Carbenoid: Difference between revisions
update with carbenoid insertion reaction |
m sp: an carbenoid→a carbenoid |
||
| Line 5: | Line 5: | ||
This complex reacts with an [[alkene]] to form a [[cyclopropane]] just as a carbene would do. |
This complex reacts with an [[alkene]] to form a [[cyclopropane]] just as a carbene would do. |
||
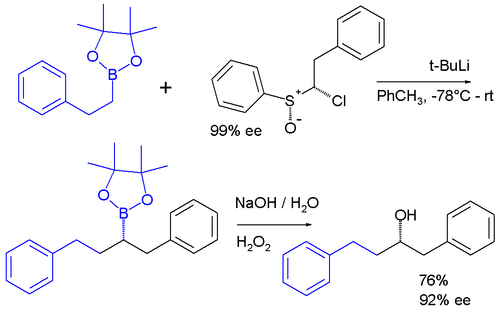
Carbenoids appear as intermediates in many other reactions. In one system |
Carbenoids appear as intermediates in many other reactions. In one system a carbenoid chloroalkyllithium reagent is prepared in situ from a [[sulfoxide]] and [[organolithium|t-BuLi]] which reacts by an [[insertion reaction]] into the C-B bond of a [[pinacol boronic ester]] <ref>Iterative Stereospecific Reagent-Controlled Homologation of Pinacol Boronates by Enantioenriched -Chloroalkyllithium Reagents Paul R. Blakemore and Matthew S. Burge [[J. Am. Chem. Soc.]]; '''2007'''; 129(11) pp 3068 - 3069; (Communication) {{DOI|10.1021/ja068808s}}</ref>. |
||
Revision as of 21:12, 5 June 2007
In chemistry a carbenoid is a reactive intermediate that shares reaction characteristics with a carbene [1]. In the Simmons-Smith reaction the carbenoid intermediate is a zinc / iodine complex that takes the form of
- I-CH2-Zn-I
This complex reacts with an alkene to form a cyclopropane just as a carbene would do.
Carbenoids appear as intermediates in many other reactions. In one system a carbenoid chloroalkyllithium reagent is prepared in situ from a sulfoxide and t-BuLi which reacts by an insertion reaction into the C-B bond of a pinacol boronic ester [2].
The enantiopurity of the chiral sulfoxide is preserved in the ultimate product after oxidation of the boronic ester to the alcohol indicating that a true carbene was never involved in the sequence.
See also
- The silicon pendant: Silylenoids
References
- ^ Organic Chemistry john McMurry Brooks /Cole Publishing Company 1988 ISBN 0-534-07968-7
- ^ Iterative Stereospecific Reagent-Controlled Homologation of Pinacol Boronates by Enantioenriched -Chloroalkyllithium Reagents Paul R. Blakemore and Matthew S. Burge J. Am. Chem. Soc.; 2007; 129(11) pp 3068 - 3069; (Communication) doi:10.1021/ja068808s