Niobium: Difference between revisions
mNo edit summary |
Plasticbot (talk | contribs) m Correcting common errors in the accessdate field of citation templates and general fixes |
||
| Line 74: | Line 74: | ||
| coauthor = Tadeu Carneiro |
| coauthor = Tadeu Carneiro |
||
| url = http://www.cbmm.com.br/portug/sources/techlib/science_techno/table_content/images/pdfs/closing.pdf |
| url = http://www.cbmm.com.br/portug/sources/techlib/science_techno/table_content/images/pdfs/closing.pdf |
||
| |
| accessdate = 2008-09-03}}</ref> It is a very important alloy addition in [[HSLA steel]]s, which are widely used as structural components in modern automobiles.<ref name = patel>{{ cite journal |
||
| journal = Metallurgist |
| journal = Metallurgist |
||
| volume = 45 |
| volume = 45 |
||
| Line 234: | Line 234: | ||
| url = |
| url = |
||
| doi = 10.1111/j.1600-0536.1998.tb05832.x }}</ref> |
| doi = 10.1111/j.1600-0536.1998.tb05832.x }}</ref> |
||
Niobium-containing compounds are relatively rarely encountered by most people, but some are toxic and should be treated with care. The short and long term exposure to niobates and niobium chloride, two chemicals which are water soluable, have been tested in rats. Rats treated with a single injection of niobium pentachloride or niobates showing show a [[Median lethal dose |
Niobium-containing compounds are relatively rarely encountered by most people, but some are toxic and should be treated with care. The short and long term exposure to niobates and niobium chloride, two chemicals which are water soluable, have been tested in rats. Rats treated with a single injection of niobium pentachloride or niobates showing show a [[Median lethal dose| LD<sub>50</sub>]] between 10 and 100 mg/kg.<ref name= Haley>{{cite journal |
||
| title = Pharmacology and toxicology of niobium chloride |
| title = Pharmacology and toxicology of niobium chloride |
||
| first = Thomas J. |
| first = Thomas J. |
||
Revision as of 06:08, 14 September 2008
 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Niobium | ||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pronunciation | /naɪˈoʊbiəm/ | |||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | Gray metallic, bluish when oxidized | |||||||||||||||||||||||||||||||||||||||||||||||
| Standard atomic weight Ar°(Nb) | ||||||||||||||||||||||||||||||||||||||||||||||||
| Niobium in the periodic table | ||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic number (Z) | 41 | |||||||||||||||||||||||||||||||||||||||||||||||
| Group | group 5 | |||||||||||||||||||||||||||||||||||||||||||||||
| Period | period 5 | |||||||||||||||||||||||||||||||||||||||||||||||
| Block | d-block | |||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Kr] 4d4 5s1 | |||||||||||||||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 12, 1 | |||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | ||||||||||||||||||||||||||||||||||||||||||||||||
| Phase at STP | solid | |||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 2750 K (2477 °C, 4491 °F) | |||||||||||||||||||||||||||||||||||||||||||||||
| Boiling point | 5017 K (4744 °C, 8571 °F) | |||||||||||||||||||||||||||||||||||||||||||||||
| Density (at 20° C) | 8.582 g/cm3[3] | |||||||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 30 kJ/mol | |||||||||||||||||||||||||||||||||||||||||||||||
| Heat of vaporization | 689.9 kJ/mol | |||||||||||||||||||||||||||||||||||||||||||||||
| Molar heat capacity | 24.60 J/(mol·K) | |||||||||||||||||||||||||||||||||||||||||||||||
Vapor pressure
| ||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | ||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | common: +5 −3,[4] −1,[5] 0,[6] +1,[6] +2,[5] +3,[5] +4[5] | |||||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | Pauling scale: 1.6 | |||||||||||||||||||||||||||||||||||||||||||||||
| Ionization energies |
| |||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius | empirical: 146 pm | |||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 164±6 pm | |||||||||||||||||||||||||||||||||||||||||||||||
| Other properties | ||||||||||||||||||||||||||||||||||||||||||||||||
| Natural occurrence | primordial | |||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | body-centered cubic (bcc) (cI2) | |||||||||||||||||||||||||||||||||||||||||||||||
| Lattice constant | a = 330.05 pm (at 20 °C)[3] | |||||||||||||||||||||||||||||||||||||||||||||||
| Thermal expansion | 7.07×10−6/K (at 20 °C)[3] | |||||||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | 53.7 W/(m⋅K) | |||||||||||||||||||||||||||||||||||||||||||||||
| Electrical resistivity | 152 nΩ⋅m (at 0 °C) | |||||||||||||||||||||||||||||||||||||||||||||||
| Magnetic ordering | paramagnetic | |||||||||||||||||||||||||||||||||||||||||||||||
| Young's modulus | 105 GPa | |||||||||||||||||||||||||||||||||||||||||||||||
| Shear modulus | 38 GPa | |||||||||||||||||||||||||||||||||||||||||||||||
| Bulk modulus | 170 GPa | |||||||||||||||||||||||||||||||||||||||||||||||
| Speed of sound thin rod | 3480 m/s (at 20 °C) | |||||||||||||||||||||||||||||||||||||||||||||||
| Poisson ratio | 0.40 | |||||||||||||||||||||||||||||||||||||||||||||||
| Mohs hardness | 6.0 | |||||||||||||||||||||||||||||||||||||||||||||||
| Vickers hardness | 870–1320 MPa | |||||||||||||||||||||||||||||||||||||||||||||||
| Brinell hardness | 735–2450 MPa | |||||||||||||||||||||||||||||||||||||||||||||||
| CAS Number | 7440-03-1 | |||||||||||||||||||||||||||||||||||||||||||||||
| History | ||||||||||||||||||||||||||||||||||||||||||||||||
| Naming | after Niobe in Greek mythology, daughter of Tantalus (tantalum) | |||||||||||||||||||||||||||||||||||||||||||||||
| Discovery | Charles Hatchett (1801) | |||||||||||||||||||||||||||||||||||||||||||||||
| First isolation | Christian Wilhelm Blomstrand (1864) | |||||||||||||||||||||||||||||||||||||||||||||||
| Recognized as a distinct element by | Heinrich Rose (1844) | |||||||||||||||||||||||||||||||||||||||||||||||
| Isotopes of niobium | ||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||
Niobium (Template:PronEng), or columbium (/kəˈlʌmbiəm/) is a chemical element that has the symbol Nb and atomic number 41. A rare, soft, gray, ductile transition metal, niobium is found in and pyrochlore, which is the main source for niobium, and columbite. It was first discovered in the latter mineral and so was initially named columbium; that mineral has since been renamed niobite. Niobium reacts similar to tantalum and is difficult to separate from it.
Brazil is the leading producer of niobium and ferroniobium, a niobium iron alloy. Niobium is used mostly used in alloys, the largest part in special steel alloy, which are used for example in gas piplines. Although only a maximum of 0.1% is used in the alloys it leads to an improved strength of the steel. The temperature stability of certain niobium alloys, so called superalloys, is used in jet engines and rocket engines. The superconducting alloys with titanium and tin are widely used in MRI scanners. Less than 10% is used for applications in welding, nuclear industries, electronics, optics, numismatics and jewelry.
Characteristics

Niobium is a lustrous, grey, ductile metal that takes on a bluish tinge when exposed to air at room temperature for extended periods.[citation needed] Niobium's chemical properties are almost identical to the chemical properties of tantalum, which appears directly below niobium in the periodic table.[8]
Chemical
When it is processed at even moderate temperatures, niobium must be placed in a protective atmosphere.[citation needed] The metal begins to oxidize in air at 200°C; its common oxidation states are +5, +4 and +3,[9] although others are also known.[clarification needed] The most stable oxidation state is +5.[9]
Niobium forms oxides in the oxidation states +5 (Nb2O5), +4 ((NbO2)), +3 (Nb2O3), and +2 (NbO). The most common ones are the white chemically inert niobium pentoxide (Nb2O5) and the dark green non-stoichiometric niobium dioxide (NbO2) (rutile-like structure).[9] Niobium pentoxide is the starting material for several of the niobates. Examples of the niobates, created by dissolving the pentoxide in basic hydroxide solutions or by melting the metal oxide with niobium pentoxide, are lithium niobate (LiNbO3) and lanthan niobate (LnNbO4). In the lithium niobate, the niobate ion NbO3− is not isolated but part of a perovskite-like structure, while the lantane niobate contains isolated NbO43− ions.[9]
The fluorides of niobium are important due to the fact that the separation of tantalum and niobium uses the fluorides of the elements as substrates.[10] Niobium forms halogen compounds in the oxidation sates of +5, +4, and +3, although multi core complexes and substoichiometric compounds are also known.[9][11]
Niobium pentafluoride (NbF5) is a white solid with a melting point of 79.0°C and niobium pentachloride (NbCl5) is a white solid with a melting point of 203.4°C. Both are hydrolyzed by water and react with additional niobium at elevated temperatures by forming the black and highly hygroscopic niobium tetrafluoride (NbF4) and niobium tetrachloride (NbCl4). While the trihalogen compounds can be obtained by reduction of the pentahalogenes with hydrogen, the dihalogen compounds do not exist.[9]
History
Niobium (Greek mythology: Niobe, daughter of Tantalus) was discovered by Charles Hatchett in 1801.[12] Hatchett found niobium in columbite ore that was sent to England in the 1750s by John Winthrop, the first governor of Connecticut, and named it columbium.[13]
There was a considerable amount of confusion[14] about the difference between the closely-related niobium and tantalum. William Hyde Wollaston compared in 1809 the oxides derived from columbite (density 5.918) and tantalite (density 7.935) and concluded from the reactions that the obtained oxides, although the density difference was significant, are identical, keeping the name tantalum.[14] This was disputed in 1846 by Heinrich Rose, who argued that there are two elements in the tantalite sample, and named these two elements after the name of the two daughters of Tantalus: niobium (after the goddness of tears Niobe, and pelopoium (after Pelops).[15][16][17] Other alleged elements were reported in this sample,[17] until Christian Wilhelm Blomstrand in 1864,[17] and Jean Charles Galissard de Marignac in 1866[18] proved that there were only two elements. In 1864, Blomstrand was the first to prepare the pure metal, reducing niobium chloride by heating it in a hydrogen atmosphere.[19] All of these discoveries caused some comments of disbelief.[20][21]
Due to the little difference between tantalum and niobium and the fact that niobium reacts with chlorine and traces of oxygen which are hardly avoidable, by forming two compounds the white volatile niobium pentachloride (NbCl5) and the non volatile niobium oxychloride (NbOCl3) lead to the claimed discoveris of the elements pelopium ilmenium and dianium, which were in fact identical to niobium or mixtures of niobium and tantalum. The difference between tantalum and niobium was unequivocally made by Henri Etienne Sainte-Claire Deville and Louis J. Troost, who determined the formulas of some of its compounds.[17] It is possible that the columbium discovered by Hatchett was probably a mixture of these two elements.[17] Which did not stop scientists to publish articles about Ilmenium until 1871.
Columbium (symbol Cb[22] was the name originally given to this element by Hatchett, and remained in use in American journals,[23] while niobium was used in Europe. To end this confusion, at the 15th Conference of the Union of Chemistry in Amsterdam in 1949, the name niobium was chosen for element #41.[17] A year later this name was officially adopted by the International Union of Pure and Applied Chemistry (IUPAC) after 100 years of controversy, despite the chronological precedence of the name Columbium. The latter name is still sometimes used in US industry.[24] This was a compromise of sorts;[citation needed] the IUPAC accepted tungsten instead of wolfram, in deference to North American usage; and niobium instead of columbium, in deference to European usage. Not everyone agreed, however, and while many leading chemical societies and government organizations refer to it by the official IUPAC name, many leading metallurgists, metal societies, and most leading American commercial producers still refer to the metal by the original "columbium."[25][17]
Even after its discovery and even after Jean Charles Galissard de Marignac was able to produce tantalum free niobium in a larger scale in 1866, it took until early 20th century when niobium was used in incendiary lamps,[8] but was quickly replaced by the even higher melting tungsten. The discovery that niobium improves the strength of steel was in the 1920s and this use is still the dominating use for niobium.[8]
Occurrence

The element is never found as a free element but does occur in minerals. Minerals that contain niobium often also contain tantalum,[10] for example columbite ((Fe,Mn)(Nb,Ta)2O6), columbite-tantalite (coltan, ((Fe,Mn)(Ta,Nb)2O6)) and pyrochlore.
Less common, although they form the largest mined niobium deposits , are the niobates of calcium, uranium, thorium and the rare earth elements like pyrochlore ((Na,Ca)2Nb2O6OH,F), and euxenite ((Y,Ca,Ce,U,Th)(Nb,Ta,Ti)2O6). These large deposits of niobium have been found associated with carbonatites (carbon-silicate igneous rocks) and as a constituent of pyrochlore.
Extensive ore reserves are located in Nigeria, Democratic Republic of Congo, and in Russia. The two largest deposits of pyrochlore were found in the 1950s in Brazil and Canada, and both countries are still the major producers of niobium mineral concentrates.[8] The largest deposits in Brazil are owned by CBMM (Companhia Brasileira de Metalurgia e Mineração) located in Araxá; Minas Gerais the other deposit is owned by Mineração Catalão located in Catalão, Goiás. The third largest producer of niobium is the Niobec Inc. mine in Saint-Honore near Chicoutimi, Quebec.[26]
Production
After the separation from the other minerals, the mixed oxides of tantalum Ta2O5 and niobium Nb2O5 are obtained. The first step in the processing is the reaction of the oxides with hydrofluoric acid.[10]
- Ta2O5 + 14HF → 2H2[TaF7] + 5H2O
- Nb2O5 + 10HF → 2H2[NbOF5] + 3H2O
The first industrial scale separation, developed by de Marignac, used the difference in solubility between the complex niobium and tantalum fluorides, dipotassium heptafluorotantalate (K2[TaF7]) and dipotassium oxypentafluoroniobate monohydrate (K2[NbOF5].H2O) in water. Newer processes use the liquid extraction of the fluorides from aqueous solution by organic solvents like cyclohexanone.[10]
The complex niobium and tantalum fluorides are extracted separately from the organic solvent with water and either precipitated by the addition of potassium fluoride to produce a potassium fluoride complex, or precipitated with ammonia as the pentoxide.[9]
- H2[TaF7] + KF → K2[TaF7]↓ + HF
- H2[NbOF5] + 10HF → K2[NbOF5]↓ + HF
- 2H2[TaF7] + 14NH4OH → Ta2O5↓ + 14NH4F + 9H2O
- 2H2[NbOF5] + 10NH4OH → Nb2O5↓ + 10NH4F + 3H2O
Several methods are used for the reduction to metallic niobium. The electrolysis of a molten mixture of K2[NbOF5] and sodium chloride is one, the other is the reduction of the fluoride with sodium. With this method niobium with a relatively high purity can be obtained. The reduction of Nb2O5 with hydrogen or carbon,[9] however in large scale production the is used. In the process involving the aluminothermic reaction a mixture of iron oxide and niobium oxide is reacted with aluminium.
- 3Nb2O5 + Fe2O3 + 12Al → 6Nb + 2Fe + 3Al2O3
To enhance the reaction small amounts of oxidizers like sodium nitrate are added. The result is aluminium oxide and ferroniobium, an alloy of iron and niobium used in the steel production.[27][28] The ferroniobium contains between 60 and 70% of niobium.Cite error: The opening <ref> tag is malformed or has a bad name (see the help page).
Without addition of iron oxide the same process is used for the production of niobium. To reach the grade for superconductive alloys further purification is necessary. Electron beam melting under vacuum is the method used by the two major distributors of niobium.[11]
Applications

It is estimated that of the 44.5 metric kilotons of niobium mined in 2006, 90% ended up in the production of steel followed by the use in superalloys.[29] The use of niobium alloys for superconductors and the use in electonic components account only for a small share of the production.[29]
Steel production
Niobium is a component of some stainless steels, which normally have a niobium content of less than 0.1%.[30] It is a very important alloy addition in HSLA steels, which are widely used as structural components in modern automobiles.[31] These niobium containing alloys are strong and are often used in pipeline construction.[32] [33]
Superalloys

Appreciable amounts of pure niobium or in the form of high-purity ferroniobium and nickel niobium are used in nickel-, cobalt-, and iron-base superalloys for such applications as jet engine components, gas turbines, rocket subassemblies, and heat-resisting and combustion equipment. The alloys contain up to 6.5% niobium.[30] One example for an nickel based niobium containing super alloy is inconel 718, it consists of 18.6 chromium 18.5% iron 5% noibium 3.1% molybdenum and 0.9% titanium and 0.4% of aluminum.[34] These superalloys are used for example, in advanced air frame systems such as those used in the Gemini program used this metal. An alloy used for liquid rocket thruster nozzles, for example the main engine of the Apollo Lunar Modules is C130, which consists of 89% niobium, 10% hafnium and 1% titanium.[35] Another niobium alloy was used for the nozzle of the Apollo Service Module. As niobium is oxidised at temperatures above 400°C a protective coating is necessary for these applications to prevent the alloy becoming brittle.[35]
Superconducting Magnets
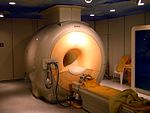
Niobium becomes a superconductor when lowered to cryogenic temperatures. At atmospheric pressure, it has the highest critical temperature of the elemental superconductors: 9.2 K.[36] Niobium has the largest magnetic penetration depth of any element.Cite error: The opening <ref> tag is malformed or has a bad name (see the help page). In addition, it is one of the three elemental superconductors that are Type II (the others being vanadium and technetium). Niobium-tin and niobium-titanium alloys are used as wires for superconducting magnets capable of producing exceedingly strong magnetic fields. These superconducting magnets are used in Magnetic resonance imaging and Nuclear magnetic resonance instruments as well as in for particle accelerators. [37]
For example the Large Hadron Collider uses 600 metric tons of superconducting strands while the International Thermonuclear Experimental Reactor is estimated to use 600 metric tonnes of Nb3Sn strands and 250 metric tonnes of NbTi strands.[38]
Numismatics


Niobium is occasionally used as a precious metal in commemorative coins, often together with silver or gold. Some of the examples are:
- The 2004 Austrian 25 euro 150 Years Semmering Alpine Railway commemorative coin. The "pill" of the coin is made of green Niobium (other coins may use other colors, like brown, purple or yellow).[39]
- In 2005, Sierra Leone made a coin honoring Pope John Paul II that contained a disc of 24 carat gold surrounded by a ring of purple-tinted niobium.[40]
- The 2006 Austrian 25 euro European Satellite Navigation commemorative coin was minted with a niobium "pill", colour gold-brown. The very high value collector's coin was minted in honour of the Galileo Positioning System.[41]
The center piece of the Austrian 25 Euro coins, shown in the images, is made frome pure niobium, the colour created by refraction of light in a thin oxide layer which is created by anodizing.
Other uses
- The metal has a low capture cross-section for thermal neutrons, [42] for which it is used in the nuclear industries.
- The electrodes in some high pressure sodium vapor lamp are made from niobium or niobium with 1% of zirconium, because niobium is resistant to the corrosive metal vapour within the lamp.[43][44]
- It is also the metal used in arc welding rods for some stabilized grades of stainless steel.
- Niobium is being evaluated as an alternative to tantalum in capacitors.[45]
- Because niobium and some niobium alloys are physiologically inert (and thus hypoallergenic), they are used in jewelry[46] and in medical devices such as pacemakers. [47] Niobium treated with sodium hydroxide forms a porous layer that aids osseointegration.[48]
- Along with titanium, tantalum, and aluminium, niobium can also be electrically heated and anodized to a wide array of colors using a process known as reactive metal anodizing. This makes it very attractive for use in jewelry.[49][50]
- Niobium is also added to glass in order to attain a higher refractive index, a property used in the optical industry to make thinner corrective glasses.
Isotopes
Naturally occurring niobium is composed of one stable isotope, 93Nb.[51] 32 radioisotopes have also been synthesized, ranging in atomic mass from 81 to 113. The most stable of these is 92Nb with a half-life of 34.7 million years. The least stable is 113Nb, with an estimated half-life of 30 ms. Isotopes that are lighter than the stable 93Nb tend to decay by β+ decay, and those that are heavier tend to decay by β- decay, with some exceptions. 81Nb, 82Nb, and 84Nb have minor β+ delayed proton emission decay paths, 91Nb decays by electron capture and positron emission, and 92Nb decays by both β+ and β- decay.[51]
At least 25 nuclear isotopes have been characterized, ranging in atomic mass from 84 to 104. Within this range, only 96Nb, 101Nb, and 103Nb do not have isomers. The most stable of niobium's isomers is 93mNb with a half-life of 16.13 years. The least stable is 84mNb with a half-life of 103 ns. All of niobium's isotopes decay by isomeric transition or beta decay except 92m1Nb, which has a minor electron capture decay path.[51]
Precautions
Niobium has no known biological role. Metallic niobium dust is an eye and skin irritant and also can be a fire hazard. However niobium metal, without compounds, is physiologically inert (and thus hypoallergenic) and harmless. It is frequently used in jewelry and was tested for medical implants.[52][53] Niobium-containing compounds are relatively rarely encountered by most people, but some are toxic and should be treated with care. The short and long term exposure to niobates and niobium chloride, two chemicals which are water soluable, have been tested in rats. Rats treated with a single injection of niobium pentachloride or niobates showing show a LD50 between 10 and 100 mg/kg.[54][55][56] For oral administration the toxicity is lower, a study with rats yielded a LD50 after 7 days of 940 mg/kg.[54]
See also
References
- ^ "Standard Atomic Weights: Niobium". CIAAW. 2017.
- ^ Prohaska, Thomas; Irrgeher, Johanna; Benefield, Jacqueline; Böhlke, John K.; Chesson, Lesley A.; Coplen, Tyler B.; Ding, Tiping; Dunn, Philip J. H.; Gröning, Manfred; Holden, Norman E.; Meijer, Harro A. J. (2022-05-04). "Standard atomic weights of the elements 2021 (IUPAC Technical Report)". Pure and Applied Chemistry. doi:10.1515/pac-2019-0603. ISSN 1365-3075.
- ^ a b c Arblaster, John W. (2018). Selected Values of the Crystallographic Properties of Elements. Materials Park, Ohio: ASM International. ISBN 978-1-62708-155-9.
- ^ Nb(–3) occurs in Cs3Nb(CO)5; see John E. Ellis (2003). "Metal Carbonyl Anions: from [Fe(CO)4]2- to [Hf(CO)6]2- and Beyond†". Organometallics. 22 (17): 3322–3338. doi:10.1021/om030105l.
- ^ a b c d Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 28. ISBN 978-0-08-037941-8.
- ^ a b Nb(0) and Nb(I) has been observed in Nb(bpy)3 and CpNb(CO)4, respectively; see Holleman, Arnold F.; Wiberg, Egon; Wiberg, Nils (2008). Lehrbuch der Anorganischen Chemie (in German) (102 ed.). Walter de Gruyter. p. 1554. ISBN 9783110206845.
- ^ Kondev, F. G.; Wang, M.; Huang, W. J.; Naimi, S.; Audi, G. (2021). "The NUBASE2020 evaluation of nuclear properties" (PDF). Chinese Physics C. 45 (3): 030001. doi:10.1088/1674-1137/abddae.
- ^ a b c d C. K. Gupta, A. K. Suri, Gupta K. Gupta (1994). Extractive Metallurgy of Niobium. CRC Press. ISBN 0849360714. Retrieved 2008-08-29.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ a b c d e f g h Holleman, A. F., Wiberg, E., Wiberg, N. (2007). Lehrbuch der Anorganischen Chemie, 102nd ed. de Gruyter. ISBN 978-3-11-017770-1.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ a b c d Donald J. Soisson, J. J. McLafferty, and James A. Pierret (1961). "Staff-Industry Collaborative Report: Tantalum and Niobium". Ind. Eng. Chem. 53 (11): 861–868. doi:10.1021/ie50623a016.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Anatoly Agulyansky (2004). The Chemistry of Tantalum and Niobium Fluoride Compounds. Elsevier. ISBN 9780444516046. Retrieved 2008-09-02.
- ^ Hatchett, Charles. "Eigenschaften und chemisches Verhalten des von Charlesw Hatchett entdeckten neuen Metalls, Columbium". Annalen der Physik. 11 (5): 120–122. doi:10.1002/andp.18020110507.
- ^ William P. Griffith and Peter J. T. Morris (2003). "Charles Hatchett FRS (1765-1847), Chemist and Discoverer of Niobium". Notes and Records of the Royal Society of London. 57 (3): 299. doi:10.1098/rsnr.2003.0216.
- ^ a b Wollaston, William Hyde (1809). "On the Identity of Columbium and Tantalum". Philosophical Transactions of the Royal Society of London. 99: 246–252.
- ^ Rose, Heinrich. "Ueber die Zusammensetzung der Tantalite und ein im Tantalite von Baiern enthaltenes neues Metall". Annalen der Physik. 139 (10): 317–341. doi:10.1002/andp.18441391006.
- ^ Heinrich Rose (1847). "Ueber die Säure im Columbit von Nordamérika". Annalen der Physik. 146 (4): 572–577. doi:10.1002/andp.18471460410.
- ^ a b c d e f g Peter van der Krogt. "Elementymology & Elements Multidict: Niobium". Retrieved 2008-09-04.
- ^ M. C. Marignac (1865–1866). "Recherches sur les combinaisons du niobium". Annales de chimie et de physique. 4 (8): 7–75.
{{cite journal}}: CS1 maint: date format (link) - ^ "niobium". Retrieved 2008-09-05.
- ^ Cite error: The named reference
=krogtwas invoked but never defined (see the help page). - ^ the science editor of the American magazine (July 1880). "A New Metallic Compound". Manufacturer and builder.
{{cite journal}}:|author=has generic name (help) - ^ Peter van der Krogt. "Elementymology & Elements Multidict: Names that did not make it". Retrieved 2008-09-04.
- ^ JACS or whoever was teh predecessor used columbium; need a ref though
- ^ Clarke, F. W. (1914). "Columbium Versus Niobium". Science. 39 (995): 139–140.
- ^ Norman N. Greenwood (2003). "Catalysis Today". Vanadium to dubnium: from confusion through clarity to complexity. 78 (1–4): 5–11. doi:10.1016/S0920-5861(02)00318-8.
- ^ J. Kouptsidis, F. Peters, D. Proch, W. Singer. "Niob für TESLA" (PDF). Retrieved 2008-09-02.
{{cite web}}: CS1 maint: multiple names: authors list (link) - ^ Geoffrey Tither. "Progress in Niobium Markets and Technology 1981–2001" (PDF). Retrieved 2008-09-02.
- ^ Claude Dufresne. "The Production of Ferroniobium at the Niobec mine 1981–2001" (PDF). Retrieved 2008-09-02.
{{cite web}}: Unknown parameter|coauthor=ignored (|author=suggested) (help) - ^ a b John F. Papp. "Niobium (Columbium ) and Tantalum" (PDF). USGS 2006 Minerals Yearbook. Retrieved 2008-09-03.
- ^ a b Friedrich Heisterkamp. "Niobium: Future Possibilities – Technology and the Market Place" (PDF). Retrieved 2008-09-03.
{{cite web}}: Unknown parameter|coauthor=ignored (|author=suggested) (help) - ^ Patel, Zh. (2001). "Niobium for Steelmaking". Metallurgist. 45 (11–12): 477–480. doi:10.1023/A:1014897029026.
{{cite journal}}:|access-date=requires|url=(help);|format=requires|url=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Eggert, Peter (1982). "Niobium: a steel additive with a future". Economic Bulletin. 19 (9): 8–11. doi:10.1007/BF02227064.
{{cite journal}}:|access-date=requires|url=(help);|format=requires|url=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Hillenbrand, Hans–Georg (2001-05-02). "Development and Production of High Strength Pipeline Steels" (PDF). EUROPIPE. Retrieved 2008-09-09.
{{cite web}}: Unknown parameter|coauthors=ignored (|author=suggested) (help)CS1 maint: multiple names: authors list (link) - ^ H. K. D. H. Bhadeshia. "Nickel Based Superalloys". University of Cambridge.
{{cite web}}: Unknown parameter|assessdate=ignored (help) - ^ a b John Hebda (2001). "Niobium alloys and high Temperature Applications" (PDF). CBMM.
{{cite web}}: Unknown parameter|assessdate=ignored (help) - ^ Peiniger, M. (1985). "A Superconducting Nb3Sn Coated Multicell Accelerating Cavity". Nuclear Science. 32 (5). doi:10.1109/TNS.1985.4334443.
{{cite journal}}: Unknown parameter|coauthor=ignored (|author=suggested) (help) - ^ B. A. Glowacki, X. -Y. Yan, D. Fray, G. Chen, M. Majoros, and Y. Shi (2002). "Niobium based intermetallics as a source of high-current/high magnetic field superconductors". Physica C: Superconductivity. 372–376 (3): 1315–1320. doi:10.1016/S0921-4534(02)01018-3.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Grunblatt, G. (2005). "A success story: LHC cable production at ALSTOM-MSA" (PDF). Fusion Engineering and Design (Proceedings of the 23rd Symposium of Fusion Technology). 75–79: 1–5. doi:10.1016/j.fusengdes.2005.06.216.
{{cite journal}}: Unknown parameter|coauthor=ignored (|author=suggested) (help) - ^ "150 Jahre Semmeringbahn". austrian mint. Retrieved 2008-09-04.
- ^ "Sierra Leone". tax free gold. Retrieved 2008-09-04.
- ^ "Europäische Satellitennavigation". austrian mint. Retrieved 2008-09-04.
- ^ Jahnke, L.P. (1960). "Columbium Alloys Today". Metal Progr. 77 (6): 69–74.
{{cite journal}}: Unknown parameter|coauthor=ignored (|author=suggested) (help) - ^ Eichelbrönner, G. (1998). "Refractory metals: crucial components for light sources". International Journal of Refractory Metals and Hard Materials. 16 (1): 5–11. doi:10.1016/S0263-4368(98)00009-2.
{{cite journal}}:|access-date=requires|url=(help);|format=requires|url=(help) - ^ Christopher A. Michaluk, Louis E. Huber, and Robert B. Ford. "Niobium and Niobium 1% Zirconium for High Pressure Sodium (HPS) Discharge Lamps" (PDF). Retrieved 2008-09-03.
{{cite web}}: CS1 maint: multiple names: authors list (link) - ^ Pozdeev, Y. (1991). "Reliability comparison of tantalum and niobium solid electrolytic capacitors". Quality and Reliability Engineering International. 14 (2): 79–82. doi:'"`UNIQ--NOWIKI-000000BA-QINU`"'.
{{cite journal}}: Check|doi=value (help); Cite has empty unknown parameter:|coauthors=(help); NOWIKI stripmarker in|doi=at position 1 (help) - ^ Azevedo, C. R. F. (2002). "Characterization of metallic piercings that caused adverse reactions during use". Journal of Failure Analysis and Prevention. 2 (4): 47–53. doi:10.1007/BF02715453.
{{cite journal}}:|access-date=requires|url=(help);|format=requires|url=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Mallela, Venkateswara Sarma (2004). "Trends in Cardiac Pacemaker Batteries" (PDF). Indian Pacing Electrophysiol J. 4 (4): 201–212. Retrieved 2008-08-29.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Godley, Reut (2004). "Bonelike apatite formation on niobium metal treated in aqueous NaOH" (PDF). Journal of Materials Science: Materials in Medicine. 15: 1073–1077. doi:10.1023/B:JMSM.0000046388.07961.81. Retrieved 2006-09-07.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Biason Gomes, M. A. (1991). "Anodization of niobium in sulphuric acid media". Journal of Applied Electrochemistry. 21 (11): 1023–1026. doi:10.1007/BF01077589.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Chiou, Y. L. (1971). "A note on the thicknesses of anodized niobium oxide films". Thin Solid Films. 8 (4): R37–R39. doi:10.1016/0040-6090(71)90027-7.
{{cite journal}}: Cite has empty unknown parameter:|coauthors=(help) - ^ a b c Georges, Audi (2003). "The NUBASE Evaluation of Nuclear and Decay Properties". Nuclear Physics A. 729. Atomic Mass Data Center: 3–128. doi:10.1016/j.nuclphysa.2003.11.001.
- ^ Vilaplana, J. (1990). "New trends in the use of metals in jewellery". Contact Dermatitis. 25 (3): 145–148. doi:10.1111/j.1600-0536.1991.tb01819.x.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Vilaplana, J. (1998). "New developments in jewellery and dental materials". Contact Dermatitis. 39 (2): 55–57. doi:10.1111/j.1600-0536.1998.tb05832.x.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b Haley, Thomas J. (1962). "Pharmacology and toxicology of niobium chloride". Toxicology and Applied Pharmacology. 4 (3): 385–392. doi:10.1016/0041-008X(62)90048-0.
{{cite journal}}: Unknown parameter|coauthor=ignored (|author=suggested) (help) - ^ Downs, William L. (1965). "The Toxicity of Niobium Salts". American Industrial Hygiene Association Journal. 26 (4): 337–346. doi:10.1080/00028896509342740.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Henry A. Schroeder, Marian Mitchener and Alexis P. Nason (1970). "Zirconium, Niobium, Antimony, Vanadium and Lead in Rats: Life term studies". Journal of Nutrition. 100 (1): 59–68.