Aza-Diels–Alder reaction: Difference between revisions
m cat |
ref format; wikilinks |
||
| Line 3: | Line 3: | ||
[[Image:Aza Diels Alder.gif|center|The Aza Diels-Alder reaction, general scope]] |
[[Image:Aza Diels Alder.gif|center|The Aza Diels-Alder reaction, general scope]] |
||
The imine is often generated [[in situ]] from an [[amine]] and [[formaldehyde]]. An example is the reaction of [[cyclopentadiene]] with [[benzylamine]] to an aza [[norbornene]] |
The imine is often generated [[in situ]] from an [[amine]] and [[formaldehyde]]. An example is the reaction of [[cyclopentadiene]] with [[benzylamine]] to an aza [[norbornene]].<ref>''N-benzyl-2-azanorbornene'' Paul A. Grieco and Scott D. Larsen [[Organic Syntheses]], Coll. Vol. 8, p.31; Vol. 68, p.206 [http://www.orgsyn.org/orgsyn/prep.asp?prep=cv8p0031 Article]</ref> |
||
[[Image:Aza-Diels-Alder phenylmethylamine.gif|center]] |
[[Image:Aza-Diels-Alder phenylmethylamine.gif|center]] |
||
In the [[enantioselective]] Diels-Alder (DA) reaction of an [[aniline]], [[formaldehyde]] and a cyclohexenone [[catalysis|catalyzed]] by (S)-[[proline]] even the diene is masked |
In the [[enantioselective]] Diels-Alder (DA) reaction of an [[aniline]], [[formaldehyde]] and a [[cyclohexenone]] [[catalysis|catalyzed]] by (S)-[[proline]] even the diene is masked.<ref>''Direct Catalytic Enantioselective Aza-Diels-Alder Reactions'' Henrik Sundén, Ismail Ibrahem, Lars Eriksson, Armando Córdova [[Angewandte Chemie International Edition]] 4877 '''2005''' [http://www3.interscience.wiley.com/cgi-bin/abstract/110546882/ABSTRACT Abstract]</ref> |
||
[[Image:Aza Diels Alder proline.gif|center|S-proline enantioselective Aza Diels-Alder reaction]] |
[[Image:Aza Diels Alder proline.gif|center|S-proline enantioselective Aza Diels-Alder reaction]] |
||
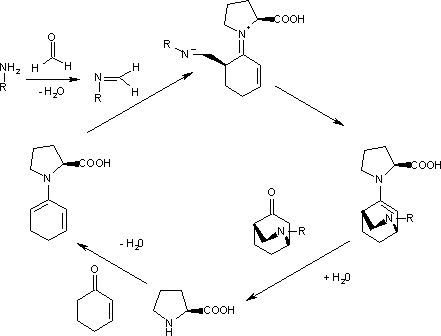
The [[catalytic cycle]] starts with the reactions of the aromatic amine with formaldehyde to the imine and the reaction of the [[ketone]] with proline to the diene. The second step, an [[endo trig cyclisation]], is driven to one of the two possible [[enantiomer]]s (99% [[enantiomeric excess|ee]]) because the imine nitrogen atom forms a [[hydrogen bond]] with the [[carboxylic acid]] group of proline on the [[Si face]]. Hydrolysis of the final complex releases the product and regenerates the catalyst. |
The [[catalytic cycle]] starts with the reactions of the aromatic amine with [[formaldehyde]] to the [[imine]] and the reaction of the [[ketone]] with [[proline]] to the diene. The second step, an [[endo trig cyclisation]], is driven to one of the two possible [[enantiomer]]s (99% [[enantiomeric excess|ee]]) because the imine nitrogen atom forms a [[hydrogen bond]] with the [[carboxylic acid]] group of proline on the [[Si face]]. Hydrolysis of the final complex releases the product and regenerates the catalyst. |
||
[[Image:Aza DA mechanism.gif|center|catalytic cycle for S-proline enantioselective Aza Diels-Alder reaction]] |
[[Image:Aza DA mechanism.gif|center|catalytic cycle for S-proline enantioselective Aza Diels-Alder reaction]] |
||
== References == |
== References == |
||
<references/> |
|||
* <sup>[[#references|1]]</sup> ''N-benzyl-2-azanorbornene'' Paul A. Grieco and Scott D. Larsen [[Organic Syntheses]], Coll. Vol. 8, p.31; Vol. 68, p.206 [http://www.orgsyn.org/orgsyn/prep.asp?prep=cv8p0031 Article] |
|||
* <sup>[[#references|2]]</sup> ''Direct Catalytic Enantioselective Aza-Diels-Alder Reactions'' Henrik Sundén, Ismail Ibrahem, Lars Eriksson, Armando Córdova [[Angewandte Chemie International Edition]] 4877 '''2005''' [http://www3.interscience.wiley.com/cgi-bin/abstract/110546882/ABSTRACT Abstract] |
|||
[[Category:Cycloadditions]] |
[[Category:Cycloadditions]] |
||
Revision as of 19:29, 14 November 2007
The Aza Diels-Alder reaction converts imines and dienes to tetrahydropyridines. This organic reaction is a modification of the Diels-Alder reaction. The nitrogen atom can be part of the diene or the dienophile.
The imine is often generated in situ from an amine and formaldehyde. An example is the reaction of cyclopentadiene with benzylamine to an aza norbornene.[1]

In the enantioselective Diels-Alder (DA) reaction of an aniline, formaldehyde and a cyclohexenone catalyzed by (S)-proline even the diene is masked.[2]

The catalytic cycle starts with the reactions of the aromatic amine with formaldehyde to the imine and the reaction of the ketone with proline to the diene. The second step, an endo trig cyclisation, is driven to one of the two possible enantiomers (99% ee) because the imine nitrogen atom forms a hydrogen bond with the carboxylic acid group of proline on the Si face. Hydrolysis of the final complex releases the product and regenerates the catalyst.
References
- ^ N-benzyl-2-azanorbornene Paul A. Grieco and Scott D. Larsen Organic Syntheses, Coll. Vol. 8, p.31; Vol. 68, p.206 Article
- ^ Direct Catalytic Enantioselective Aza-Diels-Alder Reactions Henrik Sundén, Ismail Ibrahem, Lars Eriksson, Armando Córdova Angewandte Chemie International Edition 4877 2005 Abstract
