Serotonin: Difference between revisions
→Psychedelic drugs: {{fact}} |
|||
| Line 182: | Line 182: | ||
=== Antiemetics === |
=== Antiemetics === |
||
[[5-HT3 antagonist|5-HT<sub>3</sub> antagonists]] such as [[ondansetron]], [[granisetron]], and [[tropisetron]] are important [[antiemetic]] agents. They are particularly important in treating the [[nausea]] and [[vomiting]] that occur during anticancer [[chemotherapy]] using cytotoxic drugs. Another application is in the treatment of post-operative nausea and vomiting. Applications to the treatment of depression and other mental and psychological conditions have also been investigated with some positive results. |
[[5-HT3 antagonist|5-HT<sub>3</sub> antagonists]] such as [[ondansetron]], [[granisetron]], and [[tropisetron]] are important [[antiemetic]] agents. They are particularly important in treating the [[nausea]] and [[vomiting]] that occur during anticancer [[chemotherapy]] using cytotoxic drugs. Another application is in the treatment of post-operative nausea and vomiting. Applications to the treatment of depression and other mental and psychological conditions have also been investigated with some positive results.{{Citation needed|date=July 2009}} |
||
== Pathology == |
== Pathology == |
||
Revision as of 11:13, 21 July 2009
| |||
| Names | |||
|---|---|---|---|
| IUPAC names
5-Hydroxytryptamine or
3-(2-aminoethyl)-1H-indol-5-ol | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| ECHA InfoCard | 100.000.054 | ||
| MeSH | Serotonin | ||
PubChem CID
|
|||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C10H12N2O | |||
| Molar mass | 176.215 | ||
| Appearance | White powder | ||
| Melting point | 167.5 C | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Serotonin (Template:PronEng) (5-hydroxytryptamine, or 5-HT) is a monoamine neurotransmitter. It is found extensively in the gastrointestinal tract of animals, and about 80 to 90 percent of the human body's total serotonin is located in the enterochromaffin cells in the gut, where it is used to regulate intestinal movements.[1][2] The remainder is synthesized in serotonergic neurons in the central nervous system (CNS) where it has various functions, including control of appetite, mood and anger.
Serotonin is found not only in animals, but also in fungi and plants,[3] including fruits and vegetables.
Function
Serotonin functions as a neurotransmitter in nerve systems of primitive as well as highly evolved animals. In the roundworm Caenorhabditis elegans serotonin activates the muscles used for feeding, while octopamine suppresses them.[4] Serotonin diffuses to serotonin-sensitive neurons, which control the animal's perception of nutrient availability. Artificial depletion of serotonin or increase of octopamine cues behaviour that is typical of a low-food environment: C. elegans becomes more active, and mating and egg-laying is suppressed, while the opposite occurs if serotonin is increased or octopamine is decreased in this animal.[5]
The role of serotonin in animals with a more complex nerve system is in perceptions of more complex life situations such as, for example, social rank. If a lobster is injected with serotonin, it behaves like a dominant animal, while octopamine causes subordinate behaviour.[6]
Frightened crayfish flips its tail to flee, and the effect of serotonin on this behaviour depends on the animal's social status. Serotonin inhibits the fleeing reaction in subordinates, but enhances it in socially dominant or isolated individuals. Social experience alters the proportion between different serotonin receptors which have opposing effects on the fight-or-flight response. [7]
Serotonergic signaling plays an important role in the modulation of human mood, anger and aggression. Individuals of C.elegans facing stress (eg. a low-food environment) resume normal behaviour if given serotonin-increasing drugs. The same drugs have similar effects in humans; the action of serotonin on the worms' mating and egg-laying resembles its effects on human sexuality.[citation needed]
Serotonin has broad activities in the brain, and genetic variation in serotonin receptors and the serotonin transporter, which facilitates reuptake of serotonin into presynapses, have been implicated in neurological diseases. Drugs targeting serotonin-induced pathways are being used in the treatment of many psychiatric disorders. One focus of clinical research is the influence of genetics on serotonin action and metabolism in psychiatric settings. Such studies have revealed that the variation in the promoter region of the serotonin transporter protein accounts for nearly 10% of total variance in anxiety-related personality,[8] and the effect of this gene on depression was found to interact with the environment.[9]
Levels of serotonin in the brain show association with aggression,[10] and a mutation in the gene which codes for the 5-HT2A receptor may double the risk of suicide for those with that genotype.[11]
In the ultimatum game, participants whose serotonin levels have been artificially lowered will reject unfair offers more often than players with normal serotonin levels.[12]
Serotonin also has effects on appetite, sleep and general metabolism. In the blood, the major storage site is platelets, which collect serotonin from plasma. Bleeding causes serotonin release which constricts blood vessels.[13] Irritants present in food trigger the enterochromaffin cells to release serotonin to increase peristaltic movements for emptying of the gut. Leakage of intestinal serotonin into the bloodstream at a rate faster than the platelets can absorb it increases free serotonin in the blood, which activates 5HT3 receptors in the chemoreceptor trigger zone that stimulate vomiting. [14]
Serotonin also acts as a growth factor. Liver damage increases cellular expression of 5-HT2A and 5-HT2B receptors.[15] Serotonin present in the blood then stimulates cellular growth to repair liver damage.[16] 5HT2B receptors also activate osteoblasts, which build up bone.[17] However, serotonin also activates osteoclasts, which degrade bone.[18][19]
Serotonin in the central nervous system is not essential to viability in some mammals, as shown for mice that are genetically altered so that they are unable to produce serotonin in the brain stem. These mice can live into adulthood and even give birth to live pups.[20] Although brain serotonin is not essential for viability, its ablation causes impairment such as growth retardation, 50% mortality in the first four weeks of postnatal life, and effects on various physiological and behavioral pathways that originate from the autonomic nervous system. Specifically, mice dams that lack serotonin in the brain are less able to rear pups and show more aggression towards other mice.[20]
Anatomy
Gross anatomy
The neurons of the raphe nuclei are the principal source of 5-HT release in the brain.[21] The raphe nuclei are neurons grouped into about nine pairs and distributed along the entire length of the brainstem, centered around the reticular formation.[22]
Axons from the neurons of the raphe nuclei form a neurotransmitter system, reaching large areas of the brain. Axons of neurons in the caudal raphe nuclei terminate in the following locations:
On the other hand, axons of neurons in the rostral raphe nuclei terminate in e.g.:
Thus, activation of this serotonin system has effects on large areas of the brain.
Microanatomy
Serotonin is released from serotonergic varicosities (swellings) into the extra neuronal space, but not from synaptic terminal boutons as other neurotransmitters. [citation needed] Serotonin diffuses over a relatively wide gap (>20 µm) to activate 5-HT receptors located on the dendrites, cell bodies and presynaptic terminals of adjacent neurons.
Receptors
5-HT receptors are the receptors for serotonin. They are located on the cell membrane of nerve cells and other cell types in animals and mediate the effects of serotonin as the endogenous ligand and of a broad range of pharmaceutical and hallucinogenic drugs. With the exception of the 5-HT3 receptor, a ligand gated ion channel, all other 5-HT receptors are G protein coupled seven transmembrane (or heptahelical) receptors that activate an intracellular second messenger cascade. [citation needed]
Termination
Serotonergic action is terminated primarily via uptake of 5-HT from the synapse. This is through the specific monoamine transporter for 5-HT, SERT, on the presynaptic neuron. Various agents can inhibit 5-HT reuptake including MDMA (ecstasy), amphetamine, cocaine, dextromethorphan (an antitussive), tricyclic antidepressants (TCAs) and selective serotonin reuptake inhibitors (SSRIs).
Interestingly, a 2006 study conducted by the University of Washington suggested that a newly discovered monoamine transporter, known as PMAT, may account for 'a significant percentage of 5-HT clearance.[23]' Contrasting with the high-affinity SERT, the PMAT has been identified as a low affinity transporter with an apparent Km of 114 micromoles/L for serotonin; approximately 230 times higher than that of SERT. However, the PMAT, despite its relatively low serotonergic affinity, has a considerably higher transport capacity than SERT,
"..resulting in roughly comparable uptake efficiencies to SERT in heterologous expression systems."
The study also suggests that some SSRIs, such as fluoxetine and sertraline, inhibit PMAT but at IC50 values which surpass the therapeutic plasma concentrations by up to four orders of magnitudes; therefore, SSRI monotherapy is ineffective in PMAT inhibition. At present, there are no known pharmaceuticals which would appreciably inhibit PMAT at normal therapeutic doses. The PMAT also suggestively transports dopamine and norepinephrine albeit at Km values even higher than that of 5-HT (330–15,000 micromoles/L).
Endothelial cell function and Serotonin
5-hydroxytryptamine evokes endothelial nitric oxide synthase activation and stimulates phosphorylation of p44/p42 mitogen-activated protein kinase activation in bovine aortic endothelial cell cultures.[24]
Biosynthesis
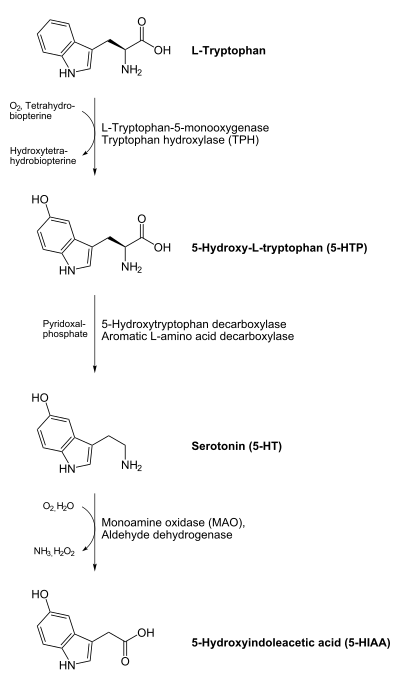
In animals including humans, serotonin is synthesized from the amino acid L-tryptophan by a short metabolic pathway consisting of two enzymes: tryptophan hydroxylase (TPH) and amino acid decarboxylase (DDC). The TPH-mediated reaction is the rate-limiting step in the pathway. TPH has been shown to exist in two forms: TPH1, found in several tissues, and TPH2, which is a brain-specific isoform[25]. There is evidence that genetic polymorphisms in both these subtypes influence susceptibility to anxiety and depression in humans. The 5-HTT gene regulates a chemical called serotonin. This chemical is found in very low amounts in people diagnosed with depression compared to other people. Serotonin works as a neurotransmitter and helps with the modulation of things such as anger, appetite, sexuality, sleep, mood, and several other things. People with depression often have impaired 5-HTT genes. There are two forms of the 5-HTT gene and everyone has two 5-HTT genes. (Levinson) There is a long form of 5-HTT and a short form of 5-HTT. Research shows that people with both 5-HTT genes being the long form are less likely to become depressed while people with one short and one long or two short forms are more likely to develop depression. Research is still being conducted to find more information.[26] There is also evidence that ovarian hormones can affect the expression of TPH in various species, suggesting a possible mechanism for postpartum depression and premenstrual stress syndrome.[citation needed] Serotonin biosynthesis in plants likewise begins with L-tryptophan, which is however first decarboxylated by tryptophan decarboxylase to give tryptamine, which is then hydroxylated by the cytochrome P450 monooxygenase, tryptamine 5-hydroxylase, yielding serotonin.[27]
Serotonin taken orally does not pass into the serotonergic pathways of the central nervous system because it does not cross the blood-brain barrier. However, tryptophan and its metabolite 5-hydroxytryptophan (5-HTP), from which serotonin is synthesized, can and do cross the blood-brain barrier. These agents are available as dietary supplements and may be effective serotonergic agents.
One product of serotonin breakdown is 5-Hydroxyindoleacetic acid (5 HIAA), which is excreted in the urine. Serotonin and 5 HIAA are sometimes produced in excess amounts by certain tumors or cancers, and levels of these substances may be measured in the urine to test for these tumors.
Drugs targeting the 5-HT system
Several classes of drugs target the 5-HT system including some antidepressants, antipsychotics, anxiolytics, antiemetics, and antimigraine drugs as well as the psychedelic drugs and empathogens.
Psychedelic drugs
The psychedelic drugs psilocin/psilocybin, DMT, mescaline, and LSD are agonists primarily at 5-HT2A receptor.[citation needed] The empathogen MDMA (ecstasy) releases serotonin from synaptic vesicles of neurons.[citation needed]
Antidepressants
The MAOIs prevent the breakdown of monoamine neurotransmitters (including serotonin), and therefore increase concentrations of the neurotransmitter in the brain. MAOI therapy is associated with many adverse drug reactions, and patients are at risk of hypertensive emergency triggered by foods with high tyramine content and certain drugs.
Some drugs inhibit the re-uptake of serotonin, making it stay in the synapse longer. The tricyclic antidepressants (TCAs) inhibit the re-uptake of both serotonin and norepinephrine. The newer selective serotonin re-uptake inhibitors (SSRIs) have fewer side-effects and fewer interactions with other drugs. The side effects that have become apparent in recent times include a decrease in bone mass in elderly and increased risk for osteoporosis. However, it is not yet clear whether it is due to SSRI action on peripheral serotonin production and or action in the gut or in the brain. [28]
Certain SSRI medications have been shown to lower serotonin levels below the baseline after chronic use; despite initial increases in serotonin. This attributes to the observation that the benefit of SSRI's may decrease in select patients after a long-term treatment. A switch in medication will usually resolve this issue; up to 70% of the time.[29] However, the novel antidepressant Tianeptine, selective serotonin reuptake enhancer, has mood elevating effects. This has given evidence to the theory that serotonin is most likely used to regulate the extent or intensity of moods.
Antiemetics
5-HT3 antagonists such as ondansetron, granisetron, and tropisetron are important antiemetic agents. They are particularly important in treating the nausea and vomiting that occur during anticancer chemotherapy using cytotoxic drugs. Another application is in the treatment of post-operative nausea and vomiting. Applications to the treatment of depression and other mental and psychological conditions have also been investigated with some positive results.[citation needed]
Pathology
Defective signalling of serotonin in the brain may be the root cause of sudden infant death syndrome (SIDS). Scientists from the European Molecular Biology Laboratory in Monterotondo, Italy,[30] genetically modified lab mice to produce low levels of the neurotransmitter serotonin. The results showed the mice suffered drops in heart rate and other symptoms of SIDS, and many of the animals died at an early age.
Researchers now believe that low levels of serotonin in the animals' brainstems, which control heartbeat and breathing, may have caused sudden death, researchers said in the July 4, 2008 issue of Science.[31]
If neurons that make serotonin — serotonergic neurons — are abnormal in infants, there is a risk of sudden infant death syndrome (SIDS).[32][33] Low levels of serotonin may also be associated with intense spiritual experiences.[34]
Recent research conducted at Rockefeller University shows that both in patients who suffer from depression and in mice that model the disorder, levels of the p11 protein are decreased. This protein is related to serotonin transmission within the brain.[35]
Obsessive-compulsive disorder (OCD) can be a debilitating disorder with the following two anxiety-related essential features: obsessions (undesirable, recurrent, disturbing thoughts) and compulsions (repetitive or ritualized behaviors). SSRIs, and other medicines which alter serotonin levels, have been approved to be used to treat symptoms of OCD.
Serotonin syndrome
Extremely high levels of serotonin can have toxic and potentially fatal effects, causing a condition known as serotonin syndrome. In practice, such toxic levels are essentially impossible to reach through an overdose of a single anti-depressant drug, but require a combination of serotonergic agents, such as an SSRI with an MAOI.[36] The intensity of the symptoms of serotonin syndrome vary over a wide spectrum, and the milder forms are seen even at non-toxic levels.[37] [citation needed]
Chronic diseases resulting from serotonin 5-HT2B overstimulation
In blood, serotonin stored in platelets is active wherever platelets bind, as a vasoconstrictor to stop bleeding, and also as a fibrocyte mitotic, to aid healing. Because of these effects, overdoses of serotonin, or serotonin agonist drugs, may cause acute or chronic pulmonary hypertension from pulmonary vasoconstriction, or else syndromes of retroperitoneal fibrosis or cardiac valve fibrosis (endocardial fibrosis) from overstimulation of serotonic growth receptors on fibrocytes.[citation needed]
Serotonin itself may cause a syndrome of cardiac fibrosis when it is eaten in large quantities in the diet (the Matoki banana of East Africa) or when it is over-secreted by certain mid-gut carcinoid tumors.[citation needed] The valvular fibrosis in such cases is typically on the right side of the heart, since excess serotonin in the serum outside platelets is metabolized in the lungs, and does not reach the left circulation.[citation needed]
Serotonergic agonist drugs in overdose in experimental animals not only cause acute (and sometimes fatal) pulmonary hypertension, but there is epidemiologic evidence that chronic use of certain of these drugs produce a chronic pulmonary hypertensive syndrome in humans.[citation needed] Some serotonergic agonist drugs also cause fibrosis anywhere in the body, particularly the syndrome of retroperitoneal fibrosis, as well as cardiac valve fibrosis.[38]
In the past, three groups of serotonergic drugs have been epidemiologically linked with these syndromes. They are the serotonergic vasoconstrictive anti-migraine drugs (ergotamine and methysergide),[38] the serotonergic appetite suppressant drugs (fenfluramine, chlorphentermine, and aminorex), and certain anti-parkinsonian dopaminergic agonists, which also stimulate serotonergic 5-HT2B receptors. These include pergolide and cabergoline, but not the more dopamine-specific lisuride.[39] As with fenfluramine, some of these drugs have been withdrawn from the market after groups taking them showed a statistical increase of one or more of the side effects described. An example is pergolide. The drug was in decreasing use since reported in 2003 to be associated with cardiac fibrosis.[40] Two independent studies published in the New England Journal of Medicine in January 2007, implicated pergolide along with cabergoline in causing valvular heart disease.[41][42] As a result of this, the FDA removed pergolide from the U.S. market in March, 2007.[43] (Since cabergoline is not approved in the U.S. for Parkinson's Disease, but for hyperprolactinemia, the drug remains on the market. Treatment for hyperprolactinemia requires lower doses than that for Parkinson's Disease, diminishing the risk of valvular heart disease).[44]
Because neither the amino acid L-tryptophan nor the SSRI-class antidepressants raise blood serotonin levels [citation needed], they are not under suspicion to cause the syndromes described. However, since 5-hydroxytryptophan (5-HTP) does raise blood serotonin levels, it is under some of the same scrutiny as actively serotonergic drugs.[citation needed]
In unicellular organisms
Serotonin is used by a variety of single-cell organisms for various purposes. Selective serotonin re-uptake inhibitors (SSRIs) have been found to be toxic to algae.[45] The gastrointestinal parasite Entamoeba histolytica secretes serotonin, causing a sustained secretory diarrhea in some patients.[46][47] Patients infected with Entamoeba histolytica have been found to have highly elevated serum serotonin levels which returned to normal following resolution of the infection.[48] Entamoeba histolytica also responds to the presence of serotonin by becoming more virulent.[49]
In plants
Serotonin is found in mushrooms and plants, including fruits and vegetables. The highest values of 25–400 mg/kg have been found in nuts of the walnut (Juglans) and hickory (Carya) genuses. Serotonin concentrations of 3–30 mg/kg have been found in plantain, pineapple, banana, kiwifruit, plums, and tomatoes. Moderate levels from 0.1–3 mg/kg have been found in a wide range of tested vegetables.[50] Serotonin is one compound of the poison contained in stinging nettles (Urtica dioica). It should be noted that serotonin, unlike its precursors 5-HTP and tryptophan, does not cross the blood–brain barrier, which means that ingesting serotonin in the diet has no effect on brain serotonin levels. Several plants contain serotonin together with a family of related tryptamines that are methylated at the amino (NH2) and hydroxy (OH) groups, are N-oxides, or miss the OH group. Examples are plants from the Anadenanthera genus that are used in the hallucinogenic yopo snuff.
In animals
Serotonin as a neurotransmitter is found in many animals, including insects. Several toad venoms, as well as that of the Brazilian wandering spider and stingray, contain serotonin and related tryptamines. It has also been identified as the trigger for swarm behaviour in locusts.[51]
History
Serotonin was originally discovered by Italian and American scientists in the late 1940s. Isolated and named in 1948 by Maurice M. Rapport, Arda Green, and Irvine Page of the Cleveland Clinic,[52] the name serotonin is something of a misnomer and reflects the circumstances of the compound's discovery. It was initially identified as a vasoconstrictor substance in blood serum – hence serotonin, a serum agent affecting vascular tone. This agent was later chemically identified as 5-hydroxytryptamine (5-HT) by Rapport, and, as the broad range of physiological roles were elucidated, 5-HT became the preferred name in the pharmacological field.
Increasing serotonin levels
This article contains instructions, advice, or how-to content. (May 2009) |
This article or section appears to contradict itself. (March 2009) |
Serotonin levels can not be increased by diet alone. For example, increasing foods rich in tryptophan (eg, meats, proteins) does not increase serotonin levels, due to competition with other amino acids.[53] What is required to increase serotonin production is an increase in the ratio of tryptophan to phenylalanine and leucine. Fruits with a good ratio include dates, papaya and banana. Foods with a lower ratio inhibit the production of serotonin. These include whole wheat and rye bread.[54] Research indicates that vigorous aerobic exercise improves mood through BDNF expression, there is however no direct evidence that this is caused by an increase in serotonin levels.[55] Research also suggests that eating a diet rich in whole grain carbohydrates and low in protein will increase serotonin by secreting insulin, which helps in amino acid competition.[53] However, increasing insulin for a long period of time can sometimes onset insulin resistance, which is related to obesity, type 2 diabetes, and lower serotonin levels. It is also believed that muscles use many of the amino acids except tryptophan, allowing men to have more serotonin than women.[56] Bright light therapy is another popular method which prevents the conversion of serotonin to melatonin.[57] A similar effect is obtained by spending more time in natural sunlight. Recently, acupuncture has been shown to stimulate the release of serotonin in lab animals.[58]
Myo-inositol, a carbocyclic polyol present in many foods, is known to play a role in serotonin modulation.[59]
References
- ^ Indiana State University
- ^ Berger M, Gray JA, Roth BL (2009). "The expanded biology of serotonin". Annual Review of Medicine. 60: 355–66. doi:10.1146/annurev.med.60.042307.110802.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Kang K, Park S, Kim YS, Lee S, Back K (2009). "Biosynthesis and biotechnological production of serotonin derivatives". Appl Microbiol Biotechnol. 83: 27–34. doi:10.1007/s00253-009-1956-1. PMID 19308403.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Timothy Niacaris,Leon Avery: "Serotonin regulates repolarization of the C. elegans pharyngeal muscle", The Journal of Experimental Biology 206, 223-231 (2003) http://jeb.biologists.org/cgi/content/full/206/2/223
- ^ Supriya Srinivasan, Leila Sadegh, Ida C. Elle, Anne G.L. Christensen,Nils J. Faergeman, Kaveh Ashrafi: "Serotonin Regulates C. elegans Fat and Feeding through Independent Molecular Mechanisms", Cell Metabolism, Volume 7, Issue 6, 533-544, 4 June 2008 http://www.cell.com/cell-metabolism/abstract/S1550-4131(08)00144-7
- ^ EA Kravitz: "Hormonal control of behavior: amines and the biasing of behavioral output in lobsters" Science 30 September 1988: Vol. 241. no. 4874, pp. 1775 - 1781 http://www.sciencemag.org/cgi/reprint/241/4874/1775.pdf
- ^ Yeh SR, Fricke RA, Edwards DH.:"The effect of social experience on serotonergic modulation of the escape circuit of crayfish." Science. 1996 Jan 19;271(5247):366-9
- ^ Lesch, K.; Bengel, D.; Heils, A.; Sabol, S. Z.; Greenberg, B. D.; Petri, S.; Clemens, R.; Müller, J. B.; Hamer, D. H.; Murphy, D. L. (1996). "Association of Anxiety-Related Traits with a Polymorphism in the Serotonin Transporter Gene Regulatory Region". Science. 274: 1527–31. doi:10.1126/science.274.5292.1527. PMID 8929413.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Caspi, A.; Sugden, K.; Moffitt, T. E.; Taylor, A.; Craig, I. W.; Harrington, W.; McClay, J.; Mill, J.; Martin, J.; Braithwaite, A.; Poulton, R. (2003). "Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene". Science. 301: 386–89. doi:10.1126/science.1083968. PMID 12869766.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Caspi N, Modai I, Barak P, Waisbourd A, Zbarsky H, Hirschmann S, Ritsner M. (2001 Mar). "Pindolol augmentation in aggressive schizophrenic patients: a double-blind crossover randomized study". Int Clin Psychopharmacol. 16(2): 111–5. PMID 11236069.
{{cite journal}}: Check date values in:|year=(help)CS1 maint: multiple names: authors list (link) CS1 maint: year (link) - ^ Basky, Greg (2000). "Suicide linked to serotonin gene". CMAJ. 162 (9): 1343.
{{cite journal}}: Unknown parameter|day=ignored (help); Unknown parameter|month=ignored (help) - ^ Crockett, M. J.; Clark, L.; Tabibnia, G.; Lieberman, M. D.; Robbins, T. W. (2008). "Serotonin modulates behavioral reactions to unfairness". Science (journal). 320 (5884): 1739. doi:10.1126/science.1155577. PMC 2504725. PMID 18535210.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Essentials of Human Anatomy & Physiology, Eighth Edition, p. 336
- ^ Dolasetron
- ^ Mickael Lesurtel, Rolf Graf, Boris Aleil, Diego J. Walther, Yinghua Tian, Wolfram Jochum, Christian Gachet, Michael Bader, Pierre-Alain Clavien: "Platelet-Derived Serotonin Mediates Liver Regeneration"Science 7 April 2006: Vol. 312. no. 5770, pp. 104 - 107 http://www.sciencemag.org/cgi/content/abstract/312/5770/104
- ^ Ramadhan B. Matondo, Carine Punt, Judith Homberg, Mathilda J. M. Toussaint, Ronald Kisjes, Suzanne J. A. Korporaal, Jan Willem N. Akkerman, Edwin Cuppen, Alain de Bruin: "Deletion of the serotonin transporter in rats disturbs serotonin homeostasis without impairing liver regeneration", Am J Physiol Gastrointest Liver Physiol 296: G963-G968, 2009. http://ajpgi.physiology.org/cgi/content/abstract/296/4/G963
- ^ Collet C, Schiltz C, Geoffroy V, Maroteaux L, Launay JM, de Vernejoul MC: "The serotonin 5-HT2B receptor controls bone mass via osteoblast recruitment and proliferation," FASEB J. 2008 Feb;22(2):418-27 http://www.fasebj.org/cgi/content/full/22/2/418
- ^ It Takes Guts To Build Bone, Scientists Discover
- ^ Cell 135, 825–837, November 28, 2008
- ^ a b Alenina N, Kikic D, Todiras M, Mosienko V, Qadri F, Plehm R, Boyé P, Vilianovitch L, Sohr R, Tenner K, Hörtnagl H, Bader M. (2009). "Growth retardation and altered autonomic control in mice lacking brain serotonin". Proc Natl Acad Sci U S A. 106: 10332–10337. doi:10.1073/pnas.0810793106. PMID 19520831.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Frazer, A.; and Hensler, J. G. (1999). "Understanding the neuroanatomical organization of serotonergic cells in the brain provides insight into the functions of this neurotransmitter". In Siegel, G. J. (ed.). Basic Neurochemistry. Agranoff, Bernard W.; Fisher, Stephen K.; Albers, R. Wayne; Uhler, Michael D. (Sixth ed.). Lippincott Williams and Wilkins. ISBN 0-397-51820-X.
In 1964, Dahlstrom and Fuxe (discussed in [2]), using the Falck-Hillarp technique of histofluorescence, observed that the majority of serotonergic soma are found in cell body groups, which previously had been designated as the raphe nuclei.
{{cite book}}: External link in|chapterurl=|chapterurl=ignored (|chapter-url=suggested) (help)CS1 maint: multiple names: authors list (link) - ^ |The Raphe nuclei group of neurons are located along the brain stem from the labels 'Mid Brain' to 'Oblongata', centered on the pons. (See relevant image.)
- ^ Mingyan Zhou, Karen Engel,1 and Joanne Wang* (2007). "Evidence for Significant Contribution of a Newly Identified Monoamine Transporter (PMAT) to Serotonin Uptake in the Human Brain". In ? (ed.). Biochemical Pharmacology. ? (Vol 73 Issue 1 ed.). Elsevier Inc. doi:10.1016/j.bcp.2006.09.008. ISBN ?. PMID 1828907.
{{cite book}}:|editor=has numeric name (help); Check|isbn=value: invalid character (help)CS1 maint: multiple names: authors list (link) CS1 maint: numeric names: authors list (link) - ^ McDuffie, J. E. (2000). "-hydroxytryptamine stimulates phosphorylation of p44/p42 mitogen-activated protein kinase activation in bovine aortic endothelial cell cultures". J. Cardiovasc. Pharmacol. 35 (3): 398. doi:10.1097/00005344-200003000-00008.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ [Walther et. al: Science 2003 Jan 3;299(5603):76: Synthesis of serotonin by a second tryptophan hydroxylase isoform.]http://www.ncbi.nlm.nih.gov/pubmed/12511643?ordinalpos=5&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_DefaultReportPanel.Pubmed_RVDocSum
- ^ Levinson, Douglas F. "The Genetics of Depression: A Review." Rev. of The Genetics of Depression.
- ^ Schröder P, Abele C, Gohr P, Stuhlfauth-Roisch U, Grosse W. (1999). "Latest on enzymology of serotonin biosynthesis in walnut seeds". Adv Exp Med Biol. 467: 637–644. PMID 10721112.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Cell. 2008 Nov 28;135(5):825-37.
- ^ Benmansour, Saloua et al., Effects of Chronic Antidepressant Treatments on Serotonin Transporter Function, Density, and mRNA Level. Journal of Neuroscience, 1999, 19(23):10494-10501. http://www.jneurosci.org/cgi/content/full/19/23/10494
- ^ Audero, Enrica; Coppi, Elisabetta; Mlinar, Boris; Rossetti, Tiziana; Caprioli, Antonio; Al Banchaabouchi, Mumna; Corradetti, Renato; Gross, Cornelius (2008). "Sporadic Autonomic Dysregulation and Death Associated with Excessive Serotonin Autoinhibition". Science. 321: 130–133. doi:10.1126/science.1157871. PMID 18599790.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Lesurtel, M.; et al. (2006). "Platelet-derived serotonin mediates liver regeneration". Science. 312 (5770): 104–7. doi:10.1126/science.1123842. PMID 16601191.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Paterson, D. S.; Trachtenberg, F. L.; Thompson, E.G.; et al. (2006). "Multiple serotonergic brainstem abnormalities in sudden infant death syndrome". JAMA. 296 (17): 2124–32. doi:10.1001/jama.296.17.2124. PMID 17077377.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Sciencedaily Report Anger and Aggression in Women: Blame It On Genetics
- ^ Farde, Lars; and Borg, Jacqueline; Section of psychiatry at Karolinska Institutet in Stockholm, Sweden 2003, the study and a vulgarized article
- ^ Svenningsson, P.; et al. (2006). "Alterations in 5-HT1B receptor function by p11 in depression-like states". Science. 311 (5757): 77–80. doi:10.1126/science.1117571. PMID 16400147.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Isbister, G. K.; Bowe, S. J.; Dawson, A.; Whyte, I. M. (2004). "Relative toxicity of selective serotonin reuptake inhibitors (SSRIs) in overdose". J. Toxicol. Clin. Toxicol. 42 (3): 277–85. doi:10.1081/CLT-120037428. PMID 15362595.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Dunkley, E. J.; Isbister, G. K.; Sibbritt, D.; Dawson, A. H.; Whyte, I. M. (2003). "The Hunter Serotonin Toxicity Criteria: simple and accurate diagnostic decision rules for serotonin toxicity". QJM. 96 (9): 635–42. doi:10.1093/qjmed/hcg109. PMID 12925718.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b Baskin, Steven I. (1991). Principles of Cardiac Toxicology. CRC Press. ISBN 0849388090.
- ^ Jähnichen, S.; Horowski, R.; Pertz, H. Template:PDFlink Presentation. Retrieved on 2007-03-30.
- ^ ADRAC (2004). "Cardiac valvulopathy with pergolide". Aust Adv Drug React Bull. 23 (4).
{{cite journal}}: Unknown parameter|month=ignored (help) Free full text from the Australian Therapeutic Goods Administration - ^ Schade, Rene; Andersohn, Frank; Suissa, Samy; Haverkamp, Wilhelm; Garbe, Edeltraut (2007-01-04), "Dopamine Agonists and the Risk of Cardiac-Valve Regurgitation", New England Journal of Medicine, 356 (1): 29–38, doi:10.1056/NEJMoa062222, PMID 17202453
{{citation}}: Check date values in:|date=(help)CS1 maint: date and year (link) - ^ Zanettini, Renzo; Antonini, Angelo; Gatto, Gemma; Gentile, Rosa; Tesei, Silvana; Pezzoli, Gianna (2007-01-04), "Valvular Heart Disease and the Use of Dopamine Agonists for Parkinson's Disease", New England Journal of Medicine, 356 (1): 39–46, doi:10.1056/NEJMoa054830, PMID 17202454
{{citation}}: Check date values in:|date=(help)CS1 maint: date and year (link) - ^ "Food and Drug Administration Public Health Advisory". 2007-03-29. Retrieved 2007-04-27.
{{cite web}}: Check date values in:|date=(help) - ^ "MedWatch - 2007 Safety Information Alerts. Permax (pergolide) and generic equivalents". U.S. Food and Drug Administration. March 29, 2007. Retrieved 2007-03-30.
- ^ Johnson, D. J.; Sanderson, H.; Brain, R. A.; Wilson, C. J.; Solomon, K. R. (2007). "Toxicity and hazard of selective serotonin reuptake inhibitor antidepressants fluoxetine, fluvoxamine, and sertraline to algae". Ecotoxicol. Environ. Saf. 67 (1): 128–39. doi:10.1016/j.ecoenv.2006.03.016. PMID 16753215.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ McGowan, K.; Kane, A.; Asarkof, N.; et al. (1983). "Entamoeba histolytica causes intestinal secretion: role of serotonin". Science. 221 (4612): 762–4. doi:10.1126/science.6308760. PMID 6308760.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ McGowan, K.; Guerina, V.; Wicks, J.; Donowitz, M. (1985). "Secretory hormones of Entamoeba histolytica". Ciba Found. Symp. 112: 139–54. PMID 2861068.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Banu, Naheed; et al. (2005). "Neurohumoral alterations and their role in amoebiasis" (PDF). Indian J. Clin Biochem. 20 (2): 142–5. doi:10.1007/BF02867414.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Acharya, D. P.; Sen, M. R.; Sen, P. C. (1989). "Effect of exogenous 5-hydroxytryptamine on pathogenicity of Entamoeba histolytica in experimental animals". Indian J. Exp. Biol. 27 (8): 718–20. PMID 2561282.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Feldman, J. M.; Lee, E. M. (1985). "Serotonin content of foods: effect on urinary excretion of 5-hydroxyindoleacetic acid". Am. J. Clin. Nutr. 42 (4): 639–43. PMID 2413754.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ BBC NEWS | Science & Environment | Locust swarms 'high' on serotonin
- ^ Rapport, Maurice M.; Green, Arda A.; Page, Irvine H. (1948). "Serum vasoconstrictor (serotonin). IV. Isolation and characterization". J. Biol. Chem. 176 (3): 1243–51. PMID 18100415.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b Young, S. N. (2007). "How to increase serotonin in the human brain without drugs" (PDF). J. Psychiatry Neurosci. 32 (6): 394–9. PMC 2077351. PMID 18043762. Retrieved 2008-12-30.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Nutritional Pharmacology Of Sleep & Depression
- ^ Exercise, Antidepressant Medications, and Enhanced Brain Derived Neurotrophic Factor Expression
- ^ http://books.google.com/books?id=2PxhgaCThccC&pg=PA49&lpg=PA49&dq=insulin+resistance+and+serotonin&source=web&ots=udW-o42zjY&sig=rtcdA8TTAkuxSxwvrR4TxYcnF30&hl=en&sa=X&oi=book_result&resnum=3&ct=result#PPA64,M1
- ^ Education : Philips
- ^ Acupuncture Stimulates the Release of Serotonin, but Not Dopamine, in the Rat Nucleus Accumbens
- ^ Clements, Rex (1980). "Myo-inositol content of common foods: development of a high-myo-inositol diet" (PDF). American Journal of Clinical Nutrition. 33 (9): 1954–1967. PMID 7416064. Retrieved 2009-05-18.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help)
External links
- PsychoTropicalResearch Extensive reviews on serotonergic drugs and Serotonin Syndrome.
- Molecule of the Month: Serotonin at University of Bristol
- Scientific America 60-Second Psych: No Fair! My Serotonin Level Is Low.
- Serotonin Test Interpretation on ClinLab Navigator.


