Lamotrigine: Difference between revisions
No edit summary |
|||
| Line 1: | Line 1: | ||
'''Lamotrigine''' (marketed as '''Lamictal''' by [[GlaxoSmithKline]]) is an [[anticonvulsant]] drug used in the treatment of [[epilepsy]] and [[bipolar disorder]]. For epilepsy it is used to treat [[seizure|partial seizure]]s, primary and secondary [[grand mal seizure|tonic-clonic seizure]]s, and seizures associated with [[Lennox-Gastaut syndrome]]. Lamotrigine also acts as a [[mood stabilizer]]. It is the only anticonvulsant mood stabilizer that treats the [[Clinical depression|depressive]] as well as the [[manic]] phases of bipolar disorders, and it is the first medication since [[Lithium]] granted [[FDA]]-approval for the maintenance treatment of bipolar I. Chemically unrelated to other anticonvulsants, lamotrigine has relatively few side-effects and does not require blood monitoring. It is a [[sodium|Na<sup>+</sup>]] channel blocker, and is inactivated by [[liver|hepatic]] glucuronidation. |
'''Lamotrigine''' (marketed as '''Lamictal''' by [[GlaxoSmithKline]]) is an [[anticonvulsant]] drug used in the treatment of [[epilepsy]] and [[bipolar disorder]]. For epilepsy it is used to treat [[seizure|partial seizure]]s, primary and secondary [[grand mal seizure|tonic-clonic seizure]]s, and seizures associated with [[Lennox-Gastaut syndrome]]. Lamotrigine also acts as a [[mood stabilizer]]. It is the only anticonvulsant mood stabilizer that treats the [[Clinical depression|depressive]] as well as the [[manic]] phases of bipolar disorders, and it is the first medication since [[Lithium]] granted [[FDA]]-approval for the maintenance treatment of [[bipolar I]]. Chemically unrelated to other anticonvulsants, lamotrigine has relatively few side-effects and does not require blood monitoring. It is a [[sodium|Na<sup>+</sup>]] channel blocker, and is inactivated by [[liver|hepatic]] glucuronidation. |
||
{| border="1" cellpadding="3" cellspacing="0" width="250px" align="right" style="border-collapse: collapse; margin: 0 0 0 0.5em" |
{| border="1" cellpadding="3" cellspacing="0" width="250px" align="right" style="border-collapse: collapse; margin: 0 0 0 0.5em" |
||
Revision as of 20:08, 21 July 2005
Lamotrigine (marketed as Lamictal by GlaxoSmithKline) is an anticonvulsant drug used in the treatment of epilepsy and bipolar disorder. For epilepsy it is used to treat partial seizures, primary and secondary tonic-clonic seizures, and seizures associated with Lennox-Gastaut syndrome. Lamotrigine also acts as a mood stabilizer. It is the only anticonvulsant mood stabilizer that treats the depressive as well as the manic phases of bipolar disorders, and it is the first medication since Lithium granted FDA-approval for the maintenance treatment of bipolar I. Chemically unrelated to other anticonvulsants, lamotrigine has relatively few side-effects and does not require blood monitoring. It is a Na+ channel blocker, and is inactivated by hepatic glucuronidation.
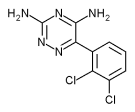
| 3,5-diamino-6-(2,3- dichlorophenyl)-as-triazine | |
| CAS number 84057-84-1 |
ATC code N03AX09 |
| Chemical formula | Template:Carbon9Template:Hydrogen7Template:Nitrogen5Template:Chlorine2 |
| Molecular weight | 256.09 |
| Bioavailability | 98% |
| Metabolism | Hepatic |
| Elimination half-life | 24-34 hours (healthy adults) |
| Excretion | Renal |
| Pregnancy category | C (USA) |
| Legal status | N/A(USA) POM (UK) |
| Routes of administration | Oral |
U.S. FDA approval history
- December 1994 - for use as adjunctive treatment for partial seizures with or without secondary generalization in adult patients (16 years of age and older).
- August 1998 - for use as adjunctive treatment of Lennox-Gastaut syndrome in pediatric and adult patients, new dosage form: chewable dispersible tablets.
- December 1998 - for use as monotherapy for treatment of partial seizures in adult patients when converting from a single enzyme-inducing anti-epileptic drug (EIAED).
- January 2003 - for use as adjunctive therapy for partial seizures in pediatric patients as young as 2 years of age.
- June 2003 - for the maintenance treatment of adults with Bipolar I Disorder to delay the time to occurrence of mood episodes (depression, mania, hypomania, mixed episodes) in patients treated for acute mood episodes with standard therapy. Additionally, the FDA has noted that findings for Lamictal maintenance treatment were more robust in bipolar depression.
- January 2004 - for use as monotherapy for treatment of partial seizures in adult patients when converting from the anti-epileptic drug valproate (including valproic acid (Depakene) and divalproex sodium (Depakote)).
Indications & Usage
The FDA approved lamotrigine (Lamictal) for the treatment of epilepsy in 1994, and bipolar I disorder in 2003 (http://www.fda.gov/cder/). Off-label uses include the treatment of peripheral neuropathy, trigeminal neuralgia, cluster headaches, migraines, and reducing neuropathic pain (Backonja, 2004; Jensen 2002; Pappagallo, 2003). Off-label psychiatric usage includes the treatment of bipolar II disorders, schizoaffective disorder, post traumatic stress disorder, and as adjunctive therapy for "treatment-resistant" unipolar depression (Barbosa, Berk & Vorster, 2003). Lamotrigine is one of a small number of FDA-approved therapies for seizures associated with Lennox-Gastaut syndrome; it is one of two approved for the maintenance treatment of bipolar disorder.
Lennox-Gastaut syndrome (LGS) is a severe form of epilepsy. Typically developing before 4 years of age, LGS is associated with developmental delays. There is no cure, treatment is often complicated, and complete recovery is rare. Symptoms include the atonic seizure (also known as a "drop attack"), during which brief loss of muscle tone and consciousness cause abrupt falls. Lamotrigine significantly reduces the frequency of LGS seizures, and is one of two medications known to decrease the severity of drop attacks (French et al., 2004). Combination with valproate is common, but this increases the risk of lamotrigine-induced rash, and necessitates reduced dosing due to the interaction of these drugs (Pellock, 1999).
Lamotrigine (Lamictal) is the first FDA-approved therapy since Lithium for maintenance treatment of bipolar I disorder (GlaxoSmithKline, 2003). These are the only true "mood stabilizers" in that they posses antidepressant as well as antimanic properties, and research has shown that of the two, lamotrigine is the more effective treatment for bipolar depression. Traditional anticonvulsant drugs are primarily antimanics. Lamotrigine treats depression without triggering mania, hypomania, mixed states, or rapid-cycling, and the 2002 American Psychiatric Association guidelines recommended lamotrigine as a first-line treatment for acute depression in bipolar disorder as well as a maintenance therapy, however lamotrigine is not indicated "on label" for treatment of acute symptoms.
Dosing
Lamotrigine is manufactured in scored tablets (25mg, 100mg, 150mg and 200mg) and chewable dispersible tablets (2mg, 5mg and 25mg). 5-week sample kits are also available; these include titration instructions and scored tablets (25mg for patients taking valproate, 25mg and 100mg for patients not taking valproate).
Recommended initial dosing begins at less than 1mg for epilepsy and 25mg for bipolar disorder, and a therapeutic response may require weeks or months of subsequent dose escalations. This conservative titration minimizes the risk of inducing a potentially serious rash. Dosing should be reduced gradually as well. Abrupt discontinuation of any anticonvulsant increases the risk of seizures, even without a history of epilepsy. Dosing depends on the metabolic effects of concomitant medications such as valproate (reducing) and carbamazepine (enhancing).
Therapeutic plasma concentrations of lamotrigine are unknown, and according to the manufacturer, dosing should be based on therapeutic response. Generally, the therapeutic range for epilepsy is 300mg-500mg a day. Antidepressant effects may begin at 100mg a day, if not earlier, and mood stabilization takes place between 100mg to 200mg a day. Clinical studies show no effective difference for depression or bipolar disorder beyond 200mg, however antimanic effects may not begin until doses of 400mg a day. GlaxoSmithKline suggests maintenance doses up to 500mg for epilepsy, and 400mg for bipolar disorder. Blood monitoring is not required.
Side Effects
Common side effects include headaches, dizziness and insomnia. In very rare cases, Lamotrigine has been known to cause the development of a dangerous rash in some people called Stevens-Johnson syndrome. The rash is more common in children, so this medication is often reserved for adults. (The rash is also more common when other anticonvulsants, particularly the valproates, have been previously prescribed, so more care is needed in those cases.)
Drug Interactions
Other medications can increase or decrease the effectiveness of lamotrigine. The valproate AEDs (divalproex, Depakote; valproate sodium, Depakon; valproic acid, Depakene) inhibit the metabolism of lamotrigine, more than doubling its half-life. The dosage of lamotrigine must be reduced in the presence of these drugs. The enzyme-inducing AEDs (including carbamazepine USP, Tegretol; oxcarbazepine, Trileptal; and phenytoin, Dilantin) enhance the metabolism of lamotrigine, and its dosage must be increased when taken with these drugs; the same consideration is required when Lamictal is taken with oral contraceptives.
References
- Backonja, M. (2004). Neuromodulating drugs for the symptomatic treatment of neuropathic pain. Cur Pain Headache Rep. 8(3):212–6
- Barbosa, L. Berk, M. Vorster, M. (2003). A double-blind, randomized, placebo-controlled trial of augmentation with lamotrigine or placebo in patients concomitantly treated with fluoxetine for resistant major depressive episodes. [Abstract]. J Clin Psychiatry. 64(4):403–407.
- Campbell, G.H. Lutsep, H.L. (2004, 16 December). Trigeminal neuralgia. J. Mendizabal, et al. (Eds). Accessed on March 22, 2005.
- Center for Drug Evaluation and Research. (2005, 01 April). A catalog of FDA approved drug products. Washington, DC: U.S. Food and Drug Administration. Accessed on April 01, 2005.
- Center for Drug Evaluation and Research. (2005, 01 April). Electronic orange book: Approved drug products. Washington, DC: U.S. Food and Drug Administration. Accessed on April 01, 2005.
- French, J.A. et al. (2004). Efficacy and tolerability of the new antiepileptic drugs II: Treatment of refractory epilepsy [electronic version]. Neurology. 62:1261–1273.
- GlaxoSmithKline. (2003, June 23). Lamictal: First medication since Lithium approved for long-term maintenance treatment of bipolar disorder. Press Release.
- GlaxoSmithKline. (2004, January 14). For epilepsy it is used to treat partial seizures, primary and secondary tonic-clonic seizures, and seizures associated with Lennox-Gastaut syndrome. Lamictal® (lamotrigine): Prescribing information. Accessed on February 02, 2005.
- GlaxoSmithKline UK. (2004, February 09). Lamictal combined tablets. electronic Medicines Compendium. Accessed on February 02, 2005.
- Glauser, T.A. Morita, D.A. (2002, January 30). Lennox-gastaut syndrome. (eds). Accessed on March 22, 2005.
- Huntington’s Outreach Project for Education. (2004, December 08). Lamotrigine disease mechanism V: Glutamate toxicity Stanford University. Accessed on March 12, 2005.
- Jensen, T.S. (2002). Anticonvulsants in neuropathic pain: rationale and clinical evidence. [Abstract]. Eur J Pain. 6 Suppl A:61–68.
- Ochoa, J.G. & Riche W. (2005, 02 March). Antiepileptic drugs: An overview. E.A. Passaro, et al (Eds). Accessed on March 12, 2005.
- Pappagallo, M. (2003). Newer antiepileptic drugs: possible uses in the treatment of neuropathic pain and migraine. [Abstract]. Clin. Ther. 25(10):2506–38.
- Pellock, J.M. (1999). Managing pediatric epilepsy syndromes with new antiepileptic drugs [Special issue, electronic version]. Pediatrics. 104(5): 1106–1116.
External links
- Center for Drug Evaluation and Research: Lamictal - documents related to the FDA approval process, including medical reviews and correspondance letters.
- lamictal.com - the GlaxoSmithKline website, with a pdf file of the current and complete prescribing information and separate sections regarding epilepsy and bipolar disorder.
- Lamictal™ Medicine Guide - from the electronic Medicines Compendium (UK), with the summary of product characteristics and pdf files of patient information leaflets.