Pregnenolone: Difference between revisions
No edit summary |
No edit summary |
||
| Line 24: | Line 24: | ||
| routes_of_administration = |
| routes_of_administration = |
||
}} |
}} |
||
'''Pregnenolone''' is a [[steroid]] hormone involved in the [[steroidogenesis]] of [[progesterone]], [[mineralocorticoids]], [[glucocorticoids]], [[androgen]]s, and [[estrogen]]s. As such it is a [[prohormone]]. Pregnenolone is a [[GABAB]] antagonist and increases neurogenesis in the hippocampus.<ref name=Mayo2005>{{cite journal |
'''Pregnenolone''' is a [[steroid]] hormone involved in the [[steroidogenesis]] of [[progesterone]], [[mineralocorticoids]], [[glucocorticoids]], [[androgen]]s, and [[estrogen]]s. As such it is a [[prohormone]]. Pregnenolone is a [[GABAB receptor|GABA<sub>B</sub>]] antagonist and increases neurogenesis in the hippocampus.<ref name=Mayo2005>{{cite journal |
||
| pmid = 15585350 |
| pmid = 15585350 |
||
| pmc = 15585350 |
| pmc = 15585350 |
||
Revision as of 09:58, 1 July 2008
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.005.135 |
| Chemical and physical data | |
| Formula | C21H32O2 |
| Molar mass | 316.483 g/mol g·mol−1 |
Pregnenolone is a steroid hormone involved in the steroidogenesis of progesterone, mineralocorticoids, glucocorticoids, androgens, and estrogens. As such it is a prohormone. Pregnenolone is a GABAB antagonist and increases neurogenesis in the hippocampus.[1]
Chemistry
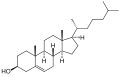
Like other steroids, pregnenolone consists of four interconnected cyclic hydrocarbons. It contains ketone and hydroxyl functional groups, two methyl branches, and a double bond at C5, in the B cyclic hydrocarbon ring. Like all steroid hormones, it is hydrophobic. Its esterified version, pregnenolone sulfate, is water-soluble.
Synthesis


Pregnenolone is synthesized from cholesterol. This conversion involves hydroxylation at the side-chain at C20 and C22 positions, with cleavage of the side-chain. The enzyme performing this task is cytochrome P450scc, located in the mitochondria, and controlled by pituitary tropic hormones, such as ACTH, FSH, LH.
Prohormone
Pregnenolone undergoes further steroid metabolism in one of three ways.
- Pregnenolone can be converted to progesterone. The critical enzyme step is two-fold using a 3-beta-hydroxysteroid dehydrogenase and a delta 4-5 isomerase. The latter transfers the double bond from C5 to C4 on the A ring. Progesterone is the entry into the delta-4-pathway, resulting in production of 17-hydroxy progesterone and androstenedione, precursor to testosterone and estrone. Aldosterone and corticosteroids are also derived from progesterone or its derivatives.
- Pregnenolone can be converted to 17-hydroxy-pregnenolone by the enzyme 17α-hydroxylase (CYP17A1). Using this pathway, termed delta-5 pathway, the next step is conversion to dehydroepiandrosterone (DHEA) using a desmolase. DHEA is the precursor of androstenedione.
- Pregnenolone can be converted to androsta-5,16-dien-3 beta-ol by 16-ene synthetase.
Neurosteroid
Pregnenolone and its sulfate, like dehydroepiandrosterone and its sulfate and progesterone, belong to the group of neurosteroids that are found in high concentrations in certain areas in the brain, and are synthesized there. Neurosteroids affect synaptic functioning, are neuroprotective, and enhance myelinization. Pregnenolone and its sulfate ester are under investigation for their potential to improve cognitive and memory functioning.[2]
Additional images
References
- ^ Mayo, W; Lemaire, V; Malaterre, J; Rodriguez, Jj; Cayre, M; Stewart, Mg; Kharouby, M; Rougon, G; Le, Moal, M; Piazza, Pv; Abrous, Dn (2005). "Pregnenolone Sulfate Enhances Neurogenesis and Psa-Ncam in Young and Aged Hippocampus". Neurobiology of Aging. 26 (1): 103–14. doi:10.1016/j.neurobiolaging.2004.03.013. PMC 15585350. PMID 15585350.
{{cite journal}}: Check|pmc=value (help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Vallée M, Mayo W, Le Moal M (2001). "Role of pregnenolone, dehydroepiandrosterone and their sulfate esters on learning and memory in cognitive aging". Brain Res Brain Res Rev. 37 (1–3): 301–12. doi:10.1016/S0165-0173(01)00135-7. PMID 11744095.
{{cite journal}}: CS1 maint: multiple names: authors list (link)