Carbon trioxide: Difference between revisions
Appearance
Content deleted Content added
No edit summary |
|||
| Line 1: | Line 1: | ||
''' |
'''Carbonate''' (CO<sub>3</sub>) is an unstable product of reactions between [[carbon dioxide]] (CO<sub>2</sub>) and [[atomic oxygen]] (O).<ref>[http://adsabs.harvard.edu/abs/1971CPL....11..593S]</ref> It is different from the [[carbonate ion]] (CO<sub>3</sub><sup>2-</sup>). It has also been detected in reactions between [[carbon monoxide]], CO, and [[molecular oxygen]], O<sub>2</sub>. Among other places it has been shown to be created in the drift zone of a negative [[corona discharge]].<ref>[http://www.springerlink.com/(3xbvg4fk245a5l45su5fwm55)/app/home/contribution.asp?referrer=parent&backto=issue,5,9;journal,147,588;linkingpublicationresults,1:106035,1]</ref> This pathway arises from reactions between carbon dioxide and atomic oxygen ions, created from molecular oxygen by free electrons in the [[plasma (physics)|plasma]]. Carbon trioxide is a [[liquid]]. |
||
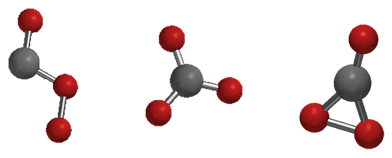
[[Image:co3-geometries.png|The Cs, D3h, and C2v isomers of carbon trioxide]] |
[[Image:co3-geometries.png|The Cs, D3h, and C2v isomers of carbon trioxide]] |
||
Revision as of 22:45, 18 January 2008
Carbonate (CO3) is an unstable product of reactions between carbon dioxide (CO2) and atomic oxygen (O).[1] It is different from the carbonate ion (CO32-). It has also been detected in reactions between carbon monoxide, CO, and molecular oxygen, O2. Among other places it has been shown to be created in the drift zone of a negative corona discharge.[2] This pathway arises from reactions between carbon dioxide and atomic oxygen ions, created from molecular oxygen by free electrons in the plasma. Carbon trioxide is a liquid.
Three possible isomers of carbon trioxide exist, denoted Cs, D3h, and C2v. The C2v state has been shown by various studies to be the ground state of the molecule.[3]
References
- Electronic structure and spectroscopy of carbon trioxide
- Sabin J. R., Kim H. (1971). "A theoretical study of the structure and properties of carbon trioxide". Chemical Physics Letters. 11 (5): 593–597. doi:10.1016/0009-2614(71)87010-0.
- Sobek V., Skalný J. D. (1993). Czechoslovak Journal of Physics. 43 (8) http://www.springerlink.com/(3xbvg4fk245a5l45su5fwm55)/app/home/contribution.asp?referrer=parent&backto=issue,5,9;journal,147,588;linkingpublicationresults,1:106035,1.
{{cite journal}}: Cite has empty unknown parameter:|coauthors=(help); Missing or empty|title=(help) - Pople J. A. , Seeger U., Seeger R., Schleyer P. v. R. (2004). "The structure of carbonate". Journal of Computational Chemistry. 1 (2): 199–203. doi:10.1002/jcc.540010215.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Moll N. G., Clutter D. R., Thompson W. E. (1966). "Carbonate: Its Production, Infrared Spectrum, and Structure Studied in a Matrix of Solid CO2". The Journal of Chemical Physics. 45 (12): 4469–4481. doi:10.1063/1.1727526.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Gimarc B. M., Chou T. S. (1968). "Geometry and Electronic Structure of Carbon Trioxide". The Journal of Chemical Physics. 49 (9): 4043–4047. doi:10.1063/1.1670715.
- DeMore W. B., Jacobsen C. W. (1969). "Formation of carbon trioxide in the photolysis of ozone in liquid carbon dioxide". Journal of Physical Chemistry. 73 (9): 2935–2938. doi:10.1021/j100843a026.
- DeMore W. B., Dede C. (1970). "Pressure dependence of carbon trioxide formation in the gas-phase reaction of O(1D) with carbon dioxide". Journal of Physical Chemistry. 74 (13): 2621–2625. doi:10.1021/j100707a006.
- Francisco J. S., Williams I. H. (1985). "A theoretical study of the force field for carbon trioxide". Chemical Physics. 95 (3). doi:10.1016/0301-0104(85)80160-9.
{{cite journal}}: Text "pages 373-383" ignored (help)