5-Hydroxytryptophan: Difference between revisions
→Psychedelic effects: Fix typo. |
→Psychedelic effects: Extend a sentence. |
||
| Line 146: | Line 146: | ||
The HTR of 5-HTP is is blocked by serotonin [[5-HT2A receptor|5-HT<sub>2A</sub> receptor]] [[receptor antagonist|antagonist]]s, which block the [[hallucinogen]]ic effects of serotonergic psychedelics in humans, is prevented by [[aromatic L-amino acid decarboxylase|aromatic <small>L</small>-amino acid decarboxylase]] (AAAD) [[aromatic L-amino acid decarboxylase inhibitor|inhibitor]]s, which convert 5-HTP into serotonin, and is potentiated by [[monoamine oxidase A]] (MAO-A) [[monoamine oxidase inhibitor|inhibitor]]s, which prevent the [[catabolism|degradation]] of serotonin and other [[endogenous]] [[substituted tryptamine|tryptamine]]s.<ref name="SchmidBohn2018">{{cite book | last=Schmid | first=Cullen L. | last2=Bohn | first2=Laura M. | title=5-HT2A Receptors in the Central Nervous System | chapter=βArrestins: Ligand-Directed Regulators of 5-HT2A Receptor Trafficking and Signaling Events | publisher=Springer International Publishing | publication-place=Cham | date=2018 | isbn=978-3-319-70472-2 | doi=10.1007/978-3-319-70474-6_2 | page=31–55}}</ref><ref name="KozlenkovGonzález-Maeso2013" /><ref name="JasterdelaFuenteRevengaGonzález-Maeso2022" /><ref name="SchmidBohn2010" /> In addition, the HTR of 5-HTP is abolished by [[indolethylamine N-methyltransferase|indolethylamine ''N''-methyltransferase]] (INMT) [[enzyme inhibitor|inhibitor]]s, which convert serotonin and other endogenous tryptamines into ''N''-[[methyl group|methylated]] tryptamines, such as [[N-Methylserotonin|''N''-methylserotonin]] (NMS; norbufotenin), [[bufotenin]] (5-hydroxy-''N'',''N''-dimethyltryptamine; 5-HO-DMT), and [[dimethyltryptamine|''N'',''N''-dimethyltryptamine]] (DMT).<ref name="KozlenkovGonzález-Maeso2013">{{cite book | last=Kozlenkov | first=Alexey | last2=González-Maeso | first2=Javier | title=The Neuroscience of Hallucinations | chapter=Animal Models and Hallucinogenic Drugs | publisher=Springer New York | publication-place=New York, NY | date=2013 | isbn=978-1-4614-4120-5 | doi=10.1007/978-1-4614-4121-2_14 | page=253–277}}</ref><ref name="HalberstadtGeyer2018">{{cite journal | vauthors = Halberstadt AL, Geyer MA | title = Effect of Hallucinogens on Unconditioned Behavior | journal = Curr Top Behav Neurosci | volume = 36 | issue = | pages = 159–199 | date = 2018 | pmid = 28224459 | pmc = 5787039 | doi = 10.1007/7854_2016_466 | url = }}</ref><ref name="SchmidBohn2010" /> These ''N''-methylated tryptamines are well-known for their psychedelic effects, whereas serotonin itself, without [[biotransformation]], does not seem to produce psychedelic effects.<ref name="KozlenkovGonzález-Maeso2013" /><ref name="KozlenkovGonzález-Maeso2013" /><ref name="SchmidBohn2010" /> 5-HTP has not been found to produce psychedelic effects in humans, which has been attributed to the high doses required to produce such effects.<ref name="KozlenkovGonzález-Maeso2013" /><ref name="JasterdelaFuenteRevengaGonzález-Maeso2022" /> The 5-HTP doses that produce the HTR in rodents are orders of magnitude higher than the doses of 5-HTP that have been used safely and therapeutically in humans.<ref name="JasterdelaFuenteRevengaGonzález-Maeso2022" /> |
The HTR of 5-HTP is is blocked by serotonin [[5-HT2A receptor|5-HT<sub>2A</sub> receptor]] [[receptor antagonist|antagonist]]s, which block the [[hallucinogen]]ic effects of serotonergic psychedelics in humans, is prevented by [[aromatic L-amino acid decarboxylase|aromatic <small>L</small>-amino acid decarboxylase]] (AAAD) [[aromatic L-amino acid decarboxylase inhibitor|inhibitor]]s, which convert 5-HTP into serotonin, and is potentiated by [[monoamine oxidase A]] (MAO-A) [[monoamine oxidase inhibitor|inhibitor]]s, which prevent the [[catabolism|degradation]] of serotonin and other [[endogenous]] [[substituted tryptamine|tryptamine]]s.<ref name="SchmidBohn2018">{{cite book | last=Schmid | first=Cullen L. | last2=Bohn | first2=Laura M. | title=5-HT2A Receptors in the Central Nervous System | chapter=βArrestins: Ligand-Directed Regulators of 5-HT2A Receptor Trafficking and Signaling Events | publisher=Springer International Publishing | publication-place=Cham | date=2018 | isbn=978-3-319-70472-2 | doi=10.1007/978-3-319-70474-6_2 | page=31–55}}</ref><ref name="KozlenkovGonzález-Maeso2013" /><ref name="JasterdelaFuenteRevengaGonzález-Maeso2022" /><ref name="SchmidBohn2010" /> In addition, the HTR of 5-HTP is abolished by [[indolethylamine N-methyltransferase|indolethylamine ''N''-methyltransferase]] (INMT) [[enzyme inhibitor|inhibitor]]s, which convert serotonin and other endogenous tryptamines into ''N''-[[methyl group|methylated]] tryptamines, such as [[N-Methylserotonin|''N''-methylserotonin]] (NMS; norbufotenin), [[bufotenin]] (5-hydroxy-''N'',''N''-dimethyltryptamine; 5-HO-DMT), and [[dimethyltryptamine|''N'',''N''-dimethyltryptamine]] (DMT).<ref name="KozlenkovGonzález-Maeso2013">{{cite book | last=Kozlenkov | first=Alexey | last2=González-Maeso | first2=Javier | title=The Neuroscience of Hallucinations | chapter=Animal Models and Hallucinogenic Drugs | publisher=Springer New York | publication-place=New York, NY | date=2013 | isbn=978-1-4614-4120-5 | doi=10.1007/978-1-4614-4121-2_14 | page=253–277}}</ref><ref name="HalberstadtGeyer2018">{{cite journal | vauthors = Halberstadt AL, Geyer MA | title = Effect of Hallucinogens on Unconditioned Behavior | journal = Curr Top Behav Neurosci | volume = 36 | issue = | pages = 159–199 | date = 2018 | pmid = 28224459 | pmc = 5787039 | doi = 10.1007/7854_2016_466 | url = }}</ref><ref name="SchmidBohn2010" /> These ''N''-methylated tryptamines are well-known for their psychedelic effects, whereas serotonin itself, without [[biotransformation]], does not seem to produce psychedelic effects.<ref name="KozlenkovGonzález-Maeso2013" /><ref name="KozlenkovGonzález-Maeso2013" /><ref name="SchmidBohn2010" /> 5-HTP has not been found to produce psychedelic effects in humans, which has been attributed to the high doses required to produce such effects.<ref name="KozlenkovGonzález-Maeso2013" /><ref name="JasterdelaFuenteRevengaGonzález-Maeso2022" /> The 5-HTP doses that produce the HTR in rodents are orders of magnitude higher than the doses of 5-HTP that have been used safely and therapeutically in humans.<ref name="JasterdelaFuenteRevengaGonzález-Maeso2022" /> |
||
The lack of the HTR and psychedelic effects with serotonin itself has been attributed to the fact that these effects appear to be dependent on activation of a population of [[intracellular]] 5-HT<sub>2A</sub> receptors expressed in [[cortex|cortical]] [[neuron]]s in the [[medial prefrontal cortex]] (mPFC) that lack the [[serotonin transporter]] (SERT).<ref name="Sapienza2023">{{cite journal | last=Sapienza | first=Jacopo | title=The Key Role of Intracellular 5-HT2A Receptors: A Turning Point in Psychedelic Research? | journal=Psychoactives | volume=2 | issue=4 | date=13 October 2023 | issn=2813-1851 | doi=10.3390/psychoactives2040018 | doi-access=free | pages=287–293}}</ref><ref name="VargasDunlapDong2023">{{cite journal | vauthors = Vargas MV, Dunlap LE, Dong C, Carter SJ, Tombari RJ, Jami SA, Cameron LP, Patel SD, Hennessey JJ, Saeger HN, McCorvy JD, Gray JA, Tian L, Olson DE | title = Psychedelics promote neuroplasticity through the activation of intracellular 5-HT2A receptors | journal = Science | volume = 379 | issue = 6633 | pages = 700–706 | date = February 2023 | pmid = 36795823 | pmc = 10108900 | doi = 10.1126/science.adf0435 | url = }}</ref> Serotonin itself is too [[hydrophilic]] to enter serotonergic neurons without the SERT, whereas serotonergic psychedelics and serotonin's ''N''-methylated [[structural analogue]]s are [[lipophilic]] and readily enter these neurons |
The lack of the HTR and psychedelic effects with serotonin itself has been attributed to the fact that these effects appear to be dependent on activation of a population of [[intracellular]] 5-HT<sub>2A</sub> receptors expressed in [[cortex|cortical]] [[neuron]]s in the [[medial prefrontal cortex]] (mPFC) that lack the [[serotonin transporter]] (SERT) and are inaccessible to serotonin.<ref name="Sapienza2023">{{cite journal | last=Sapienza | first=Jacopo | title=The Key Role of Intracellular 5-HT2A Receptors: A Turning Point in Psychedelic Research? | journal=Psychoactives | volume=2 | issue=4 | date=13 October 2023 | issn=2813-1851 | doi=10.3390/psychoactives2040018 | doi-access=free | pages=287–293}}</ref><ref name="VargasDunlapDong2023">{{cite journal | vauthors = Vargas MV, Dunlap LE, Dong C, Carter SJ, Tombari RJ, Jami SA, Cameron LP, Patel SD, Hennessey JJ, Saeger HN, McCorvy JD, Gray JA, Tian L, Olson DE | title = Psychedelics promote neuroplasticity through the activation of intracellular 5-HT2A receptors | journal = Science | volume = 379 | issue = 6633 | pages = 700–706 | date = February 2023 | pmid = 36795823 | pmc = 10108900 | doi = 10.1126/science.adf0435 | url = }}</ref> Serotonin itself is too [[hydrophilic]] to enter serotonergic neurons without the SERT, whereas serotonergic psychedelics and serotonin's ''N''-methylated [[metabolite]]s and [[structural analogue|analogue]]s are [[lipophilic]] and readily enter these neurons.<ref name="Sapienza2023" /><ref name="VargasDunlapDong2023" /> |
||
== See also == |
== See also == |
||
Revision as of 03:51, 11 September 2024
| |
| |
| Names | |
|---|---|
| IUPAC name
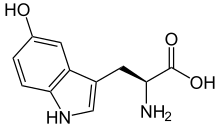
2-amino-3-(5-hydroxy-1H-indol-3-yl)propanoic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.022.193 |
| KEGG | |
| MeSH | 5-Hydroxytryptophan |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C11H12N2O3 | |
| Molar mass | 220.228 g·mol−1 |
| Density | 1.484 g/mL |
| Melting point | 298 to 300 °C (568 to 572 °F; 571 to 573 K) |
| Boiling point | 520.6 °C (969.1 °F; 793.8 K) |
| Pharmacology | |
| N06AX01 (WHO) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
5-Hydroxytryptophan (5-HTP), also known as oxitriptan, is a naturally occurring amino acid and chemical precursor as well as a metabolic intermediate in the biosynthesis of the neurotransmitter serotonin.
Uses
5-HTP is sold over the counter in the United States, France, Canada, Singapore, the Netherlands, and the United Kingdom as a dietary supplement for use as an antidepressant, appetite suppressant, and sleep aid. It is also marketed in many European countries for the indication of major depression under the trade names Cincofarm, Levothym, Levotonine, Oxyfan, Telesol, Tript-OH, and Triptum.[1]
A 2002 review concluded that although the data evaluated suggests that 5-HTP is more effective than placebo in the treatment of depression, the evidence was insufficient to be conclusive due to a lack of clinical data meeting the rigorous standards of the day.[2] More and larger studies using current methodologies are needed to determine if 5-HTP is truly effective in treating depression.[3][4] In small, controlled trials 5-HTP has also been reported to augment the antidepressant efficacy of the antidepressant clomipramine.[5][6][7] A 2020 meta-analysis found oral 5-HTP supplementation had a large effect size on depression symptom severity. However, the included studies were considered relatively weak and the methods and treatment duration varied between the seven studies examined.[8]
5-HTP use after MDMA
MDMA is an empathogenic-entactogenic and serotonergic psychotropic drug used primarily for recreational, though sometimes also therapeutic, purposes. Among users of MDMA, the serotonergic effects of the drug are often of particular interest and concern: After consuming MDMA, serotonin concentrations are greatly reduced in the brain. 5-HTP is necessary for serotonin production and its concentrations in the brain also decrease after taking MDMA.
Other usage
At high doses, or in combination with carbidopa, 5-HTP has been used to treat obesity (by promoting weight loss).[9][10]
In clinical trials of various design, 5-HTP has also been reported to treat fibromyalgia,[11] myoclonus,[12] migraine,[13] and cerebellar ataxia.[14] However, these clinical findings, as for all therapeutic findings with 5-HTP, are preliminary and need confirmation in larger trials.
Drawbacks
5-HTP's short half-life (<2h)[15] may inherently limit its therapeutic potential,[16] as systemic 5-HTP exposure levels will fluctuate substantially even with relatively frequent dosing. Such exposure fluctuations are usually associated with increased adverse event burdens resulting from Cmax (time to maximal systemic concentration) drug spikes, and decreased clinical efficacy resulting from sub-therapeutic exposure for large parts of the day, when taken as a single dose unit or at intervals significantly larger than Cmax. It has been proposed that 5-HTP dosage forms achieving prolonged delivery would be more effective,[16] as has been demonstrated many times with other pharmaceuticals with short durations of action.[17] For example, controlled release oxycodone (OxyContin) or morphine (MS-Contin) are intended to, via novel delivery mechanisms, permit pain relief for up to twelve hours with an active ingredient which only provides relief for 3–6 hours. However, the inherent variability amongst different people with respect to drug metabolism makes this task challenging.
Side effects
Potential side effects of 5-HTP include heartburn, stomach pain, nausea, vomiting, diarrhea, drowsiness, sexual problems, vivid dreams or nightmares, and muscle problems.[18]
Because 5-HTP has not been thoroughly studied in a clinical setting, possible side effects and interactions with other drugs are not well known. According to the US National Library of Medicine, 5-HTP has not been associated with serotonin syndrome or any serious adverse events in humans.[19] Across multiple studies, 5-HTP has also been reported to not cause any noticeable hematological or cardiovascular changes.[20] 5-HTP had also been associated with eosinophilia, but later studies have not found any causal connection.[21]
Interactions
When combined with antidepressants of the MAOI or SSRI class, very high parenteral doses of 5-HTP can cause acute serotonin syndrome in rats.[22][23] It is unclear if such findings have clinical relevance, as most drugs will cause serious adverse events or death in rodents at very high doses. In humans, 5-HTP has never been clinically associated with serotonin syndrome – although a case report suggests 5-HTP can precipitate mania when added to an MAOI.[24]
When combined with carbidopa (as a treatment for symptoms of Parkinson's disease), 5-HTP causes nausea and vomiting; however this can be alleviated via administration of granisetron.[25] As mentioned below under pharmacology, cases of scleroderma-like illness have been reported in patients using carbidopa and 5-HTP.[26]
Oral 5-HTP results in an increase in urinary 5-HIAA, a serotonin metabolite, indicating that 5-HTP is peripherally metabolized to serotonin, which is then metabolized. This might cause false positive results in tests looking for carcinoid syndrome.[27][28] Due to the conversion of 5-HTP into serotonin by the liver, there could be a risk of heart valve disease from serotonin's effect on the heart, as based on preclinical findings.[29][30] However, 5-HTP has not been associated with cardiac toxicity in humans.[21][20][19][31]
It has been suggested that 5-HTP may cause eosinophilia-myalgia syndrome (EMS), a serious condition which results in extreme muscle tenderness, myalgia, and blood abnormalities. However, there is evidence to show that EMS was likely caused by a contaminant in certain 5-HTP supplements.[32]
Production
5-HTP is produced from the amino acid tryptophan through the action of the enzyme tryptophan hydroxylase. Tryptophan hydroxylase is one of the biopterin-dependent aromatic amino acid hydroxylases. Production of 5-HTP is the rate-limiting step in 5-HT (serotonin) synthesis. 5-HTP is normally rapidly converted to 5-HT by amino acid decarboxylase.[33]
Absorption
After oral administration, 5-HTP is absorbed by the upper intestine.[15] The mode of absorption is not known, but presumably involves active transport via amino acid transporters. 5-HTP is adequately absorbed via oral cavity.[34] With a decarboxylase inhibitor, the bioavailability of 5-HTP can be higher than 50%.[35]
Pharmacology
The psychoactive action of 5-HTP is derived from its increase in production of serotonin in central nervous system tissue.[36]

Research shows that co-administration with carbidopa greatly increases plasma 5-HTP levels.[37] Other studies have indicated the risk of a scleroderma-like condition resulting from the combination of 5-HTP and carbidopa.[38]
Pharmacokinetics
5-HTP is rapidly absorbed with a tmax of ≈1.5 h, and rapidly eliminated with a half-life of ≈1.5 – 2 h. Co-administration of a decarboxylase inhibitor (e.g. carbidopa, benserazide) doubles the half-life of 5-HTP to ≈ 3 – 4 h,[39][15] and enhances exposure several-fold, depending on the dosing regimen.[15][40]
Metabolism
5-HTP is decarboxylated to serotonin (5-hydroxytryptamine or 5-HT) by the enzyme aromatic-L-amino-acid decarboxylase with the help of vitamin B6.[41] This reaction occurs both in nervous tissue and in the liver.[42] 5-HTP crosses the blood–brain barrier,[43] while 5-HT does not. Excess 5-HTP, especially when administered with vitamin B6, is thought to be metabolized and excreted.[44][45]
| 5-HTP | AAAD | Serotonin | |

|

| ||
| PLP | |||

| |||
Regulatory status
There are currently no approved drug products containing 5-HTP approved by the FDA.[46] All available 5-HTP products are nutraceuticals and are as such not regulated or verified for purity, integrity, or clinical efficacy or safety, mandating caution regarding human consumption.[47]
As of 25 August 2020, Hungary added 5-HTP to the controlled psychoactive substances list, prohibiting production, sale, import, storage and use, becoming the first country to do so.[48]
5-HTP slow-release
5-HTP's short half-life is impractical for chronic drug therapy. Research conducted at Duke University in mice have demonstrated that 5-HTP when administered as slow-release appears to gain drug properties.[49] Slow-release delivery attenuates or abolishes the peaks and valleys in 5-HTP exposure during treatment.[50] Slow-release delivery of 5-HTP markedly improved the safety profile of 5-HTP and conferred stable plasma exposure of 5-HTP and strong and sustained enhancement of brain serotonin function.[49] This discovery indicates that 5-HTP slow-release medications represent a new avenue for treatment of brain disorders responsive to serotonergic enhancement.
Dietary sources
Though 5-HTP is found in food only in insignificant quantities, it is a chemical involved intermediately in the metabolism of tryptophan, an amino acid found in all unfractionated foods, with lower total amino acid content correlating with increased tryptophan absorption.[51]
The seeds of the Griffonia simplicifolia, a climbing shrub native to West Africa and Central Africa, are used as an herbal supplement for their 5-HTP content.[52][53][54] In one 2010 trial, Griffonia simplicifolia extract appeared to increase satiety in overweight women.[55]
Research
Psychedelic effects
5-HTP robustly produces the head-twitch response (HTR) in rodents when administered at relatively high doses.[56][57][58][59] Similarly, intracerebroventricular injection of serotonin, but not peripheral administration of serotonin, produces the HTR.[57][56][59] The HTR is induced by serotonergic psychedelics like lysergic acid diethylamide (LSD) and psilocybin and is a behavioral proxy of psychedelic effects.[60][56]
The HTR of 5-HTP is is blocked by serotonin 5-HT2A receptor antagonists, which block the hallucinogenic effects of serotonergic psychedelics in humans, is prevented by aromatic L-amino acid decarboxylase (AAAD) inhibitors, which convert 5-HTP into serotonin, and is potentiated by monoamine oxidase A (MAO-A) inhibitors, which prevent the degradation of serotonin and other endogenous tryptamines.[57][56][58][59] In addition, the HTR of 5-HTP is abolished by indolethylamine N-methyltransferase (INMT) inhibitors, which convert serotonin and other endogenous tryptamines into N-methylated tryptamines, such as N-methylserotonin (NMS; norbufotenin), bufotenin (5-hydroxy-N,N-dimethyltryptamine; 5-HO-DMT), and N,N-dimethyltryptamine (DMT).[56][61][59] These N-methylated tryptamines are well-known for their psychedelic effects, whereas serotonin itself, without biotransformation, does not seem to produce psychedelic effects.[56][56][59] 5-HTP has not been found to produce psychedelic effects in humans, which has been attributed to the high doses required to produce such effects.[56][58] The 5-HTP doses that produce the HTR in rodents are orders of magnitude higher than the doses of 5-HTP that have been used safely and therapeutically in humans.[58]
The lack of the HTR and psychedelic effects with serotonin itself has been attributed to the fact that these effects appear to be dependent on activation of a population of intracellular 5-HT2A receptors expressed in cortical neurons in the medial prefrontal cortex (mPFC) that lack the serotonin transporter (SERT) and are inaccessible to serotonin.[62][63] Serotonin itself is too hydrophilic to enter serotonergic neurons without the SERT, whereas serotonergic psychedelics and serotonin's N-methylated metabolites and analogues are lipophilic and readily enter these neurons.[62][63]
See also
- Cardiac fibrosis
- Melatonin
- N-Acetylserotonin
- Selective serotonin reuptake inhibitor
- Serotonin
- Tryptophan
References
- ^ Swiss Pharmaceutical Society (2000). Index Nominum 2000: International Drug Directory (Book with CD-ROM). Boca Raton: Medpharm Scientific Publishers. ISBN 978-3-88763-075-1.
- ^ Shaw K, Turner J, Del Mar C (2002). Shaw KA (ed.). "Tryptophan and 5-hydroxytryptophan for depression" (PDF). The Cochrane Database of Systematic Reviews. 2010 (1): CD003198. doi:10.1002/14651858.CD003198. PMID 11869656.
- ^ "5-Hydroxytryptophan (5-HTP)". University of Maryland Medical Center. Archived from the original on 25 June 2017. Retrieved 9 January 2012.
- ^ Iovieno N, Dalton ED, Fava M, Mischoulon D (May 2011). "Second-tier natural antidepressants: review and critique". Journal of Affective Disorders. 130 (3): 343–357. doi:10.1016/j.jad.2010.06.010. PMID 20579741.
- ^ van Praag HM (1982). "Serotonin precursors in the treatment of depression". Advances in Biochemical Psychopharmacology. 34: 259–286. PMID 6753514.
- ^ van Praag HM, van den Burg W, Bos ER, Dols LC (1974). "5-hydroxytryptophan in combination with clomipramine in "therapy-resistant" depressions". Psychopharmacologia. 38 (3): 267–269. doi:10.1007/BF00421379. PMID 4547418. S2CID 11048888.
- ^ Nardini M, De Stefano R, Iannuccelli M, Borghesi R, Battistini N (1983). "Treatment of depression with L-5-hydroxytryptophan combined with chlorimipramine, a double-blind study". International Journal of Clinical Pharmacology Research. 3 (4): 239–250. PMID 6381336.
- ^ Javelle, Florian; Lampit, Amit; Bloch, Wilhelm; Häussermann, Peter; Johnson, Sheri; Zimmer, Philipp (2020). "Effects of 5-hydroxytryptophan on distinct types of depression: a systematic review and meta-analysis". Nutrition Reviews. 78 (1): 77–88. doi:10.1093/nutrit/nuz039. PMID 31504850.
- ^ Halpern B, Oliveira ES, Faria AM, Halpern A, Melo ME, Cercato C, Mancini MC (July 2010). "Combinations of drugs in the Treatment of Obesity". Pharmaceuticals. 3 (8): 2398–2415. doi:10.3390/ph3082398. PMC 4033931. PMID 27713360.
- ^ Hendricks EJ (2017). "Off-label drugs for weight management". Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy. 10: 223–234. doi:10.2147/DMSO.S95299. PMC 5473499. PMID 28652791.
- ^ Caruso I, Sarzi Puttini P, Cazzola M, Azzolini V (1990). "Double-blind study of 5-hydroxytryptophan versus placebo in the treatment of primary fibromyalgia syndrome". J Int Med Res. 18 (3): 201–209. doi:10.1177/030006059001800304. PMID 2193835. S2CID 27586915.
- ^ Thal LJ, Sharpless NS, Wolfson L, Katzman R (1980). "Treatment of myoclonus with L-5-hydroxytryptophan and carbidopa: clinical, electrophysiological, and biochemical observations". Ann Neurol. 7 (6): 570–576. doi:10.1002/ana.410070611. PMID 6969054. S2CID 11647568.
- ^ Boiardi A, Crenna P, Merati B, Negri S, Tansini E, Bussone G (1981). "5-OH-Tryptophane in migraine: clinical and neurophysiological considerations". J Neurol. 225 (1): 41–46. doi:10.1007/bf00313460. PMID 6164755. S2CID 2424064.
- ^ Trouillas P, Brudon F, Adeleine P (November 1988). "Improvement of cerebellar ataxia with levorotatory form of 5-hydroxytryptophan. A double-blind study with quantified data processing". Arch Neurol. 45 (11): 1217–1222. doi:10.1001/archneur.1988.00520350055016. PMID 3190503.
- ^ a b c d Gijsman HJ, van Gerven JM, de Kam ML, Schoemaker RC, Pieters MS, Weemaes M, de Rijk R, van der Post J, Cohen AF (April 2002). "Placebo-controlled comparison of three dose-regimens of 5-hydroxytryptophan challenge test in healthy volunteers". Journal of Clinical Psychopharmacology. 22 (2): 183–189. doi:10.1097/00004714-200204000-00012. PMID 11910264. S2CID 37414452.
- ^ a b Jacobsen JP, Krystal AD, Krishnan KR, Caron MG (November 2016). "Adjunctive 5-Hydroxytryptophan Slow-Release for Treatment-Resistant Depression: Clinical and Preclinical Rationale". Trends in Pharmacological Sciences. 37 (11): 933–944. doi:10.1016/j.tips.2016.09.001. PMC 5728156. PMID 27692695.
- ^ Thombre AG (September 2005). "Assessment of the feasibility of oral controlled release in an exploratory development setting". Drug Discovery Today. 10 (17): 1159–1166. doi:10.1016/S1359-6446(05)03551-8. PMID 16182208.
- ^ "5-HTP". U.S. National Library of Medicine. Retrieved 7 June 2015.
- ^ a b "5-Hydroxytryptophan, C11H12N2O3, CID 144 - PubChem".
- ^ a b Byerley WF, Judd LL, Reimherr FW, Grosser BI (June 1987). "5-Hydroxytryptophan: a review of its antidepressant efficacy and adverse effects". J Clin Psychopharmacol. 7 (3): 127–37. doi:10.1097/00004714-198706000-00002. PMID 3298325.
- ^ a b Das YT, Bagchi M, Bagchi D, Preuss HG (2004). "Safety of 5-hydroxy-L-tryptophan". Toxicol. Lett. 150 (1): 111–22. doi:10.1016/j.toxlet.2003.12.070. PMID 15068828.
- ^ Ma Z, Zhang G, Jenney C, Krishnamoorthy S, Tao R (July 2008). "Characterization of serotonin-toxicity syndrome (toxidrome) elicited by 5-hydroxy-l-tryptophan in clorgyline-pretreated rats". European Journal of Pharmacology. 588 (2–3): 198–206. doi:10.1016/j.ejphar.2008.04.004. PMC 4242171. PMID 18499101.
- ^ Izumi T, Iwamoto N, Kitaichi Y, Kato A, Inoue T, Koyama T (February 2006). "Effects of co-administration of a selective serotonin reuptake inhibitor and monoamine oxidase inhibitors on 5-HT-related behavior in rats". European Journal of Pharmacology. 532 (3): 258–64. doi:10.1016/j.ejphar.2005.12.075. PMID 16488409.
- ^ Pardo JV (2012). "Mania following addition of hydroxytryptophan to monoamine oxidase inhibitor". General Hospital Psychiatry. 34 (1): 102.e13–4. doi:10.1016/j.genhosppsych.2011.08.014. PMC 3253963. PMID 21963353.
- ^ Jacobs GE, Kamerling IM, de Kam ML, Derijk RH, van Pelt J, Zitman FG, van Gerven JM (January 2010). "Enhanced tolerability of the 5-hydroxytryptophane challenge test combined with granisetron". Journal of Psychopharmacology. 24 (1): 65–72. doi:10.1177/0269881108094299. PMID 18719048. S2CID 23562225.
- ^ "Carbidopa/Levodopa". Truestarhealth.com. Archived from the original on 8 January 2014. Retrieved 2014-01-09.
- ^ Joy T, Walsh G, Tokmakejian S, Van Uum SH (January 2008). "Increase of urinary 5-hydroxyindoleacetic acid excretion but not serum chromogranin A following over-the-counter 5-hydroxytryptophan intake". Canadian Journal of Gastroenterology. 22 (1): 49–53. doi:10.1155/2008/472159. PMC 2659120. PMID 18209781.
- ^ Hallin ML, Mahmoud K, Viswanath A, Gama R (January 2013). "'Sweet Dreams', 'Happy Days' and elevated 24-h urine 5-hydroxyindoleacetic acid excretion". Annals of Clinical Biochemistry. 50 (Pt 1): 80–2. doi:10.1258/acb.2012.012041. PMID 23086978. S2CID 207193834.
- ^ Gustafsson BI, Tømmerås K, Nordrum I, Loennechen JP, Brunsvik A, Solligård E, Fossmark R, Bakke I, Syversen U, Waldum H (March 2005). "Long-term serotonin administration induces heart valve disease in rats". Circulation. 111 (12): 1517–22. doi:10.1161/01.CIR.0000159356.42064.48. PMID 15781732. S2CID 3035838.
- ^ Xu J, Jian B, Chu R, Lu Z, Li Q, Dunlop J, Rosenzweig-Lipson S, McGonigle P, Levy RJ, Liang B (December 2002). "Serotonin mechanisms in heart valve disease II: the 5-HT2 receptor and its signaling pathway in aortic valve interstitial cells". The American Journal of Pathology. 161 (6): 2209–18. doi:10.1016/S0002-9440(10)64497-5. PMC 1850896. PMID 12466135.
- ^ Turner EH, Loftis JM, Blackwell AD (March 2006). "Serotonin a la carte: supplementation with the serotonin precursor 5-hydroxytryptophan". Pharmacol. Ther. 109 (3): 325–38. doi:10.1016/j.pharmthera.2005.06.004. PMID 16023217. S2CID 2563606.
- ^ Michelson D, Page SW, Casey R, Trucksess MW, Love LA, Milstien S, Wilson C, Massaquoi SG, Crofford LJ, Hallett M (December 1994). "An eosinophilia-myalgia syndrome related disorder associated with exposure to L-5-hydroxytryptophan". The Journal of Rheumatology. 21 (12): 2261–5. PMID 7699627.
- ^ Turner EH, Loftis JM, Blackwell AD (March 2006). "Serotonin a la carte: supplementation with the serotonin precursor 5-hydroxytryptophan". Pharmacology & Therapeutics. 109 (3): 325–38. doi:10.1016/j.pharmthera.2005.06.004. PMID 16023217. S2CID 2563606.
- ^ Rondanelli M, Opizzi A, Faliva M, Bucci M, Perna S (March 2012). "Relationship between the absorption of 5-hydroxytryptophan from an integrated diet, by means of Griffonia simplicifolia extract, and the effect on satiety in overweight females after oral spray administration". Eat Weight Disord. 17 (1): e22-8. doi:10.3275/8165. PMID 22142813. S2CID 10651414.
- ^ Magnussen I, Nielsen-Kudsk F (April 1980). "Bioavailability and related pharmacokinetics in man of orally administered L-5-hydroxytryptophan in steady state". Acta Pharmacologica et Toxicologica. 46 (4): 257–62. doi:10.1111/j.1600-0773.1980.tb02451.x. PMID 6966118.
- ^ "5-HTP: Uses, Side Effects, Interactions and Warnings - WebMD". Archived from the original on 16 November 2009. Retrieved 5 October 2009.
- ^ Magnussen I, Jensen TS, Rand JH, Van Woert MH (September 1981). "Plasma accumulation of metabolism of orally administered single dose L-5-hydroxytryptophan in man". Acta Pharmacologica et Toxicologica. 49 (3): 184–9. doi:10.1111/j.1600-0773.1981.tb00890.x. PMID 6175178.
- ^ Sternberg EM, Van Woert MH, Young SN, Magnussen I, Baker H, Gauthier S, Osterland CK (October 1980). "Development of a scleroderma-like illness during therapy with L-5-hydroxytryptophan and carbidopa". The New England Journal of Medicine. 303 (14): 782–7. doi:10.1056/NEJM198010023031403. PMID 6997735.
- ^ Magnussen I, Engbaek F (July 1978). "The effects of aromatic amino acid decarboxylase inhibitors on plasma concentrations of 5-hydroxytryptophan in man". Acta Pharmacologica et Toxicologica. 43 (1): 36–42. doi:10.1111/j.1600-0773.1978.tb02229.x. PMID 309271.
- ^ Westenberg HG, Gerritsen TW, Meijer BA, van Praag HM (December 1982). "Kinetics of l-5-hydroxytryptophan in healthy subjects". Psychiatry Research. 7 (3): 373–85. doi:10.1016/0165-1781(82)90074-9. PMID 6187038. S2CID 45003287.
- ^ Rahman MK, Nagatsu T, Sakurai T, Hori S, Abe M, Matsuda M (October 1982). "Effect of pyridoxal phosphate deficiency on aromatic L-amino acid decarboxylase activity with L-DOPA and L-5-hydroxytryptophan as substrates in rats". Japanese Journal of Pharmacology. 32 (5): 803–11. doi:10.1254/jjp.32.803. PMID 6983619.
- ^ Bouchard S, Bousquet C, Roberge AG (September 1981). "Characteristics of dihydroxyphenylalanine/5-hydroxytryptophan decarboxylase activity in brain and liver of cat". Journal of Neurochemistry. 37 (3): 781–7. doi:10.1111/j.1471-4159.1982.tb12555.x. PMID 6974228. S2CID 43853143.
- ^ Nakatani Y, Sato-Suzuki I, Tsujino N, Nakasato A, Seki Y, Fumoto M, Arita H (May 2008). "Augmented brain 5-HT crosses the blood-brain barrier through the 5-HT transporter in rat". The European Journal of Neuroscience. 27 (9): 2466–72. doi:10.1111/j.1460-9568.2008.06201.x. PMID 18445233. S2CID 18940166.
- ^ Bouchard S, Roberge AG (July 1979). "Biochemical properties and kinetic parameters of dihydroxyphenylalanine--5-hydroxytryptophan decarboxylase in brain, liver, and adrenals of cat". Canadian Journal of Biochemistry. 57 (7): 1014–8. doi:10.1139/o79-126. PMID 39668.
- ^ Amamoto T, Sarai K (September 1976). "On the tryptophan-serotonin metabolism in manic-depressive disorders. Changes in plasma 5-HT and 5-HIAA levels and urinary 5-HIAA excretion following oral loading of L-5HTP in patients with depression". Hiroshima Journal of Medical Sciences. 25 (2–3): 135–40. PMID 1088369.
- ^ "Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations".
- ^ "5-HTP: MedlinePlus Supplements".
- ^ MAGYARORSZÁG HIVATALOS LAPJA. Retrieved 2021-04-28.
- ^ a b Jacobsen JP, Rudder ML, Roberts W, Royer EL, Robinson TJ, Oh A, Spasojevic I, Sachs BD, Caron MG (August 2016). "SSRI Augmentation by 5-Hydroxytryptophan Slow Release: Mouse Pharmacodynamic Proof of Concept". Neuropsychopharmacology. 41 (9): 2324–34. doi:10.1038/npp.2016.35. PMC 4946063. PMID 26932820.
- ^ Thombre AG (2005). "Assessment of the feasibility of oral controlled release in an exploratory development setting". Drug Discov Today. 10 (17): 1159–66. doi:10.1016/S1359-6446(05)03551-8. PMID 16182208.
- ^ "5-Hydroxytryptophan". University of Maryland Medical Center. Archived from the original on 6 January 2010. Retrieved 21 January 2010.
- ^ "5-Hydroxytryptophan (5-HTP)". A.D.A.M., Inc. University of Maryland Medical Center. Animated Dissection of Anatomy for Medicine, Inc. (A.D.A.M., Inc.) provided health and benefits information and technology to healthcare organizations, employers, consumers, and educational institutions
- ^ Emanuele E, Bertona M, Minoretti P, Geroldi D (2010). "An open-label trial of L-5-hydroxytryptophan in subjects with romantic stress". Neuro Endocrinology Letters. 31 (5): 663–6. PMID 21178946.
- ^ "5-hydroxy-L-tryptophan", National Center for Biotechnology Information, PubChem Compound Database, September 2004CID=439280
- ^ Rondanelli M, Opizzi A, Faliva M, Bucci M, Perna S (March 2012). "Relationship between the absorption of 5-hydroxytryptophan from an integrated diet, by means of Griffonia simplicifolia extract, and the effect on satiety in overweight females after oral spray administration". Eating and Weight Disorders. 17 (1): e22–8. doi:10.3275/8165. PMID 22142813. S2CID 10651414.
- ^ a b c d e f g h Kozlenkov, Alexey; González-Maeso, Javier (2013). "Animal Models and Hallucinogenic Drugs". The Neuroscience of Hallucinations. New York, NY: Springer New York. p. 253–277. doi:10.1007/978-1-4614-4121-2_14. ISBN 978-1-4614-4120-5.
- ^ a b c Schmid, Cullen L.; Bohn, Laura M. (2018). "βArrestins: Ligand-Directed Regulators of 5-HT2A Receptor Trafficking and Signaling Events". 5-HT2A Receptors in the Central Nervous System. Cham: Springer International Publishing. p. 31–55. doi:10.1007/978-3-319-70474-6_2. ISBN 978-3-319-70472-2.
- ^ a b c d Jaster AM, de la Fuente Revenga M, González-Maeso J (July 2022). "Molecular targets of psychedelic-induced plasticity". J Neurochem. 162 (1): 80–88. doi:10.1111/jnc.15536. PMC 9068831. PMID 34741320.
- ^ a b c d e Schmid CL, Bohn LM (October 2010). "Serotonin, but not N-methyltryptamines, activates the serotonin 2A receptor via a β-arrestin2/Src/Akt signaling complex in vivo". J Neurosci. 30 (40): 13513–24. doi:10.1523/JNEUROSCI.1665-10.2010. PMC 3001293. PMID 20926677.
- ^ Canal CE, Morgan D (2012). "Head-twitch response in rodents induced by the hallucinogen 2,5-dimethoxy-4-iodoamphetamine: a comprehensive history, a re-evaluation of mechanisms, and its utility as a model". Drug Test Anal. 4 (7–8): 556–576. doi:10.1002/dta.1333. PMC 3722587. PMID 22517680.
- ^ Halberstadt AL, Geyer MA (2018). "Effect of Hallucinogens on Unconditioned Behavior". Curr Top Behav Neurosci. 36: 159–199. doi:10.1007/7854_2016_466. PMC 5787039. PMID 28224459.
- ^ a b Sapienza, Jacopo (13 October 2023). "The Key Role of Intracellular 5-HT2A Receptors: A Turning Point in Psychedelic Research?". Psychoactives. 2 (4): 287–293. doi:10.3390/psychoactives2040018. ISSN 2813-1851.
- ^ a b Vargas MV, Dunlap LE, Dong C, Carter SJ, Tombari RJ, Jami SA, Cameron LP, Patel SD, Hennessey JJ, Saeger HN, McCorvy JD, Gray JA, Tian L, Olson DE (February 2023). "Psychedelics promote neuroplasticity through the activation of intracellular 5-HT2A receptors". Science. 379 (6633): 700–706. doi:10.1126/science.adf0435. PMC 10108900. PMID 36795823.
