Bicyclic molecule: Difference between revisions
m gif → png |
m a.k.a. sounds so unprofessional... |
||
| Line 5: | Line 5: | ||
Fusion of the rings can occur in three ways: |
Fusion of the rings can occur in three ways: |
||
#Across a bond between two atoms - for example, [[decalin]], |
#Across a bond between two atoms - for example, [[decalin]], or bicyclo[4.4.0]decane, has a C-C bond shared between two [[cyclohexane]] rings; |
||
#Across a sequence of atoms ('''bridgehead''') - for example, [[norbornane]], |
#Across a sequence of atoms ('''bridgehead''') - for example, [[norbornane]], or bicyclo[2.2.1]heptane, can be viewed as a pair of [[cyclopentane]] rings that share three of the five carbon atoms; or |
||
#At a single atom ('''spirocyclic''', forming a [[spiro compound]]) |
#At a single atom ('''spirocyclic''', forming a [[spiro compound]]) |
||
Singly fused rings are the most common, and spiro rings are the least common. |
Singly fused rings are the most common, and spiro rings are the least common. |
||
Revision as of 10:19, 7 July 2009

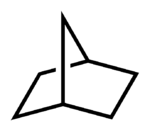

A bicyclic molecule is a molecule that features two fused rings. Bicyclic molecules occur in widely in organic and inorganic compounds.
Fusion of the rings can occur in three ways:
- Across a bond between two atoms - for example, decalin, or bicyclo[4.4.0]decane, has a C-C bond shared between two cyclohexane rings;
- Across a sequence of atoms (bridgehead) - for example, norbornane, or bicyclo[2.2.1]heptane, can be viewed as a pair of cyclopentane rings that share three of the five carbon atoms; or
- At a single atom (spirocyclic, forming a spiro compound)
Singly fused rings are the most common, and spiro rings are the least common.
Bridges
A bridge is an unbranched chain of atoms or an atom or a covalent bond connecting two bridgeheads in a polycyclic compound.
The main bridge is a bridge which connects the two main bridgeheads.
A secondary bridge is any bridge not included in the main ring or the main bridge.
An independent secondary bridge links bridgeheads which are part of the main ring or main bridge.
A dependent secondary bridge links at least one bridgehead which is part of a secondary bridge.
Nomenclature
Bicyclic molecules have a strict nomenclature.[1] On its simplest level the parent hydrocarbon is the equivalent open carbon alkane. For bridged compounds, the prefix bicyclo is added, followed by, between brackets, separated by periods, and in descending order, the number of carbon atoms between each of the bridgeheads. For example in bicyclo[2.2.1]heptane the carbon frame contains a total of 7 atoms hence the parent name heptane. This molecule has three bridges having 2, 2 and 1 carbon atoms hence the prefix bicyclo[2.2.1]. For spiro compounds, terms like spiro[2.4] are used, indicating that there are 3- and 5-membered rings (because the spiro atom itself is not counted) meeting at the spiro atom.
