Teratology: Difference between revisions
→Thalidomide: Link intercalation |
m →Research: added link |
||
| Line 28: | Line 28: | ||
=== Research === |
=== Research === |
||
Studies designed to test the teratogenic potential of environmental agents use animal model systems (e.g., rat, mouse, rabbit, dog, and monkey). Early teratologists exposed pregnant animals to environmental agents and observed the fetuses for gross visceral and skeletal abnormalities. While this is still part of the teratological evaluation procedures today, the field of Teratology is moving to a more [[molecular]] level, seeking the mechanism(s) of action by which these agents act. One example of this is the use of mammalian animal models to evaluate the molecular role of teratogens in the development of embryonic populations, such as the [[neural crest]],<ref>{{cite journal | vauthors = Cerrizuela S, Vega-Lopez GA, Aybar MJ | title = The role of teratogens in neural crest development | journal = Birth Defects Research | volume = 112 | issue = 8 | pages = 584–632 | date = May 2020 | pmid = 31926062 | doi = 10.1002/bdr2.1644 | s2cid = 210151171 }}</ref> which can lead to the development of [[neurocristopathies]]. [[Genetically modified]] mice are commonly used for this purpose. In addition, pregnancy registries are large, prospective studies that monitor exposures women receive during their pregnancies and record the outcome of their births. These studies provide information about possible risks of medications or other exposures in human pregnancies. Prenatal alcohol exposure (PAE) can produce craniofacial malformations, a phenotype that is visible in [[Fetal alcohol spectrum disorder|Fetal Alcohol Syndrome]]. Current evidence suggests that craniofacial malformations occur via: apoptosis of neural crest cells,<ref>{{cite journal | vauthors = Sulik KK, Cook CS, Webster WS | title = Teratogens and craniofacial malformations: relationships to cell death | journal = Development | volume = 103 | issue = Suppl | pages = 213–231 | date = 1988 | pmid = 3074910 | doi = 10.1242/dev.103.Supplement.213 | url = https://cdr.lib.unc.edu/downloads/f7623n81g }}</ref> interference with neural crest cell migration,<ref>{{cite journal | vauthors = Shi Y, Li J, Chen C, Gong M, Chen Y, Liu Y, Chen J, Li T, Song W | title = 5-Mehtyltetrahydrofolate rescues alcohol-induced neural crest cell migration abnormalities | journal = Molecular Brain | volume = 7 | issue = 67 | pages = 67 | date = September 2014 | pmid = 25223405 }}</ref><ref>{{cite journal | vauthors = Cartwright MM, Smith SM | title = Stage-dependent effects of ethanol on cranial neural crest cell development: partial basis for the phenotypic variations observed in fetal alcohol syndrome | journal = Alcoholism: Clinical and Experimental Research | volume = 19 | issue = 6 | pages = 1454–1462 | date = December 1995 | pmid = 8749810 | doi = 10.1111/j.1530-0277.1995.tb01007.x }}</ref> as well as the disruption of sonic hedgehog (shh) signaling.<ref>{{cite bioRxiv |biorxiv=10.1101/649673 |title=Prenatal alcohol exposure disrupts Shh pathway and primary cilia genes in the mouse neural tube |date=19 October 2019 |vauthors=Boschen KE, Fish EW, Parnell SE }}</ref> |
Studies designed to test the teratogenic potential of environmental agents use animal model systems (e.g., rat, mouse, rabbit, dog, and monkey). Early teratologists exposed pregnant animals to environmental agents and observed the fetuses for gross visceral and skeletal abnormalities. While this is still part of the teratological evaluation procedures today, the field of Teratology is moving to a more [[molecular]] level, seeking the mechanism(s) of action by which these agents act. One example of this is the use of mammalian animal models to evaluate the molecular role of teratogens in the development of embryonic populations, such as the [[neural crest]],<ref>{{cite journal | vauthors = Cerrizuela S, Vega-Lopez GA, Aybar MJ | title = The role of teratogens in neural crest development | journal = Birth Defects Research | volume = 112 | issue = 8 | pages = 584–632 | date = May 2020 | pmid = 31926062 | doi = 10.1002/bdr2.1644 | s2cid = 210151171 }}</ref> which can lead to the development of [[neurocristopathies]]. [[Genetically modified]] mice are commonly used for this purpose. In addition, pregnancy registries are large, prospective studies that monitor exposures women receive during their pregnancies and record the outcome of their births. These studies provide information about possible risks of medications or other exposures in human pregnancies. Prenatal alcohol exposure (PAE) can produce craniofacial malformations, a phenotype that is visible in [[Fetal alcohol spectrum disorder|Fetal Alcohol Syndrome]]. Current evidence suggests that craniofacial malformations occur via: apoptosis of neural crest cells,<ref>{{cite journal | vauthors = Sulik KK, Cook CS, Webster WS | title = Teratogens and craniofacial malformations: relationships to cell death | journal = Development | volume = 103 | issue = Suppl | pages = 213–231 | date = 1988 | pmid = 3074910 | doi = 10.1242/dev.103.Supplement.213 | url = https://cdr.lib.unc.edu/downloads/f7623n81g }}</ref> interference with neural crest cell migration,<ref>{{cite journal | vauthors = Shi Y, Li J, Chen C, Gong M, Chen Y, Liu Y, Chen J, Li T, Song W | title = 5-Mehtyltetrahydrofolate rescues alcohol-induced neural crest cell migration abnormalities | journal = Molecular Brain | volume = 7 | issue = 67 | pages = 67 | date = September 2014 | pmid = 25223405 }}</ref><ref>{{cite journal | vauthors = Cartwright MM, Smith SM | title = Stage-dependent effects of ethanol on cranial neural crest cell development: partial basis for the phenotypic variations observed in fetal alcohol syndrome | journal = Alcoholism: Clinical and Experimental Research | volume = 19 | issue = 6 | pages = 1454–1462 | date = December 1995 | pmid = 8749810 | doi = 10.1111/j.1530-0277.1995.tb01007.x }}</ref> as well as the disruption of [[Hedgehog_signaling_pathway|sonic hedgehog (shh) signaling]].<ref>{{cite bioRxiv |biorxiv=10.1101/649673 |title=Prenatal alcohol exposure disrupts Shh pathway and primary cilia genes in the mouse neural tube |date=19 October 2019 |vauthors=Boschen KE, Fish EW, Parnell SE }}</ref> |
||
Understanding how a '''teratogen''' causes its effect is not only important in preventing congenital abnormalities but also has the potential for developing new therapeutic drugs safe for use with pregnant women. |
Understanding how a '''teratogen''' causes its effect is not only important in preventing congenital abnormalities but also has the potential for developing new therapeutic drugs safe for use with pregnant women. |
||
Revision as of 20:02, 21 August 2024
Teratology is the study of abnormalities of physiological development in organisms during their life span. It is a sub-discipline in medical genetics which focuses on the classification of congenital abnormalities in dysmorphology caused by teratogens. Teratogens are substances that may cause non-heritable birth defects via a toxic effect on an embryo or fetus.[1] Defects include malformations, disruptions, deformations, and dysplasia that may cause stunted growth, delayed mental development, or other congenital disorders that lack structural malformations.[2] The related term developmental toxicity includes all manifestations of abnormal development that are caused by environmental insult.[3] The extent to which teratogens will impact an embryo is dependent on several factors, such as how long the embryo has been exposed, the stage of development the embryo was in when exposed, the genetic makeup of the embryo, and the transfer rate of the teratogen.[4]
Etymology
The term was borrowed in 1842 from the French tératologie, where it was formed in 1830 from the Greek τέρας teras (word stem τέρατ- terat-), meaning "sign sent by the gods, portent, marvel, monster", and -ologie (-ology), used to designate a discourse, treaty, science, theory, or study of some topic.[5]
Old literature referred to abnormalities of all kinds under the Latin term Lusus naturae (lit. "freak of nature"). As early as the 17th century, Teratology referred to a discourse on prodigies and marvels of anything so extraordinary as to seem abnormal. In the 19th century, it acquired a meaning more closely related to biological deformities, mostly in the field of botany. Currently, its most instrumental meaning is that of the medical study of teratogenesis, congenital malformations or individuals with significant malformations. Historically, people have used many pejorative terms to describe/label cases of significant physical malformations. In the 1960s, David W. Smith of the University of Washington Medical School (one of the researchers who became known in 1973 for the discovery of fetal alcohol syndrome),[6] popularized the term teratology. With the growth of understanding of the origins of birth defects, the field of teratology as of 2015[update] overlaps with other fields of science, including developmental biology, embryology, and genetics.
Until the 1940s, teratologists regarded birth defects as primarily hereditary. In 1941, the first well-documented cases of environmental agents being the cause of severe birth defects were reported.[7]
Teratogenesis
Wilson's principles
In 1959 and in his 1973 monograph Environment and Birth Defects, embryologist James Wilson put forth six principles of teratogenesis to guide the study and understanding of teratogenic agents and their effects on developing organisms.[8] These principles were derived from and expanded on by those laid forth by zoologist Camille Dareste in the late 1800s:[8][9]
- Susceptibility to teratogenesis depends on the genotype of the conceptus and the manner in which this interacts with adverse environmental factors.
- Susceptibility to teratogenesis varies with the developmental stage at the time of exposure to an adverse influence. There are critical periods of susceptibility to agents and organ systems affected by these agents.
- Teratogenic agents act in specific ways on developing cells and tissues to initiate sequences of abnormal developmental events.
- The access of adverse influences to developing tissues depends on the nature of the influence. Several factors affect the ability of a teratogen to contact a developing conceptus, such as the nature of the agent itself, route and degree of maternal exposure, rate of placental transfer and systemic absorption, and composition of the maternal and embryonic/fetal genotypes.
- There are four manifestations of deviant development (death, malformation, growth retardation and functional defect).
- Manifestations of deviant development increase in frequency and degree as dosage increases from the No Observable Adverse Effect Level (NOAEL) to a dose producing 100% lethality (LD100).
Research
Studies designed to test the teratogenic potential of environmental agents use animal model systems (e.g., rat, mouse, rabbit, dog, and monkey). Early teratologists exposed pregnant animals to environmental agents and observed the fetuses for gross visceral and skeletal abnormalities. While this is still part of the teratological evaluation procedures today, the field of Teratology is moving to a more molecular level, seeking the mechanism(s) of action by which these agents act. One example of this is the use of mammalian animal models to evaluate the molecular role of teratogens in the development of embryonic populations, such as the neural crest,[10] which can lead to the development of neurocristopathies. Genetically modified mice are commonly used for this purpose. In addition, pregnancy registries are large, prospective studies that monitor exposures women receive during their pregnancies and record the outcome of their births. These studies provide information about possible risks of medications or other exposures in human pregnancies. Prenatal alcohol exposure (PAE) can produce craniofacial malformations, a phenotype that is visible in Fetal Alcohol Syndrome. Current evidence suggests that craniofacial malformations occur via: apoptosis of neural crest cells,[11] interference with neural crest cell migration,[12][13] as well as the disruption of sonic hedgehog (shh) signaling.[14]
Understanding how a teratogen causes its effect is not only important in preventing congenital abnormalities but also has the potential for developing new therapeutic drugs safe for use with pregnant women.
Causes
Common causes of teratogenesis include:[15][16]
- Genetic disorders and chromosomal abnormalities
- Maternal health factors
- Nutrition during pregnancy (e.g., spina bifida resulting from folate deficiency[16])
- Metabolic disorders such as diabetes and thyroid disease
- Stress
- Chemical agents
- Vertically transmitted infections such as rubella and syphilis
- Ionizing radiation such as X-rays and that emitted from nuclear fallout
- Temperatures outside the accepted range for a given organism[22]
Human pregnancy
In humans, congenital disorders resulted in about 510,000 deaths globally in 2010.[23]
About 3% of newborns have a "major physical anomaly", meaning a physical anomaly that has cosmetic or functional significance.[24] Congenital disorders are responsible for 20% of infant deaths.[25] The most common congenital diseases are heart defects, Down syndrome, and neural tube defects. Trisomy 21 is the most common type of Down Syndrome. About 95% of infants born with Down Syndrome have this disorder and it consists of 3 separate copies of chromosomes. Translocation Down syndrome is not as common, as only 3% of infants with Down Syndrome are diagnosed with this type.[26] VSD, ventricular septal defect, is the most common type of heart defect in infants. If an infant has a large VSD it can result into heart failure.[27] Infants with a smaller VSD have a 96% survival rate and those with a moderate VSD have about an 86% survival rate.[citation needed] Lastly, NTD, neural tube defect, is a defect that forms in the brain and spine during early development. If the spinal cord is exposed and touching the skin it can require surgery to prevent an infection.[28]
Medicines
Acitretin
Acitretin is highly teratogenic and noted for the possibility of severe birth defects. It should not be used by pregnant women or women planning to get pregnant within 3 years following the use of acitretin. Sexually active women of childbearing age who use acitretin should also use at least two forms of birth control concurrently. Men and women who use it should not donate blood for three years after using it, because of the possibility that the blood might be used in a pregnant patient and cause birth defects. In addition, it may cause nausea, headache, itching, dry, red or flaky skin, dry or red eyes, dry or chapped lips, swollen lips, dry mouth, thirst, cystic acne or hair loss.[29][30][31]
Etretinate
Etretinate (trade name Tegison) is a medication developed by Hoffmann–La Roche that was approved by the FDA in 1986 to treat severe psoriasis. It is a second-generation retinoid.[32] It was subsequently removed from the Canadian market in 1996 and the United States market in 1998 due to the high risk of birth defects. It remains on the market in Japan as Tigason.
Vaccination
In humans, vaccination has become readily available, and is important for the prevention of various communicable diseases such as polio and rubella, among others. There has been no association between congenital malformations and vaccination — for example, a population-wide study in Finland in which expectant mothers received the oral polio vaccine found no difference in infant outcomes when compared with mothers from reference cohorts who had not received the vaccine.[33] However, on grounds of theoretical risk, it is still not recommended to vaccinate for polio while pregnant unless there is risk of infection.[34] An important exception to this relates to provision of the influenza vaccine while pregnant. During the 1918 and 1957 influenza pandemics, mortality from influenza in pregnant women was 45%. In a 2005 study of vaccination during pregnancy, Munoz et al. demonstrated that there was no adverse outcome observed in the new infants or mothers, suggesting that the balance of risk between infection and vaccination favored preventative vaccination.[35]
Reproductive hormones and hormone replacement therapy
There are a number of ways that a fetus can be affected in pregnancy, specifically due to exposure to various substances. There are a number of drugs that can do this, specifically drugs such as female reproductive hormones or hormone replacement drugs such as estrogen and progesterone that are not only essential for reproductive health, but also pose concerns when it comes to the synthetic alternatives to these. This can cause a multitude of congenital abnormalities and deformities, many of which can ultimately affect the fetus and even the mother's reproductive system in the long term. According to a study conducted from 2015 till 2018, it was found that there was an increased risk of both maternal and neonatal complications developing as a result of hormone replacement therapy cycles being conducted during pregnancy, especially in regards to hormones such as estrogen, testosterone and thyroid hormone.[36][37][38] When hormones such as estrogen and testosterone are replaced, this can cause the fetus to become stunted in growth, born prematurely with a lower birth weight, develop mental retardation, while in turn causing the mother's ovarian reserve to be depleted while increasing ovarian follicular recruitment.[39]
Withdrawn drugs
Thalidomide
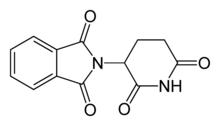
Thalidomide was once prescribed therapeutically from the 1950s to early 1960s in Europe as an anti-nausea medication to alleviate morning sickness among pregnant women. While the exact mechanism of action of thalidomide is not known, it is thought to be related to inhibition of angiogenesis through interaction with the insulin like growth factor(IGF-1) and fibroblast like growth factor 2 (FGF-2) pathways.[40] In the 1960s, it became apparent that thalidomide altered embryo development and led to limb deformities such as thumb absence, underdevelopment of entire limbs, or phocomelia.[40] Thalidomide may have caused teratogenic effects in over 10,000 babies worldwide.[41][42]
Recreational drugs
Alcohol

In the US, alcohol is subject to the FDA drug labeling Pregnancy Category X (Contraindicated in pregnancy). Alcohol is known to cause fetal alcohol spectrum disorder.
There are a wide range of affects that Prenatal Alcohol Exposure (PAE) can have on a developing fetus. Some of the most prominent possible outcomes include the development of Fetal Alcohol Syndrome, a reduction in brain volume, still births, spontaneous abortions, impairments of the nervous system, and much more.[43] Fetal Alcohol Syndrome has numerous symptoms which may include cognitive impairments and impairment of the facial features.[43] PAE remains the leading cause of birth defects and neurodevelopmental abnormalities in the United States, affecting 9.1 to 50 per 1000 live births in the U.S. and 68.0 to 89.2 per 1000 in populations with high levels of alcohol use.[44]
Tobacco
Consuming tobacco products while pregnant or breastfeeding can have significant negative impacts on the health and development of the unborn child and newborn infant.[45]
Lead exposure during pregnancy
Long before modern science, it was understood that heavy metals could cause negative effects to those who were exposed. The Greek physician Pedanius Dioscorides described the effects of lead exposure as something that "makes the mind give way." Lead exposure in adults can lead to cardiological, renal, reproductive, and cognitive issues that are often irreversible, however, lead exposure during pregnancy can be detrimental to the long-term health of the fetus.[46] Exposure to lead during pregnancy is well known to have teratogenic effects on the development of a fetus.[47] Specifically, fetal exposure to lead can cause cognitive impairment, premature births, unplanned abortions, ADHD, and much more.[48] Lead exposure during the first trimester of pregnancy leads to the greatest predictability of cognitive development issues after birth.[47]
Low socioeconomic status correlates to a higher probability of lead exposure.[49] A well-known recent example of lead poisoning - and the impacts it can have on a community - was the 2014 water crisis in Flint, Michigan. Researchers have found that female fetuses developed at a higher rate than male fetuses in Flint when compared to surrounding areas. The higher rate of female births indicated a problem because male fetuses are more sensitive to pregnancy hazards than female fetuses.[50]
Other animals
Fossil record
Evidence for congenital deformities found in the fossil record is studied by paleopathologists, specialists in ancient disease and injury. Fossils bearing evidence of congenital deformity are scientifically significant because they can help scientists infer the evolutionary history of life's developmental processes. For instance, because a Tyrannosaurus rex specimen has been discovered with a block vertebra, it means that vertebrae have been developing the same basic way since at least the most recent common ancestor of dinosaurs and mammals. Other notable fossil deformities include a hatchling specimen of the bird-like dinosaur, Troodon, the tip of whose jaw was twisted.[51] Another notably deformed fossil was a specimen of the Choristodera Hyphalosaurus, which had two heads- the oldest known example of polycephaly.[52]
Thalidomide and chick limb development
Thalidomide is a teratogen known to be significantly detrimental to organ and limb development during embryogenesis.[53] It has been observed in chick embryos that exposure to thalidomide can induce limb outgrowth deformities, due to increased oxidative stress interfering with the Wnt signaling pathway, increasing apoptosis, and damaging immature blood vessels in developing limb buds.[18][54]
Retinoic acid and mouse limb development
Retinoic acid (RA) is significant in embryonic development. It induces the function of limb patterning of a developing embryo in species such as mice and other vertebrate limbs.[55] For example, during the process of regenerating a newt limb an increased amount of RA moves the limb more proximal to the distal blastoma and the extent of the proximalization of the limb increases with the amount of RA present during the regeneration process.[55] A study looked at the RA activity intracellularly in mice in relation to human regulating CYP26 enzymes which play a critical role in metabolizing RA.[55] This study also helps to reveal that RA is significant in various aspects of limb development in an embryo, however irregular control or excess amounts of RA can have teratogenic impacts causing malformations of limb development. They looked specifically at CYP26B1 which is highly expressed in regions of limb development in mice.[55] The lack of CYP26B1 was shown to cause a spread of RA signal towards the distal section of the limb causing proximo-distal patterning irregularities of the limb.[55] Not only did it show spreading of RA but a deficiency in the CYP26B1 also showed an induced apoptosis effect in the developing mouse limb but delayed chondrocyte maturation, which are cells that secrete a cartilage matrix which is significant for limb structure.[55] They also looked at what happened to development of the limbs in wild type mice, that are mice with no CYP26B1 deficiencies, but which had an excess amount of RA present in the embryo. The results showed a similar impact to limb patterning if the mice did have the CYP26B1 deficiency meaning that there was still a proximal distal patterning deficiency observed when excess RA was present.[55] This then concludes that RA plays the role of a morphogen to identify proximal distal patterning of limb development in mice embryos and that CYP26B1 is significant to prevent apoptosis of those limb tissues to further proper development of mice limbs in vivo.
Rat development and lead exposure
There has been evidence of teratogenic effects of lead in rats as well. An experiment was conducted where pregnant rats were given drinking water, before and during pregnancy, that contained lead. Many detrimental effects, and signs of teratogenesis were found, such as negative impacts on the formation of the cerebellum, fetal mortality, and developmental issues for various parts of the body.[56]
Plants
In botany, teratology investigates the theoretical implications of abnormal specimens. For example, the discovery of abnormal flowers—for example, flowers with leaves instead of petals, or flowers with staminoid pistils—furnished important evidence for the "foliar theory", the theory that all flower parts are highly specialised leaves.[57] In plants, such specimens are denoted as 'lusus naturae' ('sports of nature', abbreviated as 'lus.'); and occasionally as 'ter.', 'monst.', or 'monstr.'.[58]
Types of deformations in plants
Plants can have mutations that leads to different types of deformations such as:
- Fasciation: Development of the apex (growing tip) in a flat plane perpendicular to the axis of elongation
- Variegation: Degeneration of genes, manifesting itself among other things by anomalous pigmentation
- Virescence: Anomalous development of a green pigmentation in unexpected parts of the plant
- Phyllody: Floral organs or fruits transformed into leaves
- Witch's broom: Unusually high multiplication of branches in the upper part of the plant, mainly in a tree
- Pelorism: Zygomorphic flower regress to their ancestral actinomorphic symmetry
- Proliferation: Repetitive growth of an entire organ, such as a flower
See also
References
- ^ Bastow BD, Holmes JL, Trupin SR, Draper JC, Matthews Jr KJ (23 February 2016). Talavera F (ed.). "Teratology and drug use during pregnancy". Medscape. WebMD. Retrieved 24 February 2016.
- ^ Gilbert SF, Epel D (2015). Ecological developmental biology: the environmental regulation of development, health, and evolution (2nd ed.). Sunderland, MA: Sinauer Associates, Inc. Publishers.
- ^ Rogers JM, Kavlock RJ (1996). "Developmental Toxicology". In Klaassen CD (ed.). Casarett and Doull's Toxicology : the basic science of poisons (5th ed.). New York: McGraw-Hill, Health Professions Division. pp. 301–331. ISBN 978-0-07-105476-8.
- ^ Macnow AS, ed. (2022). MCAT biology review 2023–2024 : online + book (2023-2024 ed.). Fort Lauderdale, Florida: Kaplan Publishing. ISBN 978-1-5062-8295-4. OCLC 1334083218.
- ^ "Teratology". Merriam-Webster Dictionary.
- ^ Jones KL, Smith DW, Ulleland CN, Streissguth P (June 1973). "Pattern of malformation in offspring of chronic alcoholic mothers". Lancet. 1 (7815): 1267–1271. doi:10.1016/S0140-6736(73)91291-9. PMID 4126070.
- ^ "Birth Defects". Howmed.net. 24 July 2011. Retrieved 1 November 2015.
Until 1940, it was assumed that congenital defects were caused primarily by hereditary factors. In 1941, the first well-documented cases were reported that an environmental agent (rubella virus) could produce severe anatomic anomalies.
- ^ a b Wilson JG (1973). Environment and Birth Defects (Environmental Science Series). London: Academic Pr. ISBN 0-12-757750-5.
- ^ "James G. Wilson's Six Principles of Teratology". The Embryo Project Encyclopedia. Retrieved 20 March 2023.
- ^ Cerrizuela S, Vega-Lopez GA, Aybar MJ (May 2020). "The role of teratogens in neural crest development". Birth Defects Research. 112 (8): 584–632. doi:10.1002/bdr2.1644. PMID 31926062. S2CID 210151171.
- ^ Sulik KK, Cook CS, Webster WS (1988). "Teratogens and craniofacial malformations: relationships to cell death". Development. 103 (Suppl): 213–231. doi:10.1242/dev.103.Supplement.213. PMID 3074910.
- ^ Shi Y, Li J, Chen C, Gong M, Chen Y, Liu Y, et al. (September 2014). "5-Mehtyltetrahydrofolate rescues alcohol-induced neural crest cell migration abnormalities". Molecular Brain. 7 (67): 67. PMID 25223405.
- ^ Cartwright MM, Smith SM (December 1995). "Stage-dependent effects of ethanol on cranial neural crest cell development: partial basis for the phenotypic variations observed in fetal alcohol syndrome". Alcoholism: Clinical and Experimental Research. 19 (6): 1454–1462. doi:10.1111/j.1530-0277.1995.tb01007.x. PMID 8749810.
- ^ Boschen KE, Fish EW, Parnell SE (19 October 2019). "Prenatal alcohol exposure disrupts Shh pathway and primary cilia genes in the mouse neural tube". bioRxiv 10.1101/649673.
- ^ "Teratogens". The Embryo Project Encyclopedia. Retrieved 27 March 2023.
- ^ a b c Gilbert-Barness E (20 March 2010). "Teratogenic causes of malformations". Annals of Clinical and Laboratory Science. 40 (2): 99–114. PMID 20421621.
- ^ Welch-Carre E (August 2005). "The neurodevelopmental consequences of prenatal alcohol exposure". Advances in Neonatal Care. 5 (4). Medscape: 217–229. doi:10.1016/j.adnc.2005.04.007. PMID 16084479. S2CID 36424689.
- ^ a b Therapontos C, Erskine L, Gardner ER, Figg WD, Vargesson N (May 2009). "Thalidomide induces limb defects by preventing angiogenic outgrowth during early limb formation". Proceedings of the National Academy of Sciences of the United States of America. 106 (21): 8573–8578. Bibcode:2009PNAS..106.8573T. doi:10.1073/pnas.0901505106. PMC 2688998. PMID 19433787.
- ^ Holt D, Webb M (April 1986). "The toxicity and teratogenicity of mercuric mercury in the pregnant rat". Archives of Toxicology. 58 (4): 243–248. doi:10.1007/BF00297114. PMID 3718227. S2CID 22045389.
- ^ Bellinger DC (June 2005). "Teratogen update: lead and pregnancy". Birth Defects Research. Part A, Clinical and Molecular Teratology. 73 (6): 409–420. doi:10.1002/bdra.20127. PMID 15880700.
- ^ Jacobson JL, Jacobson SW (May 1997). "Teratogen update: polychlorinated biphenyls". Teratology. 55 (5): 338–347. doi:10.1002/(SICI)1096-9926(199705)55:5<338::AID-TERA6>3.0.CO;2-V. PMID 9261928.
- ^ Ziskin MC, Morrissey J (June 2011). "Thermal thresholds for teratogenicity, reproduction, and development". International Journal of Hyperthermia. 27 (4): 374–387. doi:10.3109/02656736.2011.553769. PMID 21591900.
- ^ Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. (December 2012). "Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010". Lancet. 380 (9859): 2095–2128. doi:10.1016/S0140-6736(12)61728-0. hdl:10536/DRO/DU:30050819. PMC 10790329. PMID 23245604. S2CID 1541253.[permanent dead link]
- ^ Abas AK, Fausto N, Kumar V (eds.). Robbins and Cotran's Pathologic Basis of Disease (7th ed.). p. 470. ISBN 978-0-7216-0187-8.
- ^ CDC (21 December 2022). "Data & Statistics on Birth Defects | CDC". Centers for Disease Control and Prevention. Retrieved 5 February 2023.
- ^ CDC (18 November 2022). "Facts about Down Syndrome | CDC". Centers for Disease Control and Prevention. Retrieved 5 April 2023.
- ^ "Ventricular Septal Defect (VSD) (for Parents) – Nemours KidsHealth". kidshealth.org. Retrieved 5 April 2023.
- ^ "Neural Tube Defects". www.hopkinsmedicine.org. 8 August 2021. Retrieved 5 April 2023.
- ^ "Soriatane". WebMD. Retrieved 15 August 2015.
- ^ "Soriatane Side Effects". Drugs.com. Retrieved 15 August 2015.
- ^ "Soriatane (Acitretin) Drug Information: Description, User Reviews, Drug Side Effects, Interactions - Prescribing Information at RxList". RxList. Archived from the original on 2 December 2013. Retrieved 15 August 2015.
- ^ Mutschler E, Schäfer-Korting M (2001). Arzneimittelwirkungen (in German) (8 ed.). Stuttgart: Wissenschaftliche Verlagsgesellschaft. p. 728f. ISBN 3-8047-1763-2.
- ^ Harjulehto-Mervaala T, Aro T, Hiilesmaa VK, Saxén H, Hovi T, Saxén L (September 1993). "Oral polio vaccination during pregnancy: no increase in the occurrence of congenital malformations". American Journal of Epidemiology. 138 (6): 407–414. doi:10.1093/oxfordjournals.aje.a116873. PMID 8213746.
- ^ "Guidelines for Vaccinating Pregnant Women". cdc.gov. Centers for Disease Control and Prevention: Advisory Committee on Immunization Practices (ACIP). 13 January 2021.
Although no adverse effects of IPV have been documented among pregnant women or their fetuses, vaccination of pregnant women should be avoided on theoretical grounds. However, if a pregnant woman is at increased risk for infection and requires immediate protection against polio, IPV can be administered in accordance with the recommended schedules for adults.
- ^ Munoz FM, Greisinger AJ, Wehmanen OA, Mouzoon ME, Hoyle JC, Smith FA, et al. (April 2005). "Safety of influenza vaccination during pregnancy". American Journal of Obstetrics and Gynecology. 192 (4): 1098–1106. doi:10.1016/j.ajog.2004.12.019. PMID 15846187.
- ^ Zong L, Liu P, Zhou L, Wei D, Ding L, Qin Y (May 2020). "Increased risk of maternal and neonatal complications in hormone replacement therapy cycles in frozen embryo transfer". Reproductive Biology and Endocrinology. 18 (1): 36. doi:10.1186/s12958-020-00601-3. PMC 7199365. PMID 32366332.
- ^ van den Broek S, Lupattelli A, Frank AS, Haug LS, Nordeng H (June 2021). "Thyroid hormone replacement therapy in pregnancy and motor function, communication skills, and behavior of preschool children: The Norwegian Mother, Father, and Child Cohort Study". Pharmacoepidemiology and Drug Safety. 30 (6): 716–726. doi:10.1002/pds.5184. PMC 8247290. PMID 33314561.
- ^ Steckler T, Wang J, Bartol FF, Roy SK, Padmanabhan V (July 2005). "Fetal programming: prenatal testosterone treatment causes intrauterine growth retardation, reduces ovarian reserve and increases ovarian follicular recruitment". Endocrinology. 146 (7): 3185–93. doi:10.1210/en.2004-1444. PMID 15802500.
- ^ Knox RV (August 2005). "Recruitment and selection of ovarian follicles for determination of ovulation rate in the pig". Domestic Animal Endocrinology. 29 (2): 385–97. doi:10.1016/j.domaniend.2005.02.025. PMID 15998504.
- ^ a b c Stephens TD, Bunde CJ, Fillmore BJ (June 2000). "Mechanism of action in thalidomide teratogenesis". Biochemical Pharmacology. 59 (12): 1489–1499. doi:10.1016/S0006-2952(99)00388-3. PMID 10799645.
- ^ Kim JH, Scialli AR (July 2011). "Thalidomide: the tragedy of birth defects and the effective treatment of disease". Toxicological Sciences. 122 (1): 1–6. doi:10.1093/toxsci/kfr088. PMID 21507989.
- ^ Martínez-Frías ML (June 2012). "[The thalidomide experience: review of its effects 50 years later]". Medicina Clinica (in Spanish). 139 (1): 25–32. doi:10.1016/j.medcli.2011.10.011. PMID 22177324.
- ^ a b Popova S, Dozet D, Shield K, Rehm J, Burd L (September 2021). "Alcohol's Impact on the Fetus". Nutrients. 13 (10): 3452. doi:10.3390/nu13103452. PMC 8541151. PMID 34684453.
- ^ Gordis E. "Fetal Alcohol Exposure and the Brain". National Institute on Alcohol Abuse and Alcoholism.
- ^ Haustein KO (September 1999). "Cigarette smoking, nicotine and pregnancy". International Journal of Clinical Pharmacology and Therapeutics. 37 (9): 417–427. PMID 10507240.
- ^ Kasten-Jolly J, Lawrence DA (November 2017). "Sex-specific effects of developmental lead exposure on the immune-neuroendocrine network". Toxicology and Applied Pharmacology. 334: 142–157. doi:10.1016/j.taap.2017.09.009. PMID 28911972.
- ^ a b Hu H, Téllez-Rojo MM, Bellinger D, Smith D, Ettinger AS, Lamadrid-Figueroa H, et al. (November 2006). "Fetal lead exposure at each stage of pregnancy as a predictor of infant mental development". Environmental Health Perspectives. 114 (11): 1730–1735. doi:10.1289/ehp.9067. PMC 1665421. PMID 17107860.
- ^ RÍsovÁ V (September 2019). "The pathway of lead through the mother's body to the child". Interdisciplinary Toxicology. 12 (1): 1–6. doi:10.2478/intox-2019-0001. PMC 7061448. PMID 32189981.
- ^ Cory-Slechta DA, Virgolini MB, Thiruchelvam M, Weston DD, Bauter MR (May 2004). "Maternal stress modulates the effects of developmental lead exposure". Environmental Health Perspectives. 112 (6): 717–730. doi:10.1289/ehp.6481. PMC 1241967. PMID 15121516.
- ^ "Flint Water Tied to Fetal Death and Lower Fertility Rates". www.pbs.org. 22 September 2017. Retrieved 24 March 2024.
- ^ Molnar, R. E., 2001, Theropod paleopathology: a literature survey: In: Mesozoic Vertebrate Life, edited by Tanke, D. H., and Carpenter, K., Indiana University Press, p. 337-363.
- ^ Ji Q, Wu XC, Cheng YN (April 2010). "Cretaceous choristoderan reptiles gave birth to live young". Die Naturwissenschaften. 97 (4): 423–428. Bibcode:2010NW.....97..423J. doi:10.1007/s00114-010-0654-2. PMID 20179895. S2CID 8719805.
- ^ Vargesson N (June 2015). "Thalidomide-induced teratogenesis: history and mechanisms". Birth Defects Research. Part C, Embryo Today. 105 (2): 140–156. doi:10.1002/bdrc.21096. PMC 4737249. PMID 26043938.
- ^ Knobloch J, Shaughnessy JD, Rüther U (May 2007). "Thalidomide induces limb deformities by perturbing the Bmp/Dkk1/Wnt signaling pathway". FASEB Journal. 21 (7): 1410–1421. doi:10.1096/fj.06-7603com. PMID 17283219. S2CID 13467186.
- ^ a b c d e f g Yashiro K, Zhao X, Uehara M, Yamashita K, Nishijima M, Nishino J, et al. (March 2004). "Regulation of retinoic acid distribution is required for proximodistal patterning and outgrowth of the developing mouse limb". Developmental Cell. 6 (3): 411–422. doi:10.1016/S1534-5807(04)00062-0. PMID 15030763.
- ^ Mousa AM, Al-Fadhli AS, Rao MS, Kilarkaje N (January 2015). "Gestational lead exposure induces developmental abnormalities and up-regulates apoptosis of fetal cerebellar cells in rats". Drug and Chemical Toxicology. 38 (1): 73–83. doi:10.3109/01480545.2014.907578. PMID 24724870.
- ^ Glover BJ (2014). "Historical interpretations of flower induction and flower development". Understanding flowers and flowering : an integrated approach (Second ed.). Oxford: Oxford University Press. ISBN 978-0-19-966159-6.
- ^ Vázquez FM (October 2014). Turland NJ, Wiersema JH (eds.). "(023–024) Proposals to add a new Article and some Examples under Article 5". Taxon. 63 (5): 1142. doi:10.12705/635.21. ISSN 0040-0262. OCLC 6896520971. S2CID 87400780.
External links
- Society of Teratology
- European Teratology Society Archived 5 April 2010 at the Wayback Machine
- Organization of Teratology Information Specialists
- March of Dimes Foundation
- A Telling of Wonders: Teratology in Western Medicine through 1800 (New York Academy of Medicine Historical Collections)
- The Reproductive Toxicology Center Database
