref> |
||
=== Reductive deoxygenation of ketones === |
|||
[[Aromatic compound|Aromatic]] [[Ketone|ketones]] and aldehydes can be reductively [[Deoxygenation|deoxygenated]] using sodium cyanoborohydride.<ref name=":0">{{cite journal |last1=Box |first1=Vernon G. S. |last2=Meleties |first2=Panayiotis C. |date=1998-09-24 |title=Reductive, selective deoxygenation of acylbenzo[b]furans, aromatic aldehydes and ketones with NaBH3CN-TMSCl |url=https://www.sciencedirect.com/science/article/pii/S0040403998015196 |journal=Tetrahedron Letters |volume=39 |issue=39 |pages=7059–7062 |doi=10.1016/S0040-4039(98)01519-6 |issn=0040-4039}}</ref> This means that the carbonyl oxygen is being removed completely from the molecule. Deoxygenation using sodium cyanoborohydride is often done in the presence of [[trimethylsilyl chloride]], or TMSCl.<ref name=":0" /> |
|||
[[File:Reductive Deoxygenation of Ketones R.S..png|thumb|417x417px|Reductive deoxygenation of a ketone using sodium cyanoborohydride.|center]] |
|||
== Preparation == |
== Preparation == |
||
Revision as of 14:33, 1 April 2024
| |
| Names | |
|---|---|
| IUPAC name
Sodium cyanoboranuide
| |
| Other names
Sodium cyanotrihydridoborate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ECHA InfoCard | 100.043.001 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Na[BH3(CN)] | |
| Molar mass | 62.84 g·mol−1 |
| Appearance | white powder, hygroscopic |
| Density | 1.083 g/cm (25°C)3 |
| Melting point | 242 °C (468 °F; 515 K) decomposes |
| 212 g/(100 mL) (29 °C) | |
| Solubility | soluble in water, ethanol, diglyme, tetrahydrofuran, methanol slightly soluble in methanol insoluble in diethyl ether |
| Structure | |
| 4 at boron atom | |
| Tetrahedral at boron atom | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Flammable solid, fatal if swallowed, in contact with skin or if inhaled Contact with acids liberates very toxic gas Contact with water liberates highly flammable gas |
| GHS labelling: | |
  
| |
| Danger | |
| H228, H300, H310, H314, H330, H410 | |
| P210, P260, P264, P273, P280, P284 | |
| NFPA 704 (fire diamond) | |
Threshold limit value (TLV)
|
5 mg/m3 (TWA) |
| Safety data sheet (SDS) | Sigma Aldrich[1] |
| Related compounds | |
Other anions
|
Sodium borohydride |
Related compounds
|
Lithium aluminium hydride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Sodium cyanoborohydride is a chemical compound with the formula Na[BH3(CN)]. It is a colourless salt used in organic synthesis for chemical reduction including that of imines and carbonyls. Sodium cyanoborohydride is a milder reductant than other conventional reducing agents.[2]
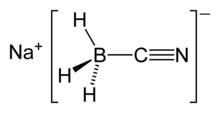
Structure
Sodium cyanoborohydride is a salt. The cationic sodium ion, [Na]+, interacts with the anionic cyanoborohydride ion, [BH3(CN)]−. The anionic component of the salt is tetrahedral at the boron atom.
The electron-withdrawing cyanide substituent draws electron density away from the negatively charged boron; thus, reducing the electrophilic capabilities of the anionic component.[2] This electronic phenomenon causes sodium cyanoborohydride to have more mild reducing qualities than other reducing agents. For example, Na[BH3(CN)] is less reducing than its counterpart sodium borohydride, containing [BH4]−.[2]
Uses
Sodium cyanoborohydride is a mild reducing agent. It is generally used for the reduction of imines. These reactions occur <pH 7 because the iminium ions are the actual substrates.[3]
Reductive amination (Borch reaction)
Reductive amination, sometimes called the Borch reaction, is the conversion of a carbonyl into an amine through an intermediate imine.[4] The carbonyl is first treated with ammonia to promote imine formation by nucleophilic attack. The imine is then reduced to an amine by sodium cyanoborohydride. This reaction works on both aldehydes and ketones. The carbonyl can be treated with ammonia, a primary amine, or a secondary amine to produce, respectively, 1°, 2°, and 3° amines.[5]
Preparation
Sodium cyanoborohydride can be purchase from most chemical suppliers. It is most commonly synthesized by the following methods:
From sodium cyanide and diborane
Sodium cyanoborohydride can be synthesized from sodium cyanide and diborane.[6]
This method of preparation can be used for other compounds of the formula RBH3CN where R is an alkali metal, a quaternary ammonium radical, or a phosphonium radical.[6] The final products are useful as hydrolysis stable reductants and as synthetic intermediates.[6]

Selectivity
Since sodium cyanoborohydride is a mild reducing agent, it gives good chemoselectivity for reaction with certain functional groups in the presence of others. For example, sodium cyanoborohydride is generally incapable of reducing amides, ethers, esters and lactones, nitriles, or epoxides.[7] Therefore, it can selectively reduce some functionalities in the presence of others.
Some examples of selective reduction include:
- Reduction of iminium ions in the presence of carbonyls[7]
- Reduction of aldehydes in the presence of ketones and esters.[8]
- Reduction of aldehydes in the presence of thioesters[7]
The selectivity of this reducing agent makes it an important tool in organic synthesis. It allows for specific modifications to be made to complex organic molecules.
History
Georg Wittig was the first to synthesize a cyanoborohydride by treating lithium borohydride with hydrogen cyanide in 1951.[7] The corresponding compound, sodium cyanoborohydride, was synthesized following a similar rationale by reacting sodium borohydride with hydrogen cyanide.[9] The synthesis was later refined to use sodium cyanide and borane in THF making the process safer.[9]
See also
- Sodium triacetoxyborohydride – a milder reductant, but unstable in water
- Sodium borohydride – a stronger, cheaper reductant
References
- ^ Sigma-Aldrich Co., Sodium cyanoborohydride. Retrieved on 2014-11-09.
- ^ a b c Baxter, Ellen W.; Reitz, Allen B. (9 January 2002). "Reductive Aminations of Carbonyl Compounds with Borohydride and Borane Reducing Agents". Organic Reactions: 1–714. doi:10.1002/0471264180.or059.01. ISBN 0-471-26418-0.
- ^ Hutchins, Robert O.; Hutchins, Marygail K.; Crawley, Matthew L.; Mercado-Marin, Eduardo V.; Sarpong, Richmond (2016). "Sodium Cyanoborohydride". Encyclopedia of Reagents for Organic Synthesis. pp. 1–14. doi:10.1002/047084289X.rs059.pub3. ISBN 978-0-470-84289-8.
- ^ Richard F. Borch (1988). "Reductive Amination with Sodium Cyanoborohydride: N,N-Dimethylcyclohexylamine". Organic Syntheses; Collected Volumes, vol. 6, p. 499.
- ^ Richard F. Borch and Mark D. Bernstein and H. Dupont Durst (1971). "Cyanohydridoborate Anion as a Selective Reducing Agent". J. Am. Chem. Soc. 93 (12): 2897–2904. doi:10.1021/ja00741a013.
- ^ a b c Hui, Benjamin C. (October 1980). "Synthesis and properties of borohydride derivatives". Inorganic Chemistry. 19 (10): 3185–3186. doi:10.1021/ic50212a075. ISSN 0020-1669.
- ^ a b c d LANE, Clinton F. (1975). "Sodium Cyanoborohydride - A Highly Selective Reducing Agent for Organic Functional Groups". Synthesis. 1975 (3): 135–146. doi:10.1055/s-1975-23685. ISSN 0039-7881. S2CID 95157786.
- ^ Paul, Avishek; Shipman, Michael A.; Onabule, Dolapo Y.; Sproules, Stephen; Symes, Mark D. (2021-04-15). "Selective aldehyde reductions in neutral water catalysed by encapsulation in a supramolecular cage". Chemical Science. 12 (14): 5082–5090. doi:10.1039/D1SC00896J. ISSN 2041-6539. PMC 8179549. PMID 34163748.
- ^ a b Abdel-Magid, Ahmed F., ed. (1996-08-13). Reductions in Organic Synthesis: Recent Advances and Practical Applications. ACS Symposium Series. Vol. 641. Washington, DC: American Chemical Society. doi:10.1021/bk-1996-0641.ch001. ISBN 978-0-8412-3381-2.

