Scandium(III) trifluoromethanesulfonate: Difference between revisions
Appearance
Benjah-bmm27 (talk | contribs) + example |
Rifleman 82 (talk | contribs) m moved Scandium(III) triflate to Scandium(III) trifluoromethanesulfonate: avoid abbreviations, which are so meaningful to non inorg chemists |
(No difference)
| |
Revision as of 12:16, 6 May 2009

| |
| Identifiers | |
|---|---|
| ECHA InfoCard | 100.157.499 |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| Properties | |
| C3F9O9S3Sc | |
| Molar mass | 492.163239 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Scandium triflate or Sc(OTf)3 is a chemical compound composed of scandium with triflate counterions. Scandium triflate is used as a reagent in organic chemistry as a Lewis acid. Compared to other Lewis acids this reagent is stable towards water and can often be used in an organic reaction as a true catalyst rather than one used in stoichiometric amounts. The compound is prepared by reaction of scandium oxide with triflic acid.
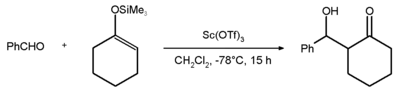
An example of the use of scandium triflate is the Mukaiyama aldol addition reaction between benzaldehyde and the silyl enol ether of cyclohexanone with an 81% chemical yield.[1]
External links
- Deborah Longbottom (1999). "SYNLETT Spotlight 12: Scandium Triflate". Synlett (12): 2023. doi:10.1055/s-1999-5997.
References
- ^ S. Kobayashi (1999). "Scandium Triflate in Organic Synthesis". Eur. J. Org. Chem. 1999: 15–27. doi:10.1002/(SICI)1099-0690(199901)1999:1<15::AID-EJOC15>3.0.CO;2-B.
Wikimedia Commons has media related to Scandium(III) triflate.
